Network between Cytokines, Cortisol and Occupational Stress in Gas and Oilfield Workers
Abstract
1. Introduction
2. Results
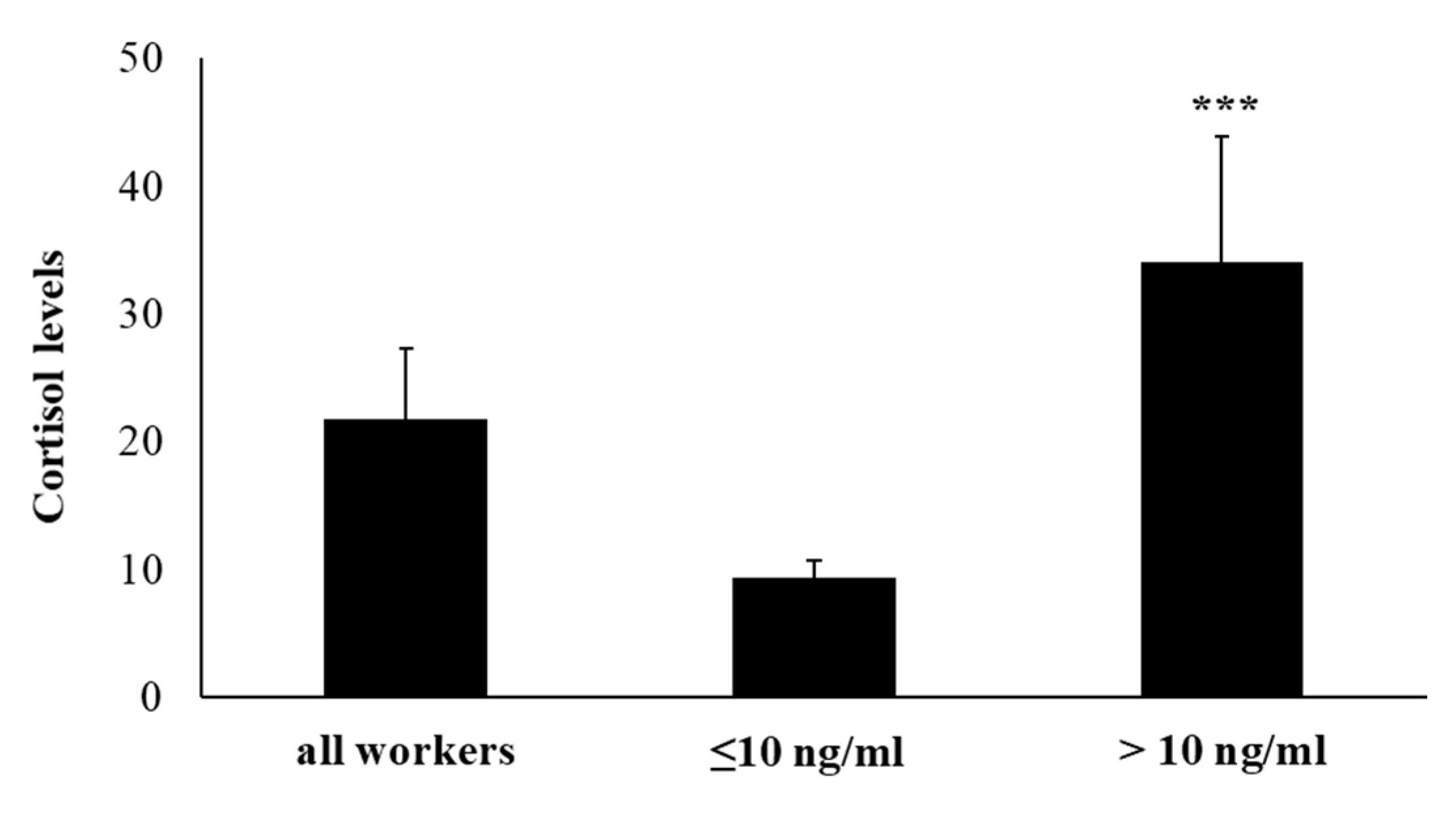
2.1. Salivary Cortisol Levels
2.2. Perception of Anxiety, Job Stress, and Physical Symptoms
2.3. Hematological Blood Parameters
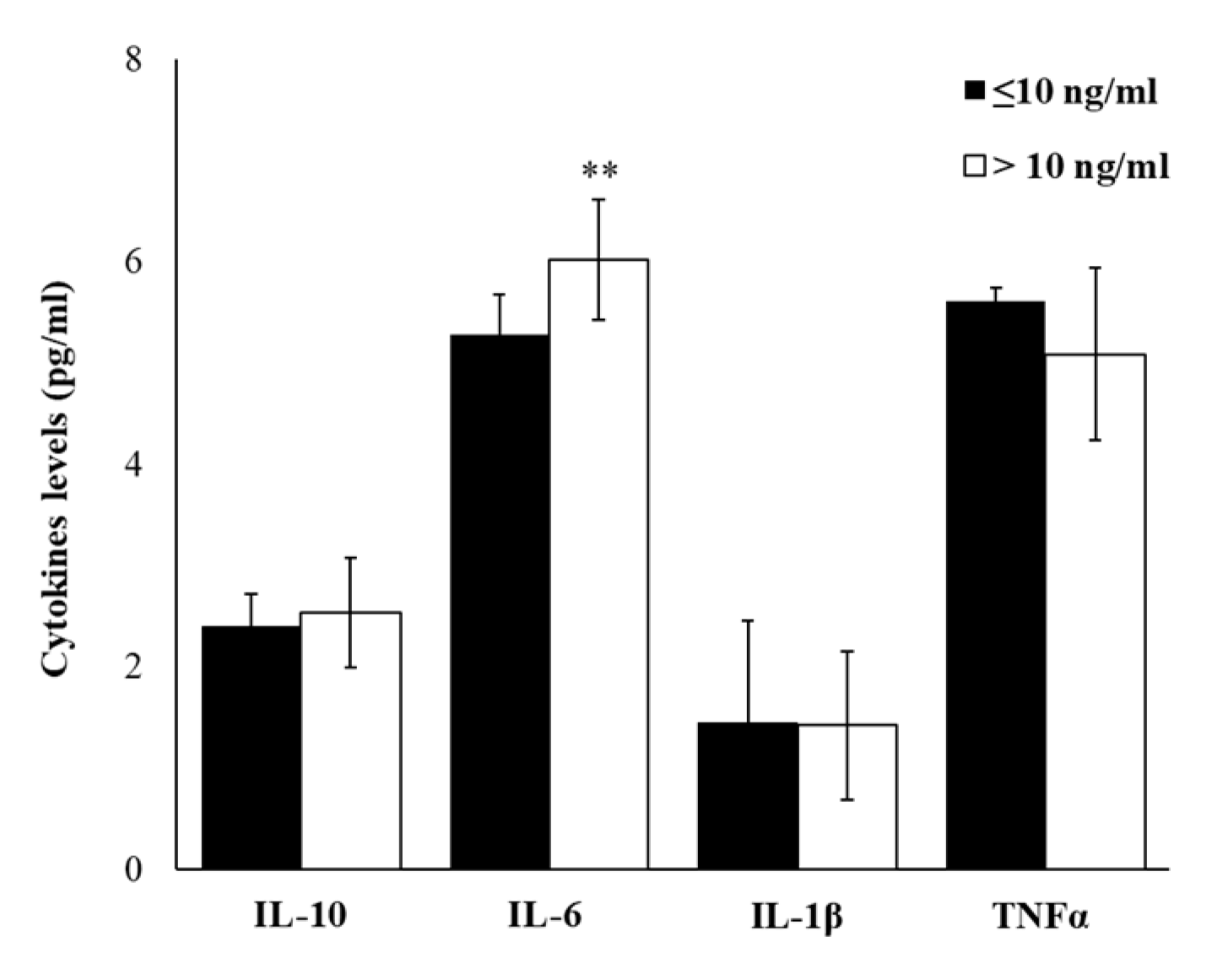
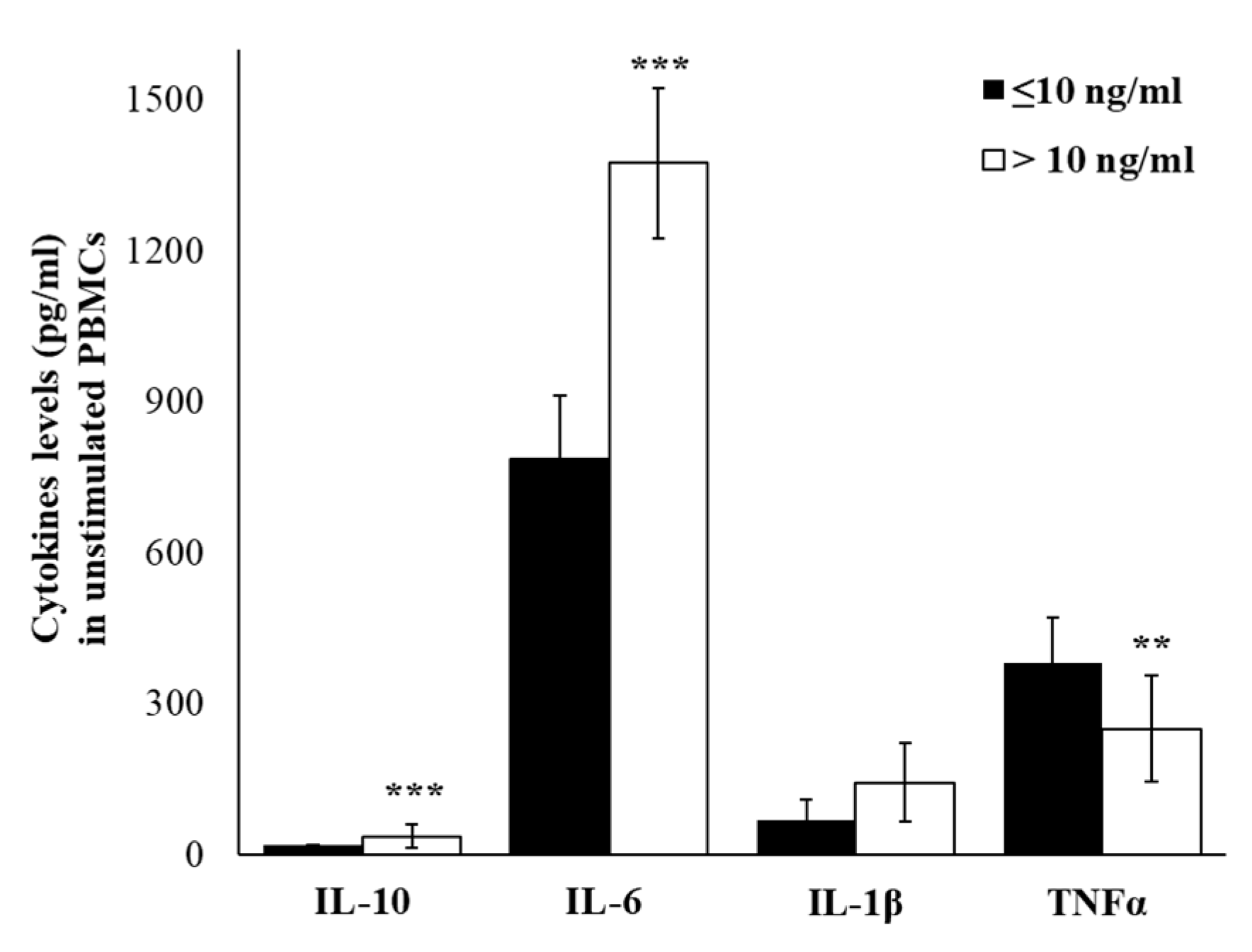
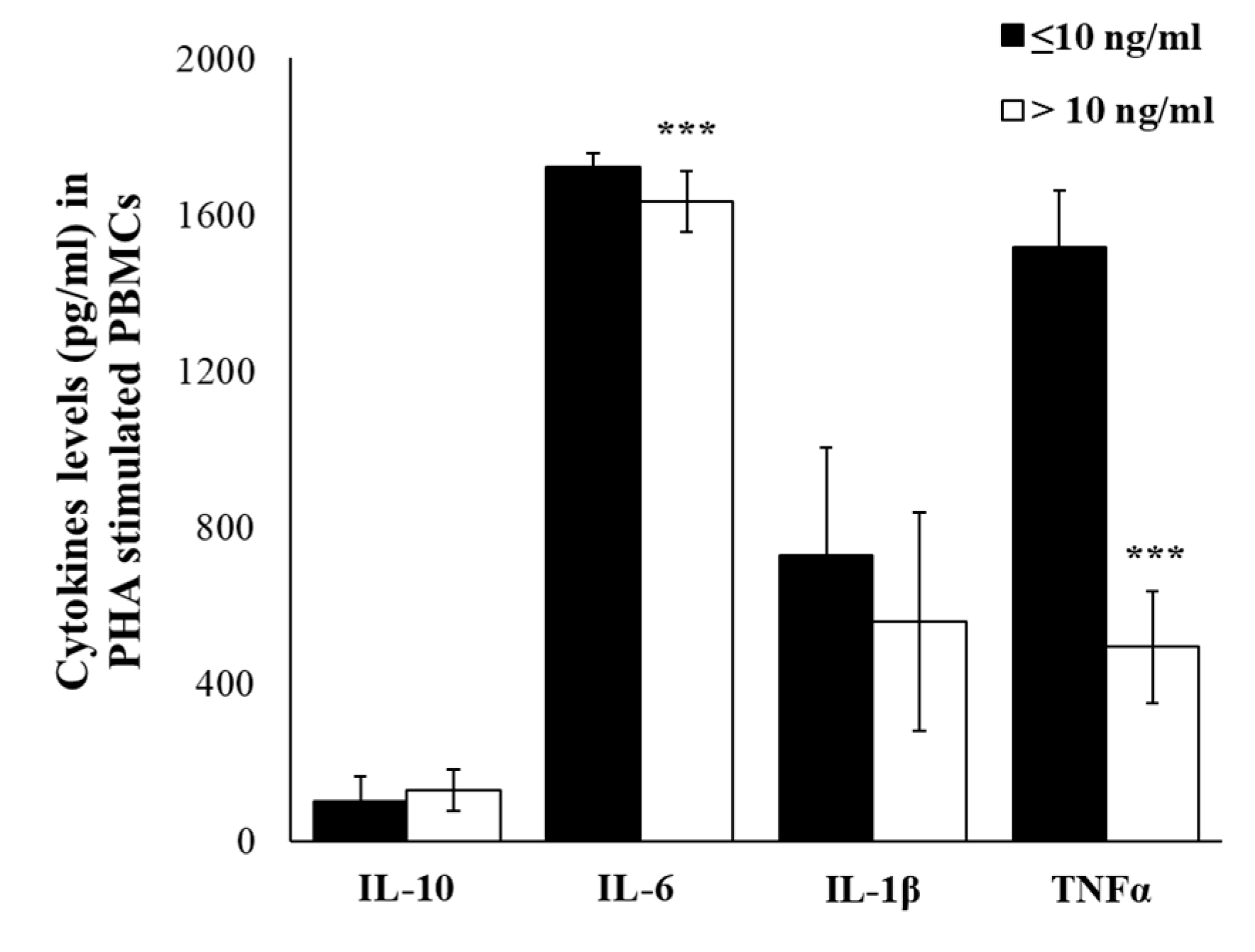
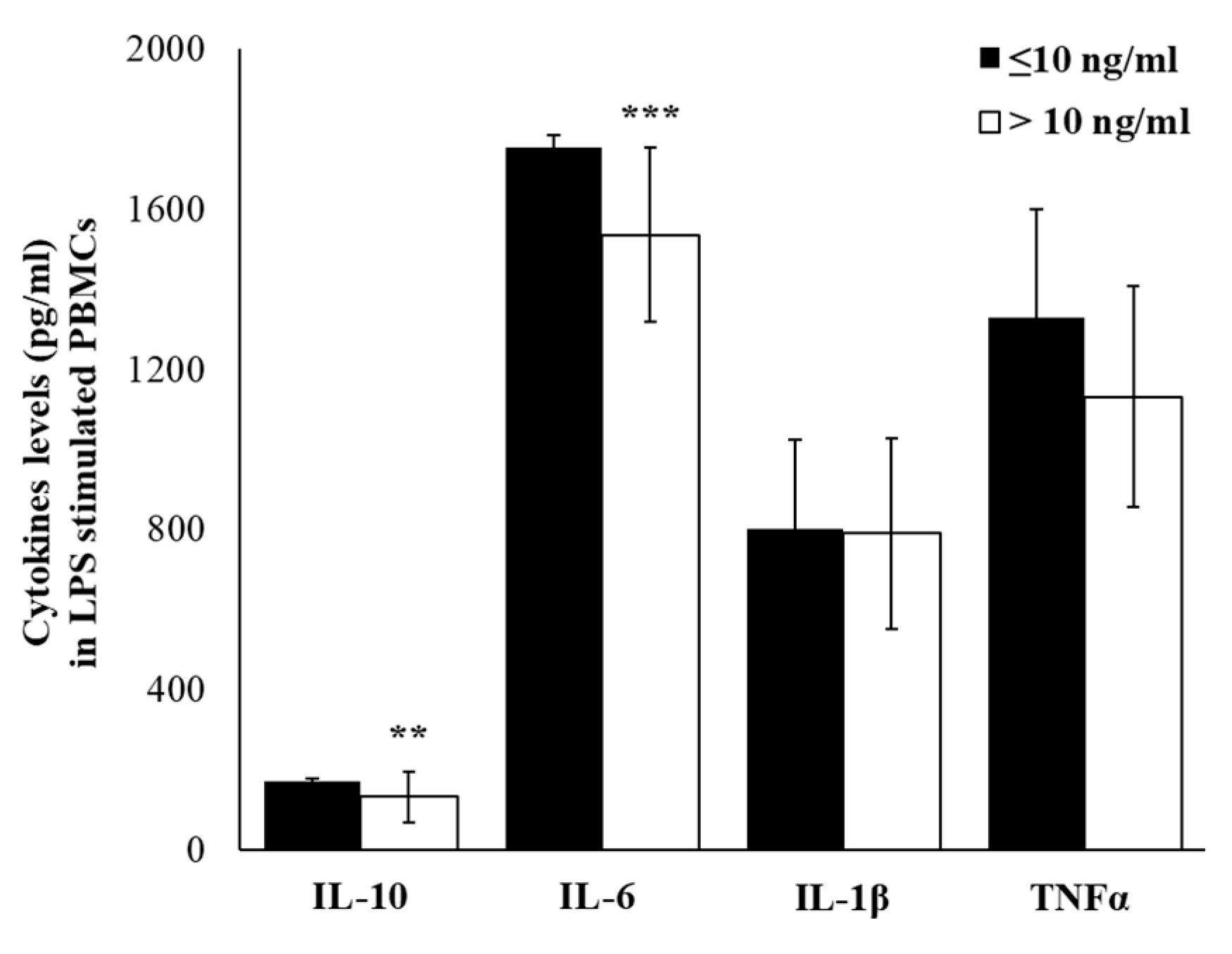
2.4. Plasma Cytokine Profile
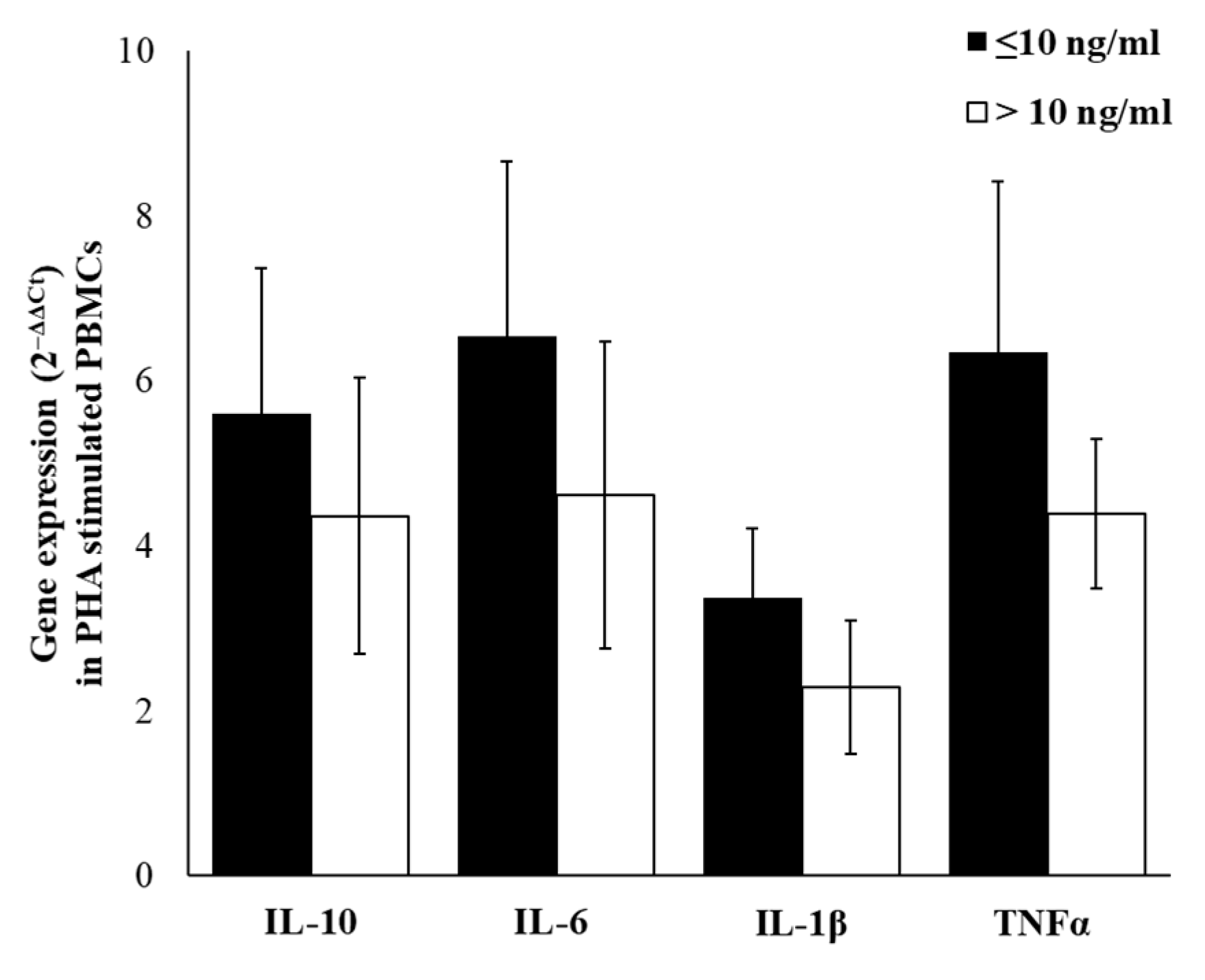
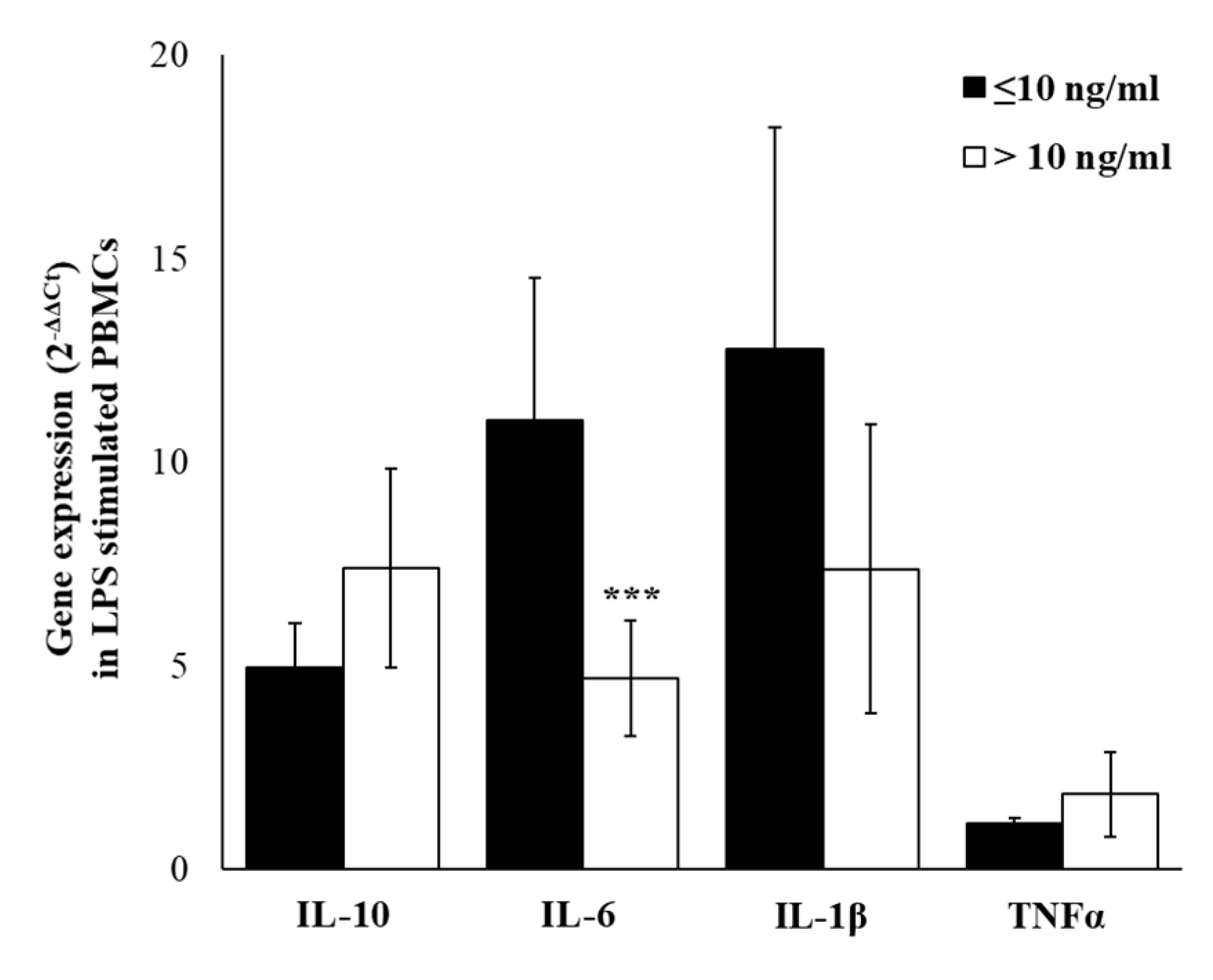
2.5. Cytokines Gene-Expression
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Characteristics of Enrolled Subjects
4.3. Analysis of Anxiety, Occupational Stress, and Perception of Symptoms
4.4. Saliva Sampling and Cortisol Analysis
4.5. Blood Sampling
4.6. Circulating and Released Cytokines Analysis
4.7. Cytokine Gene Expression
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | Central Nervous System |
| Th | T helper lymphocytes |
| IFN | Interferon |
| IL | Interleukin |
| TNF | Tumor Necrosis Factor |
| HPA | Hypothalamic–Pituitary–Adrenal |
| STAI | State-trait-anxiety inventory |
| JD | Job Demand |
| DL | Decision Latitude |
| SS | Social Support |
| JI | Job Insecurity |
| BMI | Body Max Index |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PHA | Phytohaemagglutinin |
| LPS | Lipopolysaccharide |
| JCQ | Job Content Questionnaire |
| NF-κB | nuclear transcription factor- κB |
| AD | Alzheimer’s disease |
| PD | Psychological Demands |
References
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef]
- Stow, J.L.; Low, P.C.; Offenhäuser, C.; Sangermani, D. Cytokine secretion in macrophages and other cells: Pathways and mediators. Immunobiology 2009, 214, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Maes, M. The role of the cytokine network in psychological stress. Acta Neuropsychiatr 2003, 15, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Miller, A.H. Cytokines and psychopathology: Lessons from interferon-alpha. Biol. Psychiatry 2004, 56, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, H.O.; del Rey, A.; Sorkin, E. Lymphokine-containing supernatants from con A-stimulated cells increase corticosterone blood levels. J. Immunol. 1981, 126, 385–387. [Google Scholar]
- Irwin, M.R.; Cole, S.W. Reciprocal regulation of the neural and innate immune systems. Nat. Rev. Immunol. 2011, 11, 625–632. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Caso, J.R.; Munhoz, C.D.; Sapolsky, R.M. The stressed CNS: When glucocorticoids aggravate inflammation. Neuron 2009, 64, 33–39. [Google Scholar] [CrossRef]
- Boscolo, P.; Di Donato, A.; Di Giampaolo, L.; Forcella, L.; Reale, M.; Dadorante, V.; Alparone, F.; Pagliaro, S.; Kouri, M.; Magrini, A.; et al. Blood natural killer activity is reduced in men with occupational stress and job insecurity working in a university. Int. Arch. Occup. Environ. Health 2009, 82, 787–794. [Google Scholar] [CrossRef]
- Boscolo, P.; Forcella, L.; Reale, M.; Vianale, G.; Battisti, U.; Bonfiglioli, R.; Cortini, M.; Di Giampaolo, L.; Di Donato, A.; Salerno, S. Job strain in different types of employment affects the immune response. Work 2012, 41, 2950–2954. [Google Scholar] [CrossRef]
- Bellingrath, S.; Rohleder, N.; Kudielka, B.M. Healthy working school teachers with high effort-reward-imbalance and overcommitment show increased pro-inflammatory immune activity and a dampened innate immune defence. Brain Behav. Immun. 2010, 24, 1332–1339. [Google Scholar] [CrossRef]
- Nakata, A. Psychosocial job stress and immunity: A systematic review. Methods Mol. Biol. 2012, 934, 39–75. [Google Scholar] [PubMed]
- Bresić, J.; Knezević, B.; Milosević, M.; Tomljanović, T.; Golubić, R.; Golubović, R.; Mustajbegović, J. Stress and work ability in oil industry workers. Arh. Hig. Rada. Toksikol. 2007, 58, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.V.; Lipscomb, J. Long working hours, occupational health and the changing nature of work organization. Am. J. Ind. Med. 2006, 49, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Parkes, K.R. Psychosocial aspects of stress, health and safety on North Sea installations. Scand. J. Work Environ. Health 1998, 24, 321–333. [Google Scholar] [CrossRef]
- Riethmeister, V.; Brouwer, S.; van der Klink, J.; Bültmann, U. Work, eat and sleep: Towards a healthy ageing at work program offshore. BMC Public Health 2016, 16, 134. [Google Scholar] [CrossRef]
- Carotenuto, A.; Molino, I.; Fasanaro, A.M.; Amenta, F. Psychological stress in seafarers: A review. Int. Marit. Health 2012, 63, 188–194. [Google Scholar]
- Nielsen, M.B.; Tvedt, S.D.; Matthiesen, S.B. Prevalence and occupational predictors of psychological distress in the offshore petroleum industry: A prospective study. Int. Arch. Occup. Environ. Health 2013, 86, 875–885. [Google Scholar] [CrossRef]
- Liberman, A.C.; Budziñski, M.L.; Sokn, C.; Gobbini, R.P.; Steininger, A.; Arzt, E. Regulatory and Mechanistic Actions of Glucocorticoids on T and Inflammatory Cells. Front. Endocrinol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar]
- Wolf, J.M.; Rohleder, N.; Bierhaus, A.; Nawroth, P.P.; Kirschbaum, C. Determinants of the NF-kappaB response to acute psychosocial stress in humans. Brain Behav. Immun. 2009, 23, 742–749. [Google Scholar] [CrossRef]
- Wolkow, A.; Aisbett, B.; Reynolds, J.; Ferguson, S.A.; Main, L.C. Relationships between inflammatory cytokine and cortisol responses in firefighters exposed to simulated wildfire suppression work and sleep restriction. Physiol. Rep. 2015, 3, e12604. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Towards a unified model of neuroendocrine-immune interaction. Immunol. Cell Biol. 2001, 79, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, P.H.; von Känel, R.; Emini, L.; Suter, T.; Fontana, A.; Ehlert, U. Variations in anticipatory cognitive stress appraisal and differential proinflammatory cytokine expression in response to acute stress. Brain Behav. Immun. 2007, 21, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.P.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef]
- Malamud, D. Saliva as a diagnostic fluid. Dent. Clin. N. Am. 2011, 55, 159–178. [Google Scholar] [CrossRef]
- Reale, M.; Gonzales-Portillo, I.; Borlongan, C.V. Saliva, an easily accessible fluid as diagnostic tool and potent stem cell source for Alzheimer’s Disease: Present and future applications. Brain Res. 2020, 1727, 146535. [Google Scholar] [CrossRef]
- Vining, R.F.; McGinley, R.A.; Maksvytis, J.J.; Ho, K.Y. Salivary cortisol: A better measure of adrenal cortical function than serum cortisol. Ann. Clin. Biochem. 1983, 20, 329–335. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Hellhammer, D.H. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 1994, 19, 313–333. [Google Scholar] [CrossRef]
- Jessop, D.S.; Turner-Cobb, J.M. Measurement and meaning of salivary cortisol: A focus on health and disease in children. Stress 2008, 11, 1–14. [Google Scholar] [CrossRef]
- El-Farhan, N.; Rees, D.A.; Evans, C. Measuring cortisol in serum, urine and saliva-are our assays good enough? Ann. Clin. Biochem. 2017, 54, 308–322. [Google Scholar] [CrossRef]
- Adam, E.K.; Kumari, M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 2009, 34, 1423–1436. [Google Scholar] [CrossRef]
- Shinkai, S.; Watanabe, S.; Kurokawa, Y.; Torii, J. Salivary cortisol for monitoring circadian rhythm variation in adrenal activity during shiftwork. Int. Arch. Occup. Environ. Health 1993, 64, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.J.; Ellis, J.G.; Barclay, N.L.; Wetherell, M.A. Assessing the daily stability of the cortisol awakening response in a controlled environment. BMC Psychol. 2016, 4, 3. [Google Scholar] [CrossRef]
- Stachowicz, M.; Lebiedzińska, A. The effect of diet components on the level of cortisol. Eur. Food Res. Technol. 2016, 242, 2001–2009. [Google Scholar] [CrossRef]
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef]
- Hellhammer, J.; Fries, E.; Schweisthal, O.W.; Schlotz, W.; Stone, A.A.; Hagemann, D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology 2007, 32, 80–86. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Reale, M.; Costantini, E.; Di Nicola, M.; Porfilio, I.; de Andrés, C.; Fernández-Paredes, L.; Sánchez-Ramón, S.; Pasquali, L. Profiling of Canonical and Non-Traditional Cytokine Levels in Interferon-β-Treated Relapsing-Remitting-Multiple Sclerosis Patients. Front. Immunol. 2018, 9, 1240. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Setting the cytokine trap for autoimmunity. Nat. Med. 2003, 9, 20–22. [Google Scholar] [CrossRef]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef]
- Szekely, Y.; Arbel, Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiol. Ther. 2018, 7, 25–44. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.J.H.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K.; Rubin, S.M.; Ding, J.; Simonsick, E.M.; Harris, T.B.; et al. Inflammatory markers and onset of cardiovascular events: Results from the Health ABC study. Circulation 2003, 108, 2317–2322. [Google Scholar] [CrossRef]
- Stoner, L.; Lucero, A.A.; Palmer, B.R.; Jones, L.M.; Young, J.M.; Faulkner, J. Inflammatory biomarkers for predicting cardiovascular disease. Clin. Biochem. 2013, 46, 1353–1371. [Google Scholar] [CrossRef] [PubMed]
- Clinton, J.M.; Davis, C.J.; Zielinski, M.R.; Jewett, K.A.; Krueger, J.M. Biochemical regulation of sleep and sleep biomarkers. J. Clin. Sleep Med. 2011, 7, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Maier, S.F. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002, 82, 981–1011. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.R.; Coats, B.D.; Lewis, S.S.; Zhang, Y.; Sprunger, D.B.; Rezvani, N.; Baker, E.M.; Jekich, B.M.; Wieseler, J.L.; Somogyi, A.A.; et al. Proinflammatory cytokines oppose opioid-induced acute and chronic analgesia. Brain Behav. Immun. 2008, 22, 1178–1189. [Google Scholar] [CrossRef]
- Mika, J.; Zychowska, M.; Makuch, W.; Rojewska, E.; Przewlocka, B. Neuronal and immunological basis of action of antidepressants in chronic pain-clinical and experimental studies. Pharmacol. Rep. 2013, 65, 1611–1621. [Google Scholar] [CrossRef]
- Sedger, L.M.; McDermott, M.F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants-past, present and future. Cytokine Growth Factor Rev. 2014, 25, 453–472. [Google Scholar] [CrossRef]
- Reale, M.; Greig, N.H.; Kamal, M.A. Peripheral chemo-cytokine profiles in Alzheimer’s and Parkinson’s diseases. Mini Rev. Med. Chem. 2009, 9, 1229–1241. [Google Scholar] [CrossRef]
- Cacciatore, I.; Marinelli, L.; Fornasari, E.; Cerasa, L.S.; Eusepi, P.; Türkez, H.; Pomilio, C.; Reale, M.; D’Angelo, C.; Costantini, E.; et al. Novel NSAID-Derived Drugs for the Potential Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2016, 17, 1035. [Google Scholar] [CrossRef]
- Decourt, B.; Lahiri, D.K.; Sabbagh, M.N. Targeting Tumor Necrosis Factor Alpha for Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 412–425. [Google Scholar] [CrossRef]
- Hennessy, E.; Gormley, S.; Lopez-Rodriguez, A.B.; Murray, C.; Murray, C.; Cunningham, C. Systemic TNF-α produces acute cognitive dysfunction and exaggerated sickness behavior when superimposed upon progressive neurodegeneration. Brain Behav. Immun. 2017, 59, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Patruno, A.; De Lutiis, M.A.; Pesce, M.; Felaco, M.; Di Giannantonio, M.; Di Nicola, M.; Grilli, A. Dysregulation of chemo-cytokine production in schizophrenic patients versus healthy controls. BMC Neurosci. 2011, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kálmán, J.; Juhász, A.; Laird, G.; Dickens, P.; Járdánházy, T.; Rimanóczy, A.; Boncz, I.; Parry-Jones, W.L.; Janka, Z. Serum interleukin-6 levels correlate with the severity of dementia in Down syndrome and in Alzheimer’s disease. Acta Neurol. Scand. 1997, 96, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S.; McEwen, B.S. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain Behav. Immun. 1997, 11, 286–306. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J. Neurohormonal-cytokine interactions: Implications for inflammation, common human diseases and well-being. Neurochem. Int. 2008, 52, 40–51. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Chrousos, G.P. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. 2002, 966, 290–303. [Google Scholar] [CrossRef]
- Kunz-Ebrecht, S.R.; Mohamed-Ali, V.; Feldman, P.J.; Kirschbaum, C.; Steptoe, A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav. Immun. 2003, 17, 373–383. [Google Scholar] [CrossRef]
- DeSantis, A.S.; DiezRoux, A.V.; Hajat, A.; Aiello, A.E.; Golden, S.H.; Jenny, N.S.; Seeman, T.E.; Shea, S. Associations of salivary cortisol levels with inflammatory markers: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology 2012, 37, 1009–1018. [Google Scholar] [CrossRef]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar] [CrossRef]
- Hänsel, A.; Hong, S.; Cámara, R.J.A.; von Känel, R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci. Biobehav. Rev. 2010, 35, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Plessow, F.; Kirschbaum, C.; Stalder, T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: Evaluation of salivary cortisol pulse detection in panel designs. Psychosom Med. 2013, 75, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [PubMed]
- Stephens, M.A.C.; Wand, G. Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol. Res. 2012, 34, 468–483. [Google Scholar] [PubMed]
- Tsigos, C.; Chrousos, G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Webster Marketon, J.I.; Glaser, R. Stress hormones and immune function. Cell. Immunol. 2008, 252, 16–26. [Google Scholar] [CrossRef]
- Turnbull, A.V.; Rivier, C.L. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: Actions and mechanisms of action. Physiol. Rev. 1999, 79, 1–71. [Google Scholar] [CrossRef]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Møller, K.; Pedersen, B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, 433–437. [Google Scholar] [CrossRef]
- Paik, I.H.; Toh, K.Y.; Lee, C.; Kim, J.J.; Lee, S.J. Psychological stress may induce increased humoral and decreased cellular immunity. Behav. Med. 2000, 26, 139–141. [Google Scholar] [CrossRef]
- Assaf, A.M.; Al-Abbassi, R.; Al-Binni, M. Academic stress-induced changes in Th1- and Th2-cytokine response. Saudi Pharm. J. 2017, 25, 1237–1247. [Google Scholar] [CrossRef]
- Marshall, G.D.; Montgomery, D.D.; Roy, S.R.; Brock, J.B. Effects of Stress Hormones on Regulatory T cell (Treg) and Intracellular TH1/TH2 Cytokine Levels in Normal Human PBMC. J. Allergy Clin. Immunol. 2007, 119, S93. [Google Scholar] [CrossRef]
- Kang, D.H.; Fox, C. Th1 and Th2 cytokine responses to academic stress. Res. Nurs. Health 2001, 24, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Reber, S.O.; Botteron, C.; Barth, T.; Peterlik, D.; Uschold, N.; Männel, D.N.; Lechner, A. Chronic psychosocial stress promotes systemic immune activation and the development of inflammatory Th cell responses. Brain Behav. Immun. 2010, 24, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Gebauer, G.; Lindemann, A.; Brach, M.; Mertelsmann, R. Interleukin-2 and interferon-gamma recruit different subsets of human peripheral blood monocytes to secrete interleukin-1 beta and tumour necrosis factor-alpha. Clin. Exp. Immunol. 1989, 77, 97–100. [Google Scholar] [PubMed]
- Mire-Sluis, A.R.; Wickremasinghe, R.G.; Hoffbrand, A.V.; Timms, A.M.; Francis, G.E. Human T lymphocytes stimulated by phytohaemagglutinin undergo a single round of cell division without a requirement for interleukin-2 or accessory cells. Immunology 1987, 60, 7–12. [Google Scholar] [PubMed]
- Maes, M.; Song, C.; Lin, A.; De Jongh, R.; Van Gastel, A.; Kenis, G.; Bosmans, E.; De Meester, I.; Benoy, I.; Neels, H.; et al. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine 1998, 10, 313–318. [Google Scholar] [CrossRef]
- DeVon, H.A.; Piano, M.R.; Rosenfeld, A.G.; Hoppensteadt, D.A. The association of pain with protein inflammatory biomarkers: A review of the literature. Nurs. Res. 2014, 63, 51–62. [Google Scholar] [CrossRef]
- Grütz, G. New insights into the molecular mechanism of interleukin-10-mediated immunosuppression. J. Leukoc. Biol. 2005, 77, 3–15. [Google Scholar] [CrossRef]
- Lian, Y.; Gu, Y.; Han, R.; Jiang, Y.; Guan, S.; Xiao, J.; Liu, J. Effect of Changing Work Stressors and Coping Resources on Psychological Distress. J. Occup. Environ. Med. 2016, 58, e256–e263. [Google Scholar] [CrossRef]
- Miller, G.E.; Cohen, S.; Ritchey, A.K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol. 2002, 21, 531–541. [Google Scholar] [CrossRef]
- Stansfeld, S.; Candy, B. Psychosocial work environment and mental health—A meta-analytic review. Scand J. Work Environ. Health 2006, 32, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; D’Angelo, C.; Costantini, E.; Di Nicola, M.; Yarla, N.S.; Kamal, M.A.; Salvador, N.; Perry, G. Expression Profiling of Cytokine, Cholinergic Markers, and Amyloid-β Deposition in the APPSWE/PS1dE9 Mouse Model of Alzheimer’s Disease Pathology. J. Alzheimers Dis. 2018, 62, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Di Nicola, M.; Velluto, L.; D’Angelo, C.; Costantini, E.; Lahiri, D.K.; Kamal, M.A.; Yu, Q.; Greig, N.H. Selective acetyl- and butyrylcholinesterase inhibitors reduce amyloid-β ex vivo activation of peripheral chemo-cytokines from Alzheimer’s disease subjects: Exploring the cholinergic anti-inflammatory pathway. Curr. Alzheimer Res. 2014, 11, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Iarlori, C.; Feliciani, C.; Gambi, D. Peripheral chemokine receptors, their ligands, cytokines and Alzheimer’s disease. J. Alzheimers Dis. 2008, 14, 147–159. [Google Scholar] [CrossRef]
- Baldasseroni, A.; Camerino, D.; Cenni, P.; Cesana, G.C.; Fattorini, E.; Ferrario, M.; Mariani, M.; Tartaglia, R. La valutazione dei fattori psicosociali. Job Content Quest. Fogli di Inf. ISPESL 2001, 3, 20–32. [Google Scholar]
- Habibi, E.; Poorabdian, S.; Shakerian, M. Job strain (demands and control model) as a predictor of cardiovascular risk factors among petrochemical personnel. J. Educ. Health Promot. 2015, 4, 16. [Google Scholar]
- Karasek, R.; Brisson, C.; Kawakami, N.; Houtman, I.; Bongers, P.; Amick, B. The Job Content Questionnaire (JCQ): An instrument for internationally comparative assessments of psychosocial job characteristics. J. Occup. Health Psychol. 1998, 3, 322–355. [Google Scholar] [CrossRef]
- Luchman, J.N.; González-Morales, M.G. Demands, control, and support: A meta-analytic review of work characteristics interrelationships. J. Occup. Health Psychol. 2013, 18, 37–52. [Google Scholar] [CrossRef]
- Spielberger, C.D. The Corsini encyclopedia of psychology. In State-trait Anxiety Inventory; Weiner, I.B., Craighead, W.E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]

| Parameters | Total Workers | Cortisol ≤10 ng/mL | Cortisol >10 ng/mL | p-value |
|---|---|---|---|---|
| Number of workers | 80 | 31 | 49 | |
| Age (years) | 36.8 ± 9.3 | 37.9 ± 9.6 | 35.8 ± 9.3 | 0.325 |
| Weight (kg) | 91.3 ± 12.3 | 84.0 ± 9.9 | 98.5 ± 14.7 | <0.001 |
| Height (cm) | 176.3 ± 19.9 | 180.3 ± 6.4 | 172.3 ± 33.5 | 0.191 |
| BMI (kg/m2) | 27.2 ± 2.9 | 26.0 ± 2.5 | 28.5 ± 3.4 | <0.001 |
| Systolic blood pressure (mmHg) | 124.3 ± 8.8 | 125.2 ± 11.7 | 123.4 ± 12.2 | 0.517 |
| Diastolic blood pressure (mmHg) | 72.8± 11.5 | 72.5 ± 11.6 | 73.0 ±11.3 | 0.841 |
| STAI X-1 | 44.1 ± 4.4 | 43.5 ± 3.2 | 44.7 ± 5.7 | 0.305 |
| STAI X-2 | 38.6 ± 5.3 | 37.6 ± 5.2 | 39.5 ± 5.1 | 0.100 |
| Job Demand (JD) | 34.2 ± 4.3 | 30.9 ± 4.6 | 37.6 ± 3.9 | <0.001 |
| Decision Latitude (DL) | 64.5 ± 6.4 | 62.83 ± 5.6 | 66.2 ± 7.2 | 0.027 |
| JD/DL (Job Strain) | 0.53 ± 0.07 | 0.49 ± 0.08 | 0.56 ± 0.07 | <0.001 |
| Social Support (SS) | 22.1 ±2.8 | 22.8 ± 3.0 | 21.4 ± 2.7 | 0.041 |
| Job Insecurity (JI) | 22.1 ±4.8 | 23.0 ± 5.0 | 21.1 ± 4.6 | 0.084 |
| Parameters | Total Workers | Cortisol ≤10 ng/mL | Cortisol >10 ng/mL | p-value |
|---|---|---|---|---|
| WBC (103/µL) | 6.75 ± 1.82 | 6.56 ± 1.92 | 6.94 ± 1.72 | 0.739 |
| RBC (106/µL) | 5.01 ±0.56 | 4.96 ± 0.33 | 5.07 ± 0.23 | 0.677 |
| HGB (g/dL−1) | 15.39 ±1.70 | 15.34 ± 0.82 | 15.45 ± 0.88 | 0.868 |
| Platelets (103/µL) | 218.32 ± 36.68 | 223.1 ± 46.05 | 213.54 ± 27.31 | 0.054 |
| % limphocytes | 36.14 ± 7.69 | 36.83 ± 8.31 | 35.41 ± 7.08 | 0.803 |
| % monocytes | 8.94 ± 1.73 | 8.96 ± 1.94 | 8.92 ± 1.53 | 0.967 |
| % neutrophils | 53.28 ± 8.41 | 52.91 ± 9.31 | 53.64 ± 7.52 | 0.868 |
| % eosinophils | 1.75 ± 0.85 | 1.61 ± 0.62 | 1.94 ± 1.08 | 0.008 |
| % basophils | 0.06 ± 0.04 | 0.06 ± 0.04 | 0.06 ± 0.04 | 1.000 |
| Behavior Data | |
|---|---|
| Current smoker, n (%) | 15 (18.7%) |
| Cigarettes/d range | 3–5 |
| Alcohol consumption, n (%) | 25 (31.25%) |
| Wine or beer glasses/d | One or twice a day |
| Complaints | |
| Diarrhea, n (%) | 6 (7.55 %) |
| Abdominal pain, n (%) | 5 (6.25%) |
| Headaches and dizziness, n (%) | 4 (5 %) |
| Cold, n (%) | 2 (2.5 %) |
| Educational grade | |
| Secondary school, n (%) | 65 (81.25%) |
| high school, n (%) | 15 (18.75%) |
| Occupational grade | |
| Coordinator, n (%) | 9 (11.25%) |
| Supervisor, n (%) | 12 (15%) |
| Operator, n (%) | 59 (73.75%) |
| Marital status | |
| Single, n (%) | 30 (37.5%) |
| Married, n (%) | 50 (62.5%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reale, M.; Costantini, E.; D’Angelo, C.; Coppeta, L.; Mangifesta, R.; Jagarlapoodi, S.; Di Nicola, M.; Di Giampaolo, L. Network between Cytokines, Cortisol and Occupational Stress in Gas and Oilfield Workers. Int. J. Mol. Sci. 2020, 21, 1118. https://doi.org/10.3390/ijms21031118
Reale M, Costantini E, D’Angelo C, Coppeta L, Mangifesta R, Jagarlapoodi S, Di Nicola M, Di Giampaolo L. Network between Cytokines, Cortisol and Occupational Stress in Gas and Oilfield Workers. International Journal of Molecular Sciences. 2020; 21(3):1118. https://doi.org/10.3390/ijms21031118
Chicago/Turabian StyleReale, Marcella, Erica Costantini, Chiara D’Angelo, Luca Coppeta, Rocco Mangifesta, Srinivas Jagarlapoodi, Marta Di Nicola, and Luca Di Giampaolo. 2020. "Network between Cytokines, Cortisol and Occupational Stress in Gas and Oilfield Workers" International Journal of Molecular Sciences 21, no. 3: 1118. https://doi.org/10.3390/ijms21031118
APA StyleReale, M., Costantini, E., D’Angelo, C., Coppeta, L., Mangifesta, R., Jagarlapoodi, S., Di Nicola, M., & Di Giampaolo, L. (2020). Network between Cytokines, Cortisol and Occupational Stress in Gas and Oilfield Workers. International Journal of Molecular Sciences, 21(3), 1118. https://doi.org/10.3390/ijms21031118







