Electrophysiologic Effects of Growth Hormone Post-Myocardial Infarction
Abstract
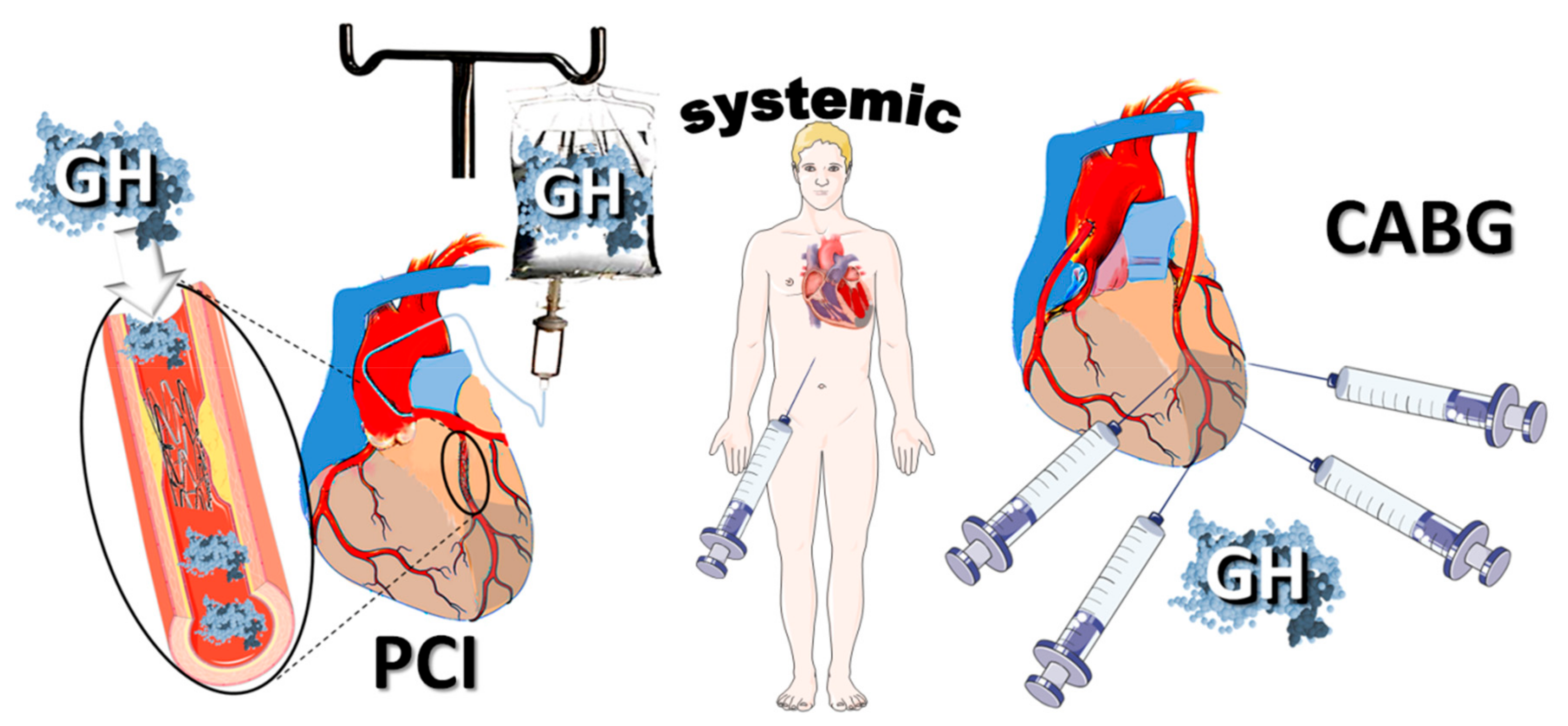
1. Introduction
2. Acute Myocardial Infarction
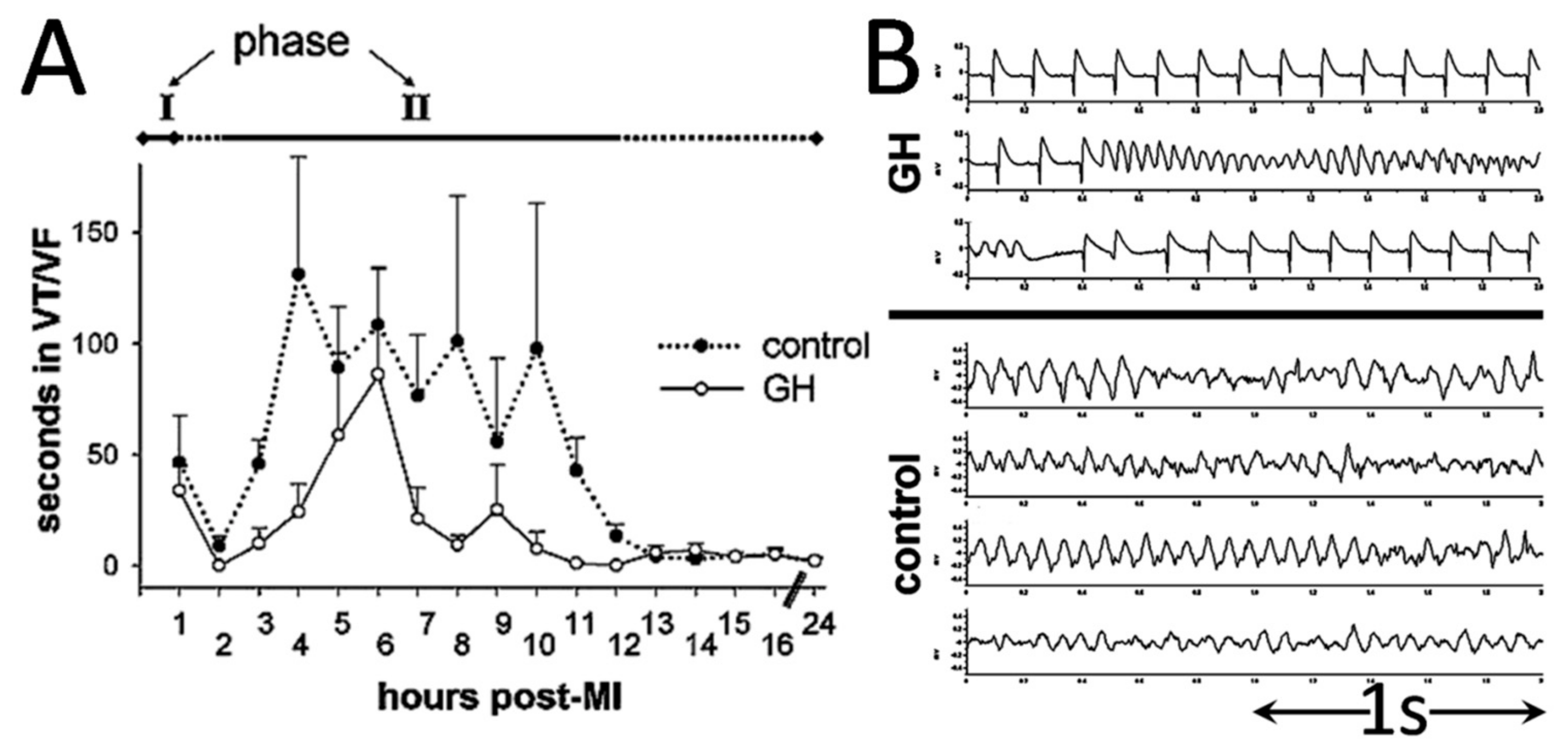
2.1. Primary Ventricular Tachyarrhythmias
2.2. Growth Hormone Confers Antiarrhythmic Actions
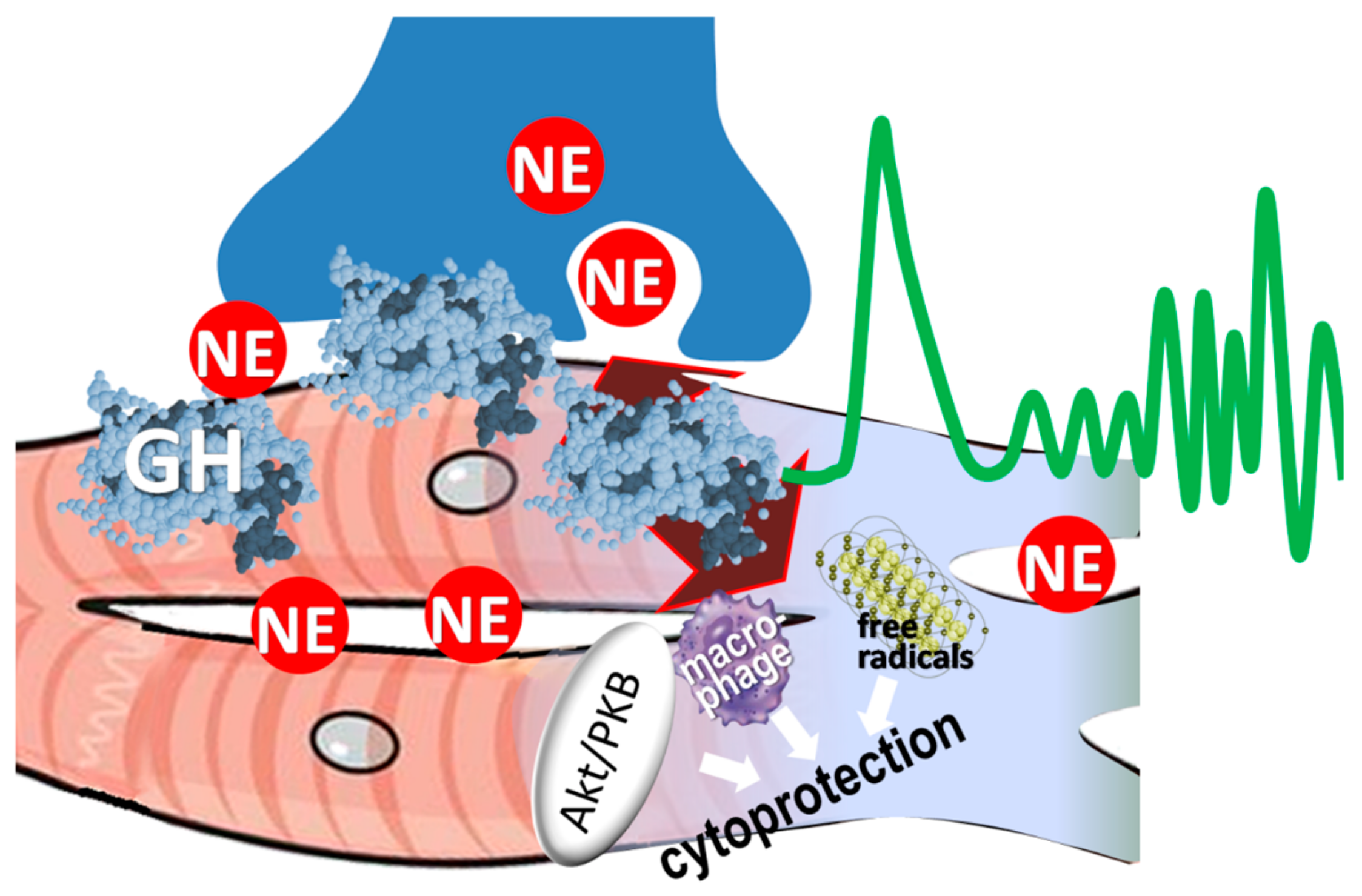
2.3. Cytoprotective Effects
2.4. GH and Myocardial Norepinephrine Content
2.5. Local Norepinephrine Release During Acute Myocardial Infarction
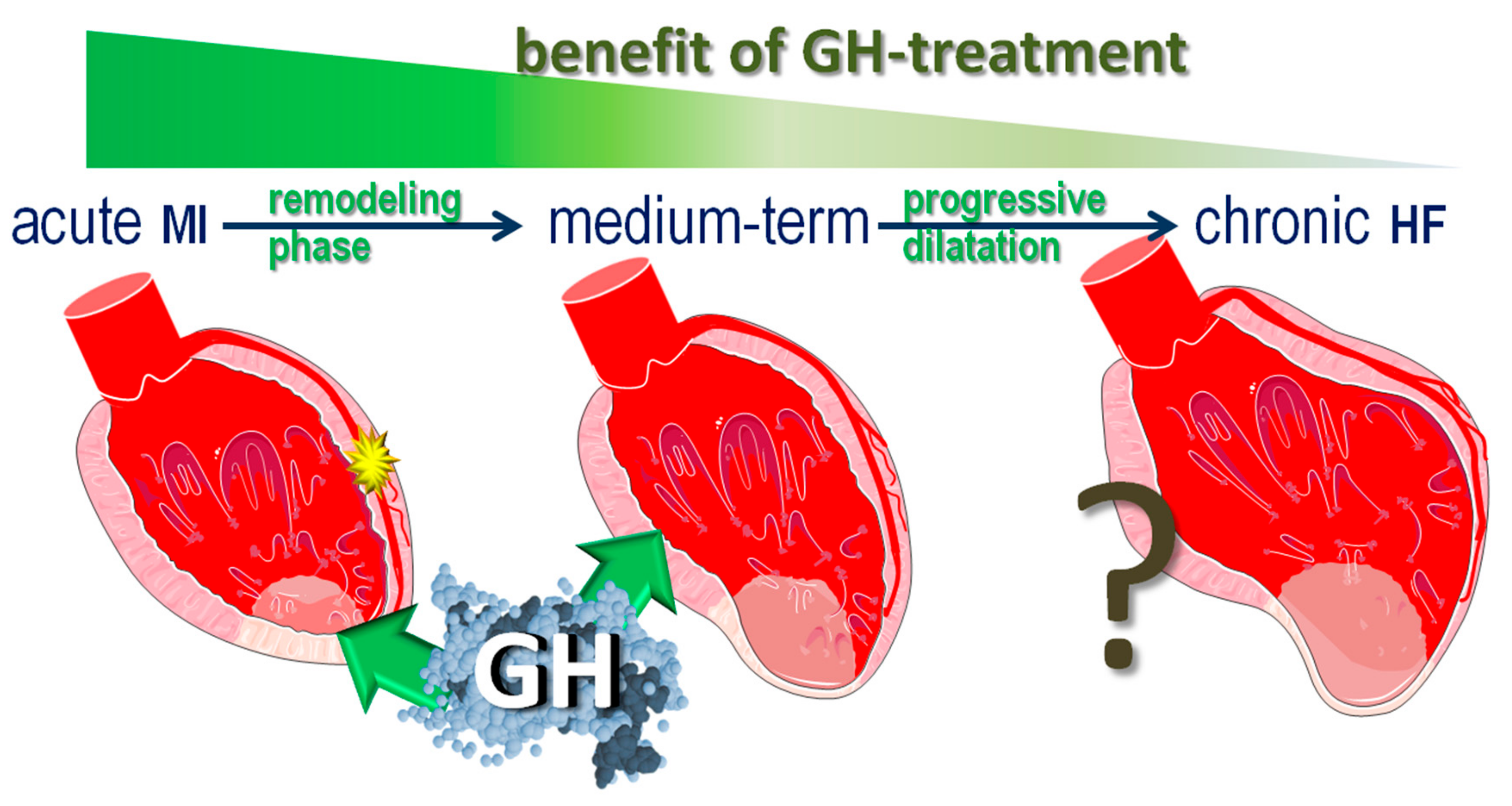
3. Structural Remodeling
3.1. Pathophysiology
3.2. GH in Post-MI Remodeling
4. Electrophysiologic Remodeling
4.1. Pathophysiology
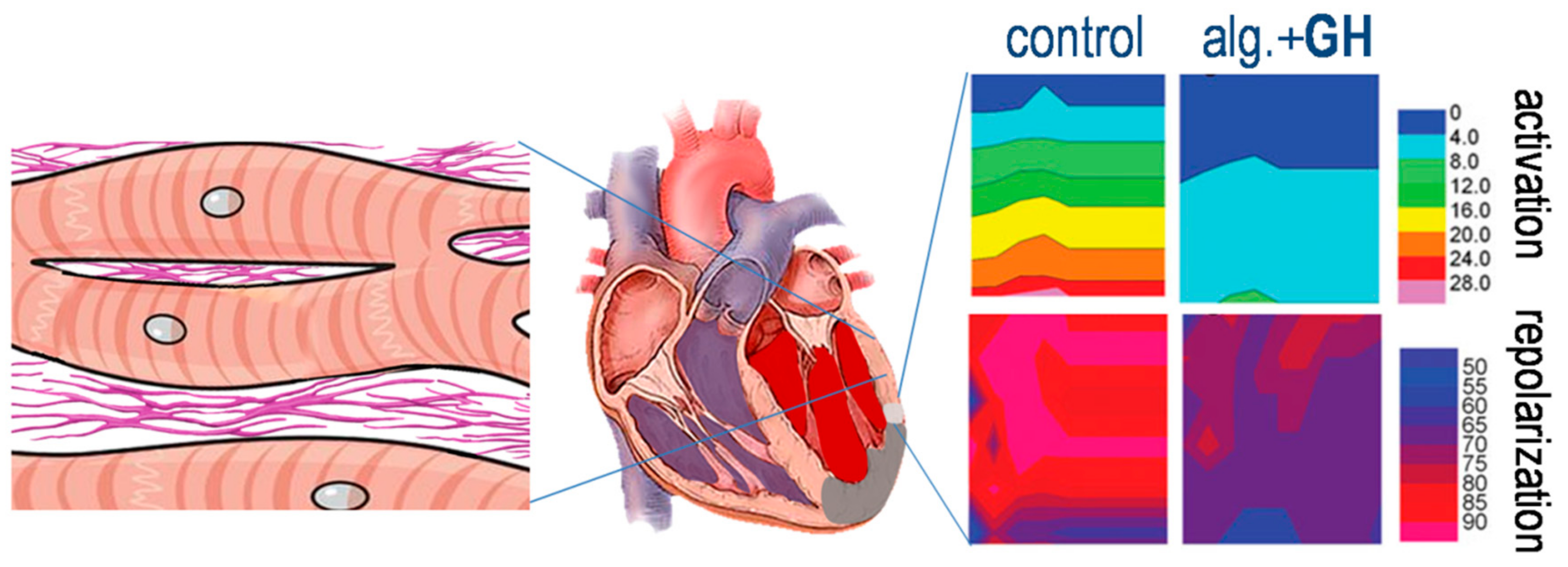
4.2. Growth Hormone and Electrophysiologic Remodeling
4.3. Hemodynamic Improvement
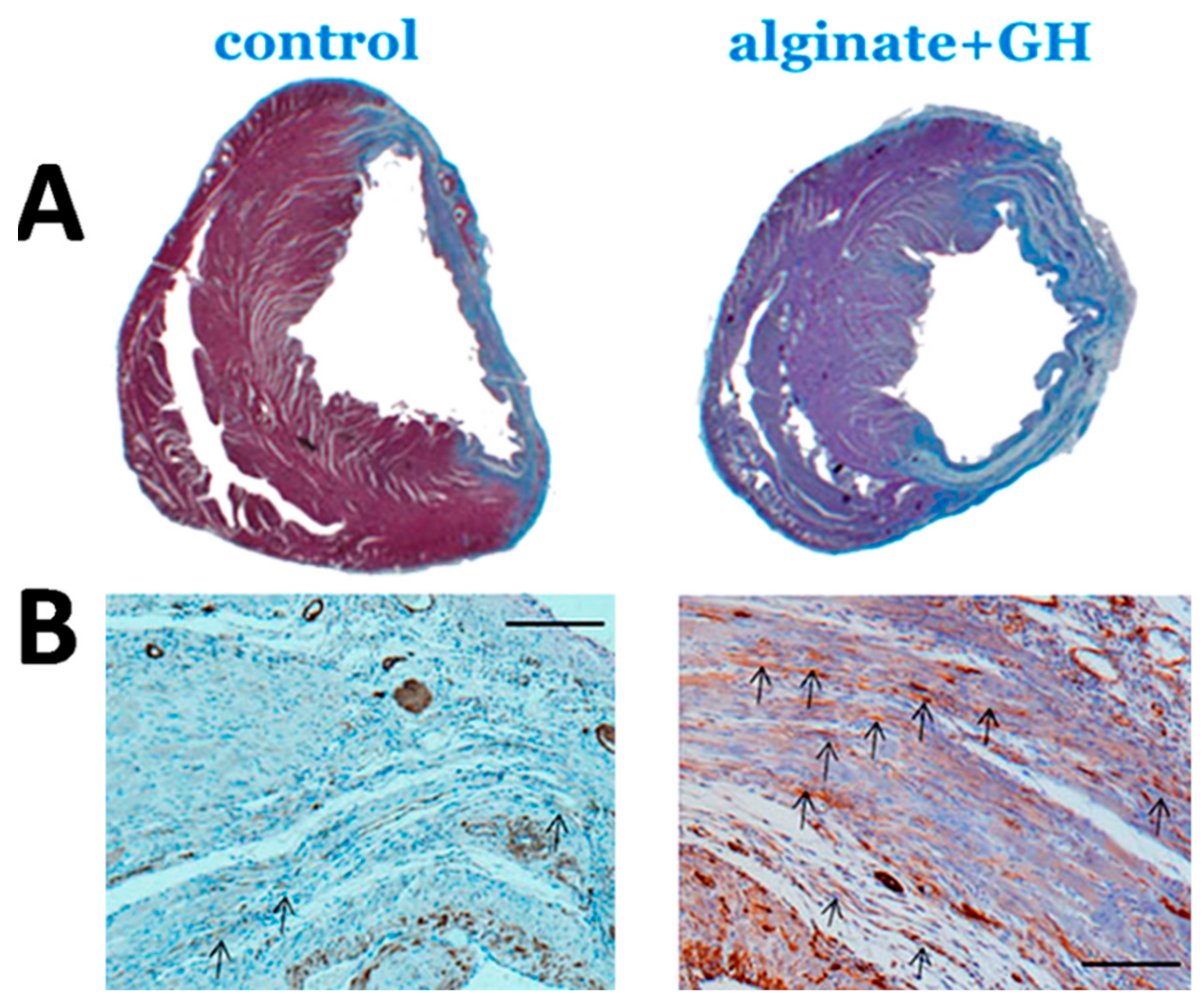
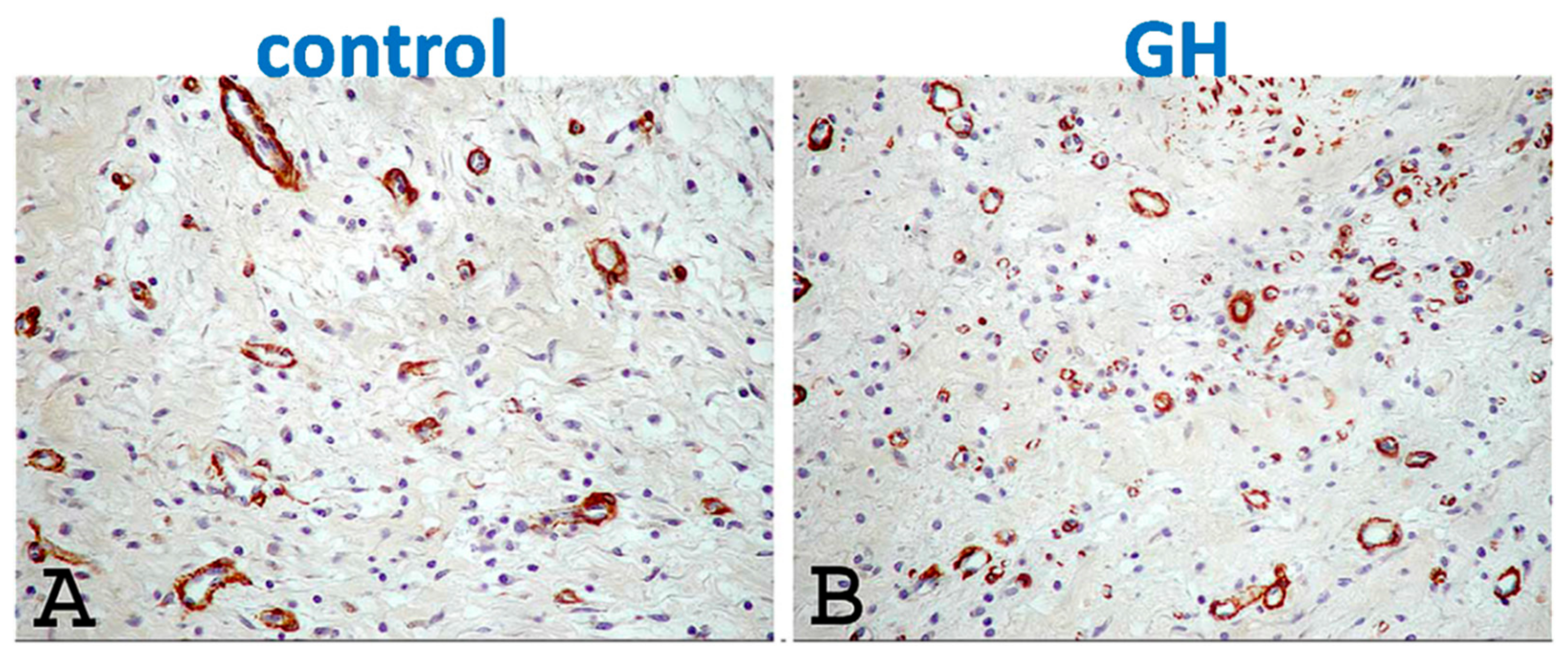
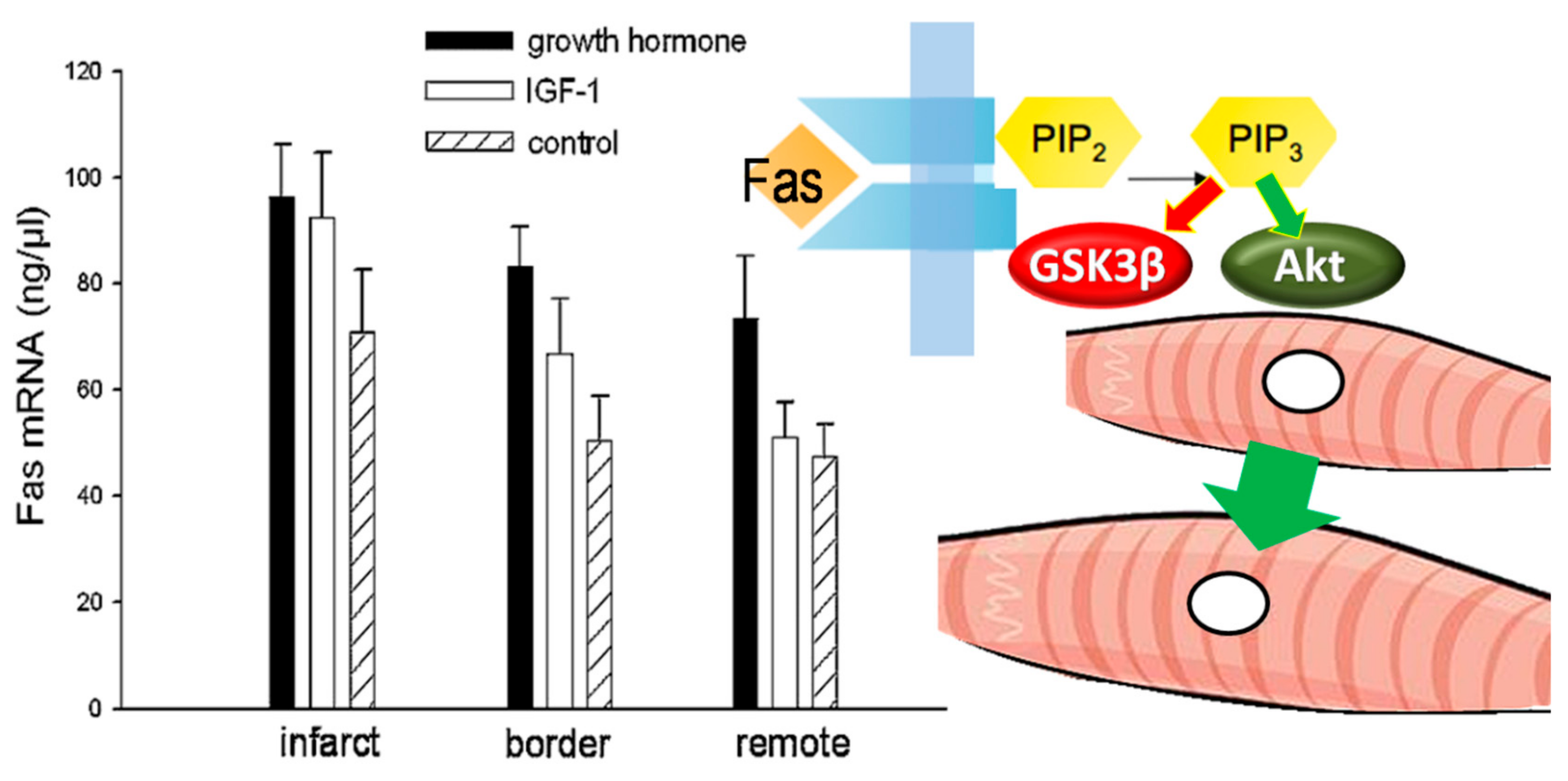
4.4. Attenuated Fibrotic Response in the Remote Myocardium
4.5. Preservation of the Peri-Infarct-Area
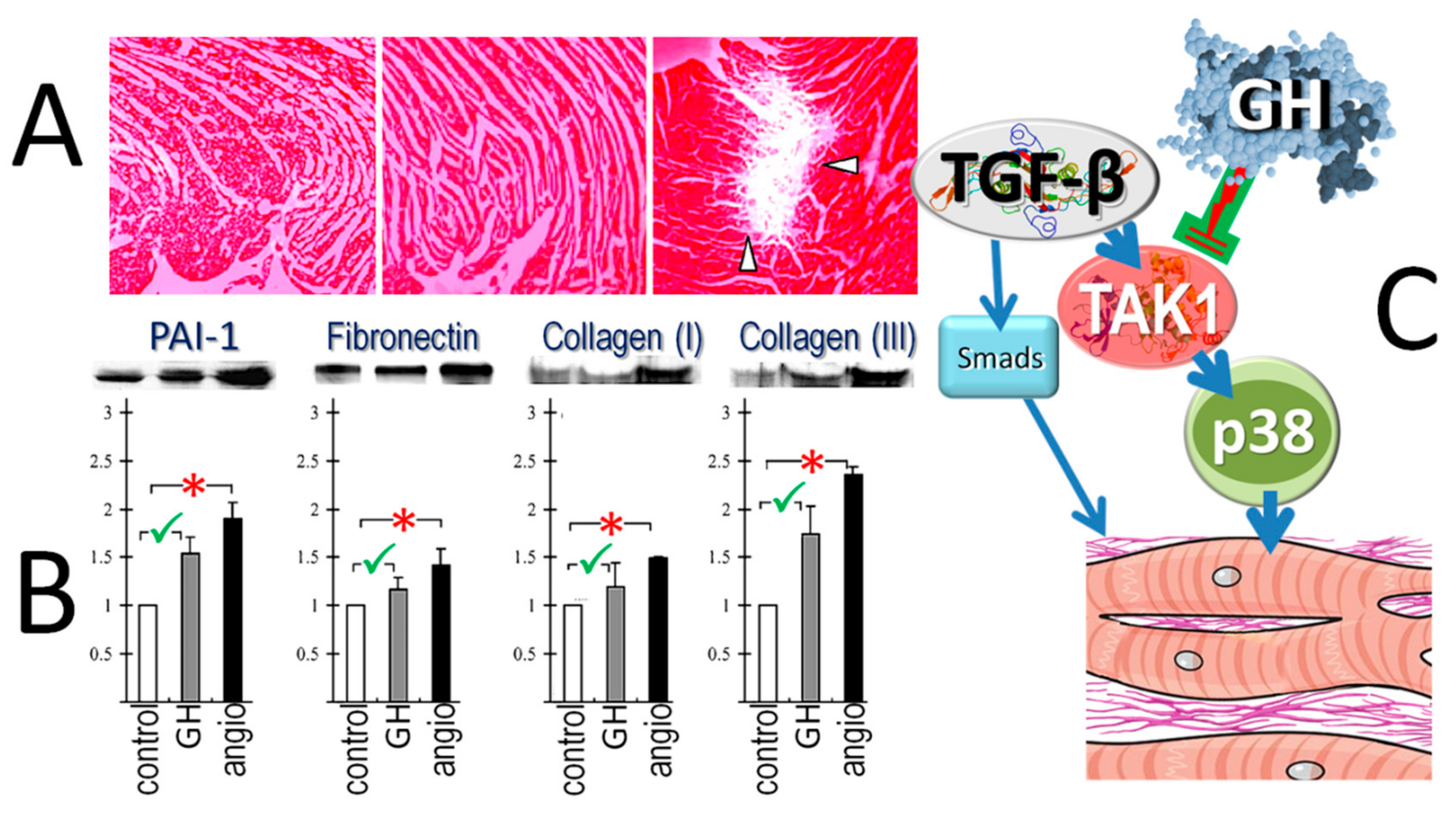
4.6. Differential Growth Hormone-Effects on Fibrosis
5. Chronic Phase Clinical Trials
6. Future Directions
7. Conclusions
Funding
Conflicts of Interest
References
- Kolettis, T.M. Ventricular arrhythmias during acute ischemia/infarction: Mechanisms and management. In Cardiac Arrhythmias: From Basic Mechanism to State-of-the-Art Management; Kibos, A.S., Knight, B.P., Essebag, V., Fishberger, S.B., Slevin, M., Tintoiu, I.C., Eds.; Springer-Verlag: London, UK, 2014; pp. 237–251. [Google Scholar] [CrossRef]
- Kolettis, T.M. Coronary artery disease and ventricular tachyarrhythmia: Pathophysiology and treatment. Curr. Opin. Pharmacol. 2013, 13, 210–217. [Google Scholar] [CrossRef]
- Raben, M.S. Chromatographic separation of growth hormone and other peptides. Proc. Soc. Exp. Biol. Med. 1956, 93, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Mathews, L.S.; Enberg, B.; Norstedt, G. Regulation of rat growth hormone receptor gene expression. J. Biol. Chem. 1989, 264, 9905–9910. [Google Scholar] [PubMed]
- Devesa, J.; Almenglo, C.; Devesa, P. Multiple effects of growth hormone in the body: Is it really the hormone for growth? Clin. Med. Insights Endocrinol. Diabetes 2016, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Mitsi, A.C.; Hatzistergos, K.; Baltogiannis, G.G.; Kolettis, T.M. Early, selective growth hormone administration may ameliorate left ventricular remodeling after myocardial infarction. Med. Hypotheses 2005, 64, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial, I. Fourth universal definition of myocardial infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Gheeraert, P.J.; De Buyzere, M.L.; Taeymans, Y.M.; Gillebert, T.C.; Henriques, J.P.; De Backer, G.; De Bacquer, D. Risk factors for primary ventricular fibrillation during acute myocardial infarction: A systematic review and meta-analysis. Eur. Heart J. 2006, 27, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Clements-Jewery, H.; Hearse, D.J.; Curtis, M.J. Phase 2 ventricular arrhythmias in acute myocardial infarction: A neglected target for therapeutic antiarrhythmic drug development and for safety pharmacology evaluation. Br. J. Pharmacol. 2005, 145, 551–564. [Google Scholar] [CrossRef]
- Piccini, J.P.; Berger, J.S.; Brown, D.L. Early sustained ventricular arrhythmias complicating acute myocardial infarction. Am. J. Med. 2008, 121, 797–804. [Google Scholar] [CrossRef]
- Castagnino, H.E.; Milei, J.; Toranzos, F.A.; Weiss, V.; Beigelman, R. Bivalent effects of human growth hormone in experimental myocardial infarcts. Protective when administered alone and aggravating when combined with beta blockers. Jpn. Heart J. 1990, 31, 845–855. [Google Scholar] [CrossRef][Green Version]
- Otani, H.; Yamamura, T.; Nakao, Y.; Hattori, R.; Kawaguchi, H.; Osako, M.; Imamura, H. Insulin-like growth factor-I improves recovery of cardiac performance during reperfusion in isolated rat heart by a wortmannin-sensitive mechanism. J. Cardiovasc. Pharmacol. 2000, 35, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Opitz, C.F.; Mitchell, G.F.; Pfeffer, M.A.; Pfeffer, J.M. Arrhythmias and death after coronary artery occlusion in the rat. Continuous telemetric ECG monitoring in conscious, untethered rats. Circulation 1995, 92, 253–261. [Google Scholar] [CrossRef]
- Elaiopoulos, D.A.; Tsalikakis, D.G.; Agelaki, M.G.; Baltogiannis, G.G.; Mitsi, A.C.; Fotiadis, D.I.; Kolettis, T.M. Growth hormone decreases phase II ventricular tachyarrhythmias during acute myocardial infarction in rats. Clin. Sci. (Lond.) 2007, 112, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Råmunddal, T.; Gizurarson, S.; Lorentzon, M.; Omerovic, E. Antiarrhythmic effects of growth hormone—In vivo evidence from small-animal models of acute myocardial infarction and invasive electrophysiology. J. Electrocardiol. 2008, 41, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Kolettis, T.M.; Agelaki, M.G.; Baltogiannis, G.G.; Vlahos, A.P.; Mourouzis, I.; Fotopoulos, A.; Pantos, C. Comparative effects of acute vs. chronic oral amiodarone treatment during acute myocardial infarction in rats. Europace 2007, 9, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Elaiopoulos, D.A.; Kolettis, T.M. Antiarrhythmic actions of growth hormone during acute myocardial infarction. J. Electrocardiol. 2009, 42, 298–299. [Google Scholar] [CrossRef]
- Costoya, J.A.; Finidori, J.; Moutoussamy, S.; Searis, R.; Devesa, J.; Arce, V.M. Activation of growth hormone receptor delivers an antiapoptotic signal: Evidence for a role of Akt in this pathway. Endocrinology 1999, 140, 5937–5943. [Google Scholar] [CrossRef]
- Jin, H.; Yang, R.; Lu, H.; Ogasawara, A.K.; Li, W.; Ryan, A.; Peale, F.; Paoni, N.F. Effects of early treatment with growth hormone on infarct size, survival, and cardiac gene expression after acute myocardial infarction. Growth Horm. IGF Res. 2002, 12, 208–215. [Google Scholar] [CrossRef]
- Castagnino, H.E.; Lago, N.; Centrella, J.M.; Calligaris, S.D.; Fariña, S.; Sarchi, M.I.; Cardinali, D.P. Cytoprotection by melatonin and growth hormone in early rat myocardial infarction as revealed by Feulgen DNA staining. Neuro. Endocrinol. Lett. 2002, 23, 391–395. [Google Scholar]
- Hatzistergos, K.E.; Mitsi, A.C.; Zachariou, C.; Skyrlas, A.; Kapatou, E.; Agelaki, M.G.; Fotopoulos, A.; Kolettis, T.M.; Malamou-Mitsi, V. Randomised comparison of growth hormone versus IGF-1 on early post-myocardial infarction ventricular remodelling in rats. Growth Horm. IGF Res. 2008, 18, 157–165. [Google Scholar] [CrossRef]
- Reimer, K.A.; Lowe, J.E.; Rasmussen, M.M.; Jennings, R.B. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation 1977, 56, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Cittadini, A.; Isgaard, J.; Monti, M.G.; Casaburi, C.; Di Gianni, A.; Serpico, R.; Iaccarino, G.; Sacca, L. Growth hormone prolongs survival in experimental postinfarction heart failure. J. Am. Coll. Cardiol. 2003, 41, 2154–2163. [Google Scholar] [CrossRef]
- Masternak, M.M.; Bartke, A. Growth hormone, inflammation and aging. Pathobiol. Aging Age Relat. Dis. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Kontonika, M.; Barka, E.; Roumpi, M.; Vilaeti, A.D.; Baltogiannis, G.G.; Vlahos, A.P.; Agathopoulos, S.; Kolettis, T.M. Intra-myocardial growth hormone administration ameliorates arrhythmogenesis during ischemia-reperfusion in rats. J. Electrocardiol. 2017, 50, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Kolettis, T.M.; Kontonika, M.; Valenti, M.C.; Vilaeti, A.D.; Baltogiannis, G.G.; Papalois, A.; Kyriakides, Z.S. Arrhythmogenesis after acute myocardial necrosis with and without preceding ischemia in rats. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Greenwood, J.P.; Stoker, J.B.; Mary, D.A.; Gilbey, S.G. Sympathetic nerve hyperactivity is associated with increased peripheral vascular resistance in hypopituitary patients with growth hormone deficiency. Clin. Endocrinol. (Oxf.) 2002, 56, 759–763. [Google Scholar] [CrossRef]
- Sverrisdόttir, Y.B.; Elam, M.; Herlitz, H.; Bengtsson, B.Å.; Johannsson, G. Intense sympathetic nerve activity in adults with hypopituitarism and untreated growth hormone deficiency. J. Clin. Endocrinol. Metab. 1998, 83, 1881–1885. [Google Scholar] [CrossRef]
- Omerovic, E.; Bollano, E.; Mobini, R.; Kujacic, V.; Madhu, B.; Soussi, B.; Fu, M.; Hjalmarson, Å.; Waagstein, F.; Isgaard, J. Growth hormone improves bioenergetics and decreases catecholamines in postinfarct rat hearts. Endocrinology 2000, 141, 4592–4599. [Google Scholar] [CrossRef]
- Schömig, A. Adrenergic mechanisms in myocardial infarction: Cardiac and systemic catecholamine release. J. Cardiovasc. Pharmacol. 1988, 12 (Suppl. 1), S1–S7. [Google Scholar] [CrossRef]
- Ravingerova, T.; Slezak, J.; Tribulova, N.; Dzurba, A.; Uhrik, B.; Ziegelhoffer, A. High arrythmogenesis during early reperfusion of ischaemic myocardium: Participation of oxygen free radicals. J. Basic Clin. Physiol. Pharmacol. 1993, 4, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Schömig, A.; Haass, M.; Richardt, G. Catecholamine release and arrhythmias in acute myocardial ischaemia. Eur. Heart J. 1991, 12 (Suppl. F), 38–47. [Google Scholar] [CrossRef]
- Rump, A.F.; Klaus, W. Evidence for norepinephrine cardiotoxicity mediated by superoxide anion radicals in isolated rabbit hearts. Naunyn. Schmiedebergs. Arch. Pharmacol. 1994, 349, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Agathopoulos, S.; Kolettis, T.M. Novel strategies for cardiac repair post-myocardial infarction. Curr. Pharm. Des. 2014, 20, 1925–1929. [Google Scholar] [CrossRef] [PubMed]
- Sutton, M.G.; Sharpe, N. Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy. Circulation 2000, 101, 2981–2988. [Google Scholar] [CrossRef]
- Friberg, L.; Werner, S.; Eggertsen, G.; Ahnve, S. Growth hormone and insulin-like growth factor-1 in acute myocardial infarction. Eur. Heart J. 2000, 21, 1547–1554. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Wang, X.; Leri, A.; Jana, K.P.; Liu, Y.; Kajstura, J.; Baserga, R.; Anversa, P. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J. Clin. Invest. 1997, 100, 1991–1999. [Google Scholar] [CrossRef]
- Cittadini, A.; Grossman, J.D.; Napoli, R.; Katz, S.E.; Strömer, H.; Smith, R.J.; Clark, R.; Morgan, J.P.; Douglas, P.S. Growth hormone attenuates early left ventricular remodeling and improves cardiac function in rats with large myocardial infarction. J. Am. Coll. Cardiol. 1997, 29, 1109–1116. [Google Scholar] [CrossRef]
- Daskalopoulos, E.P.; Vilaeti, A.D.; Barka, E.; Mantzouratou, P.; Kouroupis, D.; Kontonika, M.; Tourmousoglou, C.; Papalois, A.; Pantos, C.; Blankesteijn, W.M.; et al. Attenuation of post-infarction remodeling in rats by sustained myocardial growth hormone administration. Growth Factors 2015, 33, 250–258. [Google Scholar] [CrossRef]
- Badimon, L.; Borrell, M. Microvasculature recovery by angiogenesis after myocardial infarction. Curr. Pharm. Des. 2018, 24, 2967–2973. [Google Scholar] [CrossRef]
- Shinde, A.V.; Frangogiannis, N.G. Fibroblasts in myocardial infarction: A role in inflammation and repair. J. Mol. Cell. Cardiol. 2014, 70, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Mitsi, A.C.; Hatzistergos, K.E.; Niokou, D.; Pappa, L.; Baltogiannis, G.G.; Tsalikakis, D.G.; Papalois, A.; Kyriakides, Z.S.; Malamou-Mitsi, V.; Kolettis, T.M. Early, intracoronary growth hormone administration attenuates ventricular remodeling in a porcine model of myocardial infarction. Growth Horm. IGF Res. 2006, 16, 93–100. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.F.; Leblond, A.L.; Kelly, G.; Kumar, A.H.; Metharom, P.; Buneker, C.K.; Alizadeh-Vikali, N.; Hristova, I.; Hynes, B.G.; O’Connor, R.; et al. Potent long-term cardioprotective effects of single low-dose insulin-like growth factor-1 treatment postmyocardial infarction. Circ. Cardiovasc. Interv. 2011, 4, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Caplice, N.M.; DeVoe, M.C.; Choi, J.; Dahly, D.; Murphy, T.; Spitzer, E.; Van Geuns, R.; Maher, M.M.; Tuite, D.; Kerins, D.M.; et al. Randomized placebo controlled trial evaluating the safety and efficacy of single low-dose intracoronary insulin-like growth factor following percutaneous coronary intervention in acute myocardial infarction (RESUS-AMI). Am. Heart J. 2018, 200, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kontonika, M.; Barka, E.; Roumpi, M.; La Rocca, V.; Lekkas, P.; Daskalopoulos, E.P.; Vilaeti, A.D.; Baltogiannis, G.G.; Vlahos, A.P.; Agathopoulos, S.; et al. Prolonged intra-myocardial growth hormone administration ameliorates post-infarction electrophysiologic remodeling in rats. Growth Factors 2017, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Christia, P.; Bujak, M.; Gonzalez-Quesada, C.; Chen, W.; Dobaczewski, M.; Reddy, A.; Frangogiannis, N.G. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J. Histochem. Cytochem. 2013, 61, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Mayoux, E.; Ventura-Clapier, R.; Timsit, J.; Behar-Cohen, F.; Hoffmann, C.; Mercadier, J.J. Mechanical properties of rat cardiac skinned fibers are altered by chronic growth hormone hypersecretion. Circ. Res. 1993, 72, 57–64. [Google Scholar] [CrossRef]
- Timsit, J.; Riou, B.; Bertherat, J.; Wisnewsky, C.; Kato, N.S.; Weisberg, A.S.; Lubetzki, J.; Lecarpentier, Y.; Winegrad, S.; Mercadier, J.J. Effects of chronic growth hormone hypersecretion on intrinsic contractility, energetics, isomyosin pattern, and myosin adenosine triphosphatase activity of rat left ventricle. J. Clin. Invest. 1990, 86, 507–515. [Google Scholar] [CrossRef]
- Ueyama, T.; Ohkusa, T.; Yano, M.; Matsuzaki, M. Growth hormone preserves cardiac sarcoplasmic reticulum Ca2+ release channels (ryanodine receptors) and enhances cardiac function in cardiomyopathic hamsters. Cardiovasc. Res. 1998, 40, 64–73. [Google Scholar] [CrossRef][Green Version]
- Grimm, D.; Cameron, D.; Griese, D.P.; Riegger, G.A.; Kromer, E.P. Differential effects of growth hormone on cardiomyocyte and extracellular matrix protein remodeling following experimental myocardial infarction. Cardiovasc. Res. 1998, 40, 297–306. [Google Scholar] [CrossRef][Green Version]
- Brüel, A.; Oxlund, H. The effect of growth hormone on rat myocardial collagen. Growth Horm. IGF Res. 1999, 9, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Beranek, J.T. Does growth hormone reduce fibrosis? Cardiovasc. Res. 1999, 43, 252–254. [Google Scholar] [CrossRef]
- Daskalopoulos, E.P.; Hermans, K.C.; Blankesteijn, W.M. Cardiac (myo)fibroblast: Novel strategies for its targeting following myocardial infarction. Curr. Pharm. Des. 2014, 20, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, C.; Benamer, N.; Morley, G.E. The cardiac fibroblast: Functional and electrophysiological considerations in healthy and diseased hearts. J. Cardiovasc. Pharmacol. 2011, 57, 380–388. [Google Scholar] [CrossRef]
- Wollert, K.C.; Heineke, J.; Westermann, J.; Ludde, M.; Fiedler, B.; Zierhut, W.; Laurent, D.; Bauer, M.K.; Schulze-Osthoff, K.; Drexler, H. The cardiac Fas(APO-1/CD95)receptor/Fas ligand system: Relation to diastolic wall stress in volume-overload hypertrophy in vivo and activation of the transcription factor AP-1 in cardiac myocytes. Circulation 2000, 101, 1172–1178. [Google Scholar] [CrossRef]
- Badorff, C.; Ruetten, H.; Mueller, S.; Stahmer, M.; Gehring, D.; Jung, F.; Ihling, C.; Zeiher, A.M.; Dimmeler, S. Fas receptor signaling inhibits glycogen synthase kinase 3 beta and induces cardiac hypertrophy following pressure overload. J. Clin. Invest. 2002, 109, 373–381. [Google Scholar] [CrossRef]
- Filippatos, G.; Leche, C.; Sunga, R.; Tsoukas, A.; Anthopoulos, P.; Joshi, I.; Bifero, A.; Pick, R.; Uhal, B.D. Expression of FAS adjacent to fibrotic foci in the failing human heart is not associated with increased apoptosis. Am. J. Physiol. 1999, 277, H445–H451. [Google Scholar] [CrossRef]
- Imanishi, R.; Ashizawa, N.; Ohtsuru, A.; Seto, S.; Akiyama-Uchida, Y.; Kawano, H.; Kuroda, H.; Nakashima, M.; Saenko, V.A.; Yamashita, S.; et al. GH suppresses TGF-beta-mediated fibrosis and retains cardiac diastolic function. Mol. Cell. Endocrinol. 2004, 218, 137–146. [Google Scholar] [CrossRef]
- Cheng, W.; Kajstura, J.; Nitahara, J.A.; Li, B.; Reiss, K.; Liu, Y.; Clark, W.A.; Krajewski, S.; Reed, J.C.; Olivetti, G.; et al. Programmed myocyte cell death affects the viable myocardium after infarction in rats. Exp. Cell. Res. 1996, 226, 316–327. [Google Scholar] [CrossRef]
- Genth-Zotz, S.; Zotz, R.; Geil, S.; Voigtlander, T.; Meyer, J.; Darius, H. Recombinant growth hormone therapy in patients with ischemic cardiomyopathy: Effects on hemodynamics, left ventricular function, and cardiopulmonary exercise capacity. Circulation 1999, 99, 18–21. [Google Scholar] [CrossRef]
- Isgaard, J.; Bergh, C.H.; Caidahl, K.; Lomsky, M.; Hjalmarson, A.; Bengtsson, B.A. A placebo-controlled study of growth hormone in patients with congestive heart failure. Eur. Heart J. 1998, 19, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Osterziel, K.J.; Strohm, O.; Schuler, J.; Friedrich, M.; Hanlein, D.; Willenbrock, R.; Anker, S.D.; Poole-Wilson, P.A.; Ranke, M.B.; Dietz, R. Randomised, double-blind, placebo-controlled trial of human recombinant growth hormone in patients with chronic heart failure due to dilated cardiomyopathy. Lancet 1998, 351, 1233–1237. [Google Scholar] [CrossRef]
- Cittadini, A.; Saldamarco, L.; Marra, A.M.; Arcopinto, M.; Carlomagno, G.; Imbriaco, M.; Del Forno, D.; Vigorito, C.; Merola, B.; Oliviero, U.; et al. Growth hormone deficiency in patients with chronic heart failure and beneficial effects of its correction. J. Clin. Endocrinol. Metab. 2009, 94, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Cittadini, A.; Marra, A.M.; Arcopinto, M.; Bobbio, E.; Salzano, A.; Sirico, D.; Napoli, R.; Colao, A.; Longobardi, S.; Baliga, R.R.; et al. Growth hormone replacement delays the progression of chronic heart failure combined with growth hormone deficiency: An extension of a randomized controlled single-blind study. JACC Heart Fail. 2013, 1, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Barka, E.; Papayannis, D.K.; Kolettis, T.M.; Agathopoulos, S. Optimization of Ca(2+) content in alginate hydrogel injected in myocardium. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 223–231. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamatis, K.V.; Kontonika, M.; Daskalopoulos, E.P.; Kolettis, T.M. Electrophysiologic Effects of Growth Hormone Post-Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 918. https://doi.org/10.3390/ijms21030918
Stamatis KV, Kontonika M, Daskalopoulos EP, Kolettis TM. Electrophysiologic Effects of Growth Hormone Post-Myocardial Infarction. International Journal of Molecular Sciences. 2020; 21(3):918. https://doi.org/10.3390/ijms21030918
Chicago/Turabian StyleStamatis, Konstantinos V., Marianthi Kontonika, Evangelos P. Daskalopoulos, and Theofilos M. Kolettis. 2020. "Electrophysiologic Effects of Growth Hormone Post-Myocardial Infarction" International Journal of Molecular Sciences 21, no. 3: 918. https://doi.org/10.3390/ijms21030918
APA StyleStamatis, K. V., Kontonika, M., Daskalopoulos, E. P., & Kolettis, T. M. (2020). Electrophysiologic Effects of Growth Hormone Post-Myocardial Infarction. International Journal of Molecular Sciences, 21(3), 918. https://doi.org/10.3390/ijms21030918





