Evolution of Protein Structure and Stability in Global Warming
Abstract
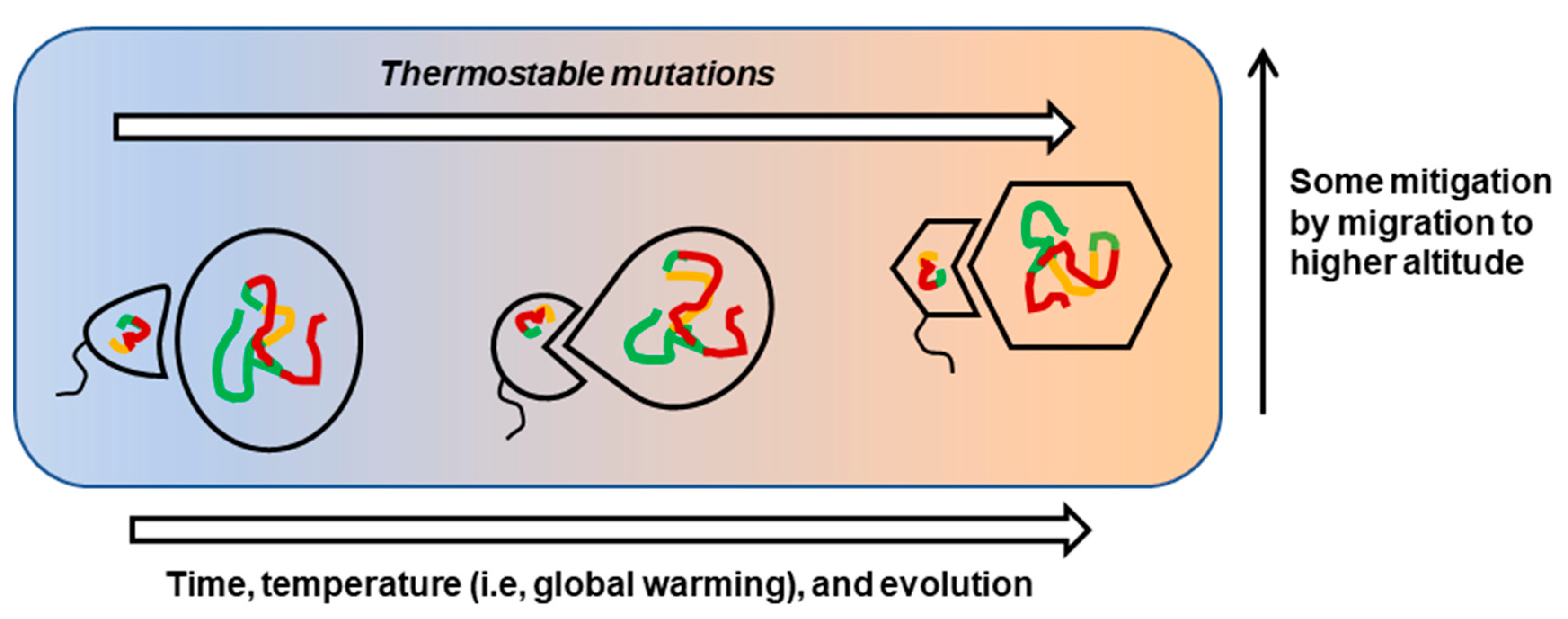
1. Introduction
2. Rationale of Choice of Heat-Relevant Organisms and Macromolecules
2.1. Choice of Organisms
2.2. Choice of Proteins and Their Analysis
2.3. Ancestral Sequence Reconstruction
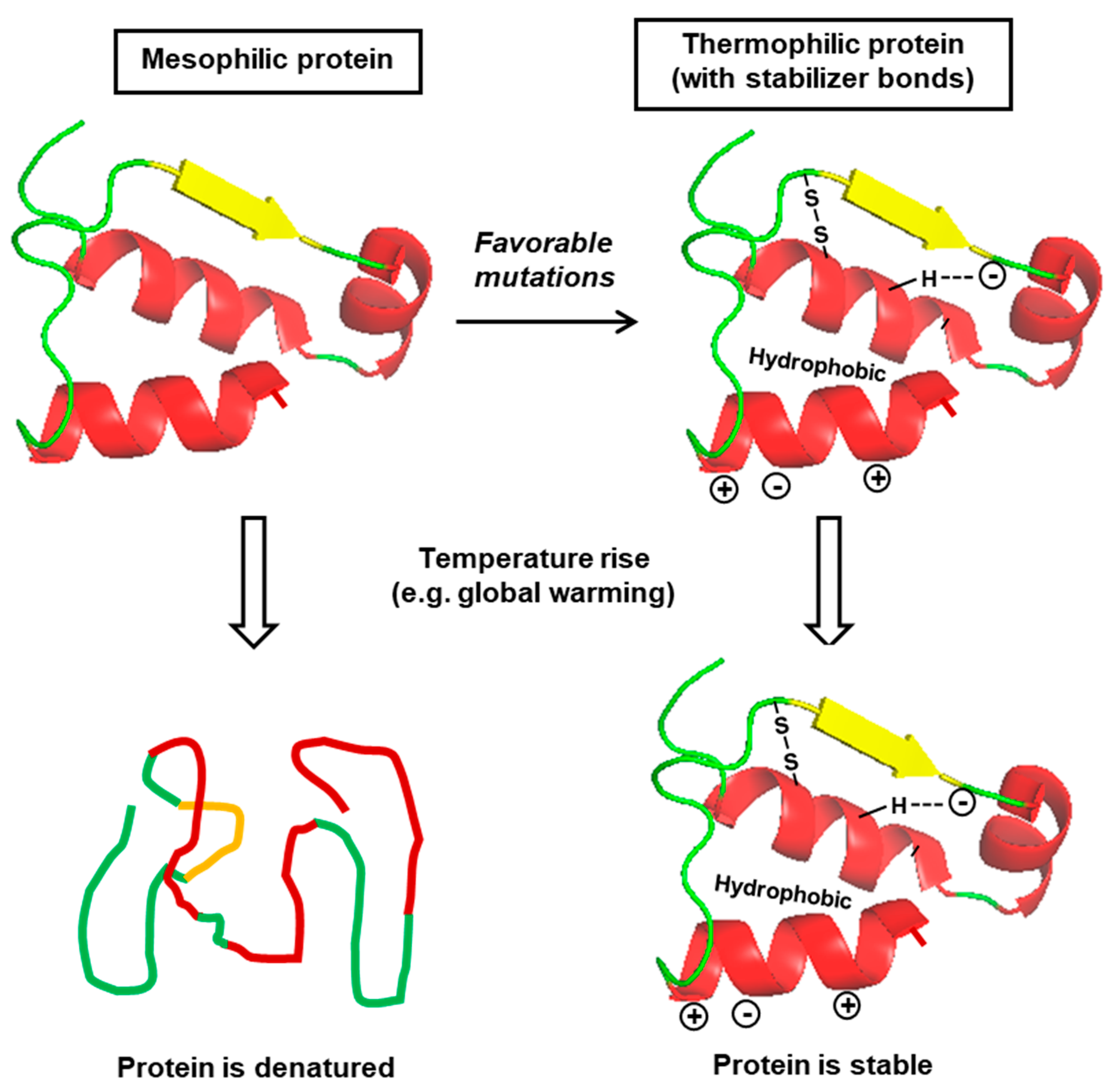
3. Role of Specific Amino Acid Residues and Interactions
3.1. Amino Acid Composition
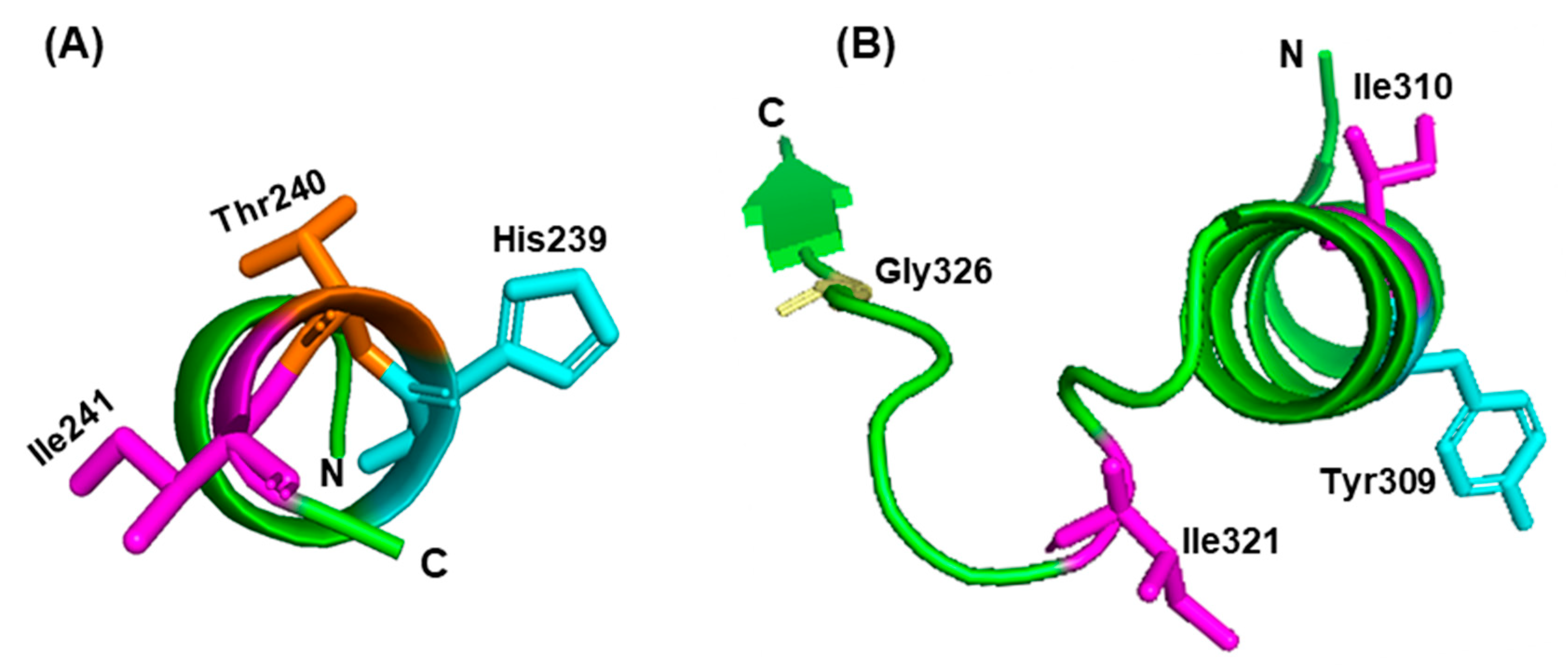
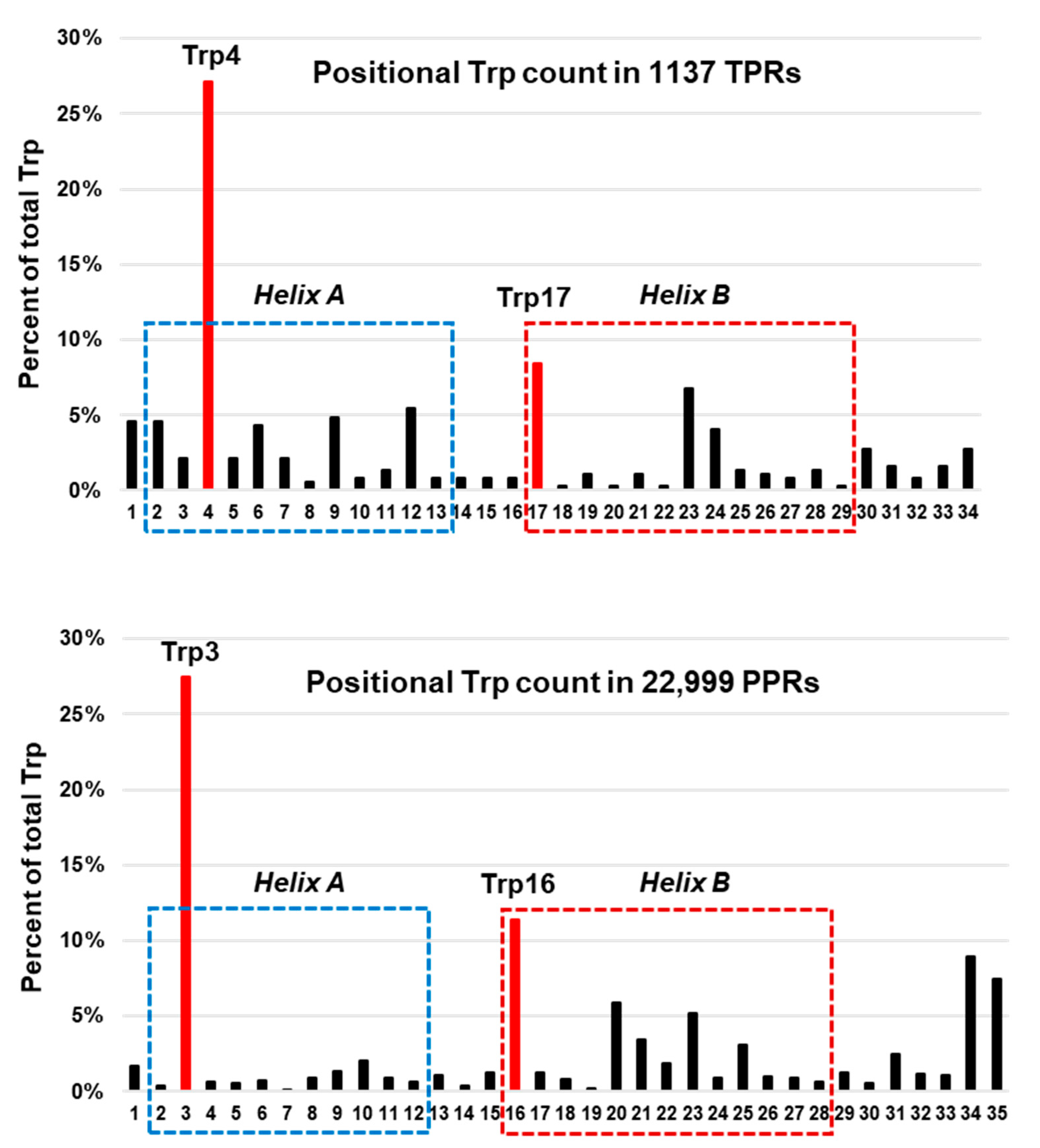
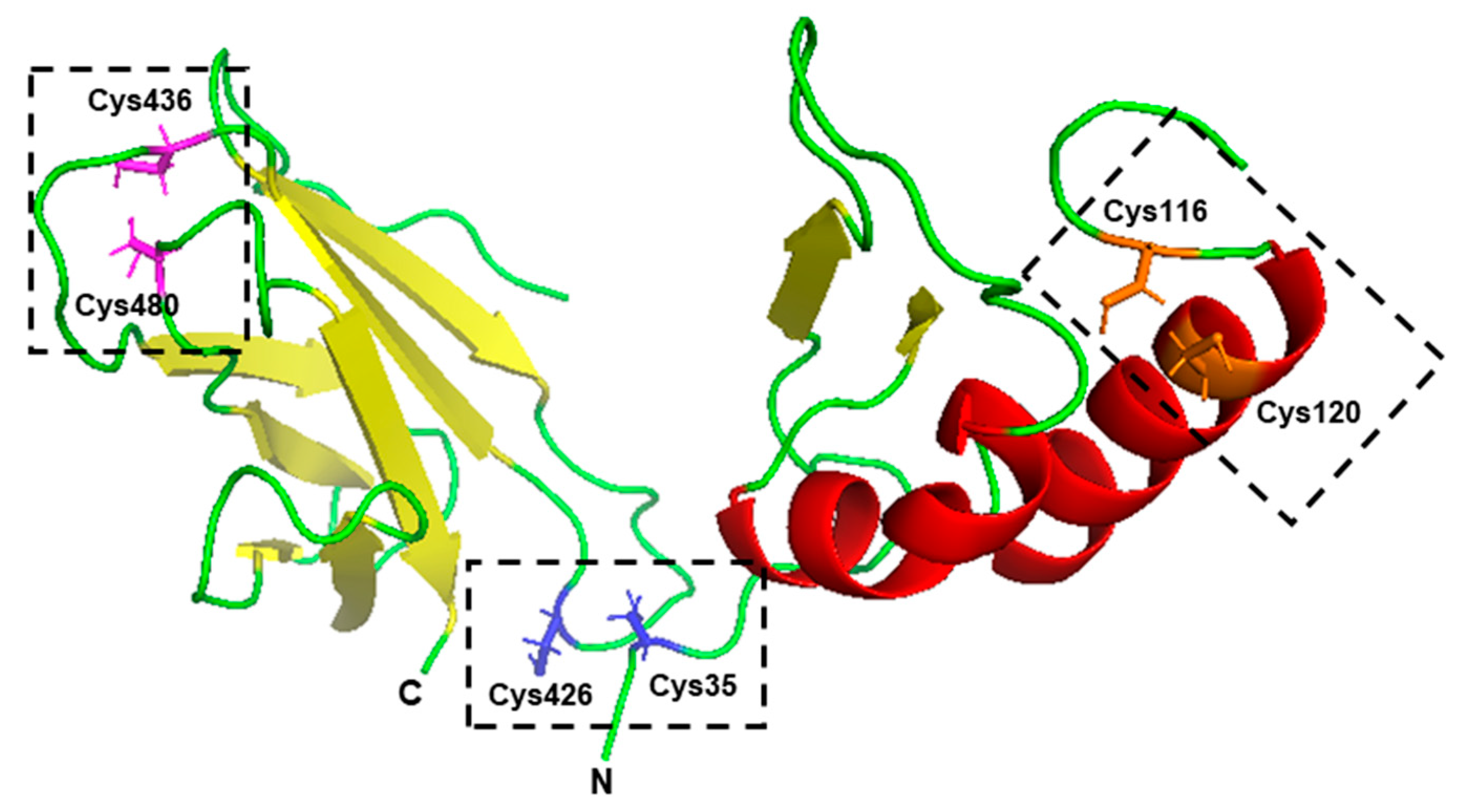
3.2. Role of Specific Amino Acids
4. Prospects of Mutational Survival in Global Warming
4.1. Designer Thermostable Organisms?
4.2. Practical Applications of the Molecular Knowledge of Thermophilia
4.3. Biological Changes in Global Warming
4.3.1. Individual Species
4.3.2. Host–Pathogen Interactions
5. Summary
Funding
Conflicts of Interest
References
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Klausmeyer, K.R.; Shaw, M.R. Climate change, habitat loss, protected areas and the climate adaptation potential of species in Mediterranean ecosystems worldwide. PLoS ONE 2009, 4, e6392. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000, 15, 56–61. [Google Scholar] [CrossRef]
- Benton, M.J. Hyperthermal-driven mass extinctions: Killing models during the Permian–Triassic mass extinction. Philos. Trans. A Math. Phys. Eng. Sci. 2018, 376, 20170076. [Google Scholar] [CrossRef]
- Madeira, D.; Araújo, J.E.; Vitorino, R.; Capelo, J.L.; Vinagre, C.; Diniz, M.S. Ocean warming alters cellular metabolism and induces mortality in fish early life stages: A proteomic approach. Environ. Res. 2016, 148, 164–176. [Google Scholar] [CrossRef]
- Traboni, C.; Mammola, S.D.; Ruocco, M.; Ontoria, Y.; Ruiz, J.M.; Procaccini, G.; Marín-Guirao, L. Investigating cellular stress response to heat stress in the seagrass Posidonia oceanica in a global change scenario. Mar. Environ. Res. 2018, 141, 12–23. [Google Scholar] [CrossRef]
- Faldyn, M.J.; Hunter, M.D.; Elderd, B.D. Climate change and an invasive, tropical milkweed: An ecological trap for monarch butterflies. Ecology 2018, 99, 1031–1038. [Google Scholar] [CrossRef]
- Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Normand, S.; Rüger, N.; Beck, P.S.; Blach-Overgaard, A.; Blok, D.; Cornelissen, J.H.C.; Forbes, B.C.; et al. Plant functional trait change across a warming tundra biome. Nature 2018, 562, 57–62. [Google Scholar] [CrossRef]
- Nolan, C.; Overpeck, J.T.; Allen, J.R.; Anderson, P.M.; Betancourt, J.L.; Binney, H.A.; Brewer, S.; Bush, M.B.; Chase, B.M.; Cheddadi, R.; et al. Past and future global transformation of terrestrial ecosystems under climate change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef]
- Grubb, M. The economics of the Kyoto protocol. World Econ. 2003, 4, 143–189. [Google Scholar]
- Spash, C.L. The brave new world of carbon trading. New Political Econ. 2010, 15, 169–195. [Google Scholar] [CrossRef]
- Rossati, A. Global warming and its health impact. Int. J. Occup. Environ. Med. 2017, 8, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Stetter, K.O. History of discovery of the first hyperthermophiles. Extremophiles 2006, 10, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Goncearenco, A.; Ma, B.G.; Berezovsky, I.N. Molecular mechanisms of adaptation emerging from the physics and evolution of nucleic acids and proteins. Nucleic Acids Res. 2014, 42, 2879–2892. [Google Scholar] [CrossRef] [PubMed]
- Brininger, C.; Spradlin, S.; Cobani, L.; Evilia, C. The more adaptive to change, the more likely you are to survive: Protein adaptation in extremophiles. Semin. Cell. Dev. Biol. 2018, 84, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Zeldes, B.M.; Keller, M.W.; Loder, A.J.; Straub, C.T.; Adams, M.W.W.; Kelly, R.M. Extremely thermophilic microorganisms as metabolic engineering platforms for production of fuels and industrial chemicals. Front. Microbiol. 2015, 6, 1209. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Fact. 2007, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Bharadwaj, G.; Bhat, M.K. Thermophilic fungi: Their physiology and enzymes. Microbiol. Mol. Biol. Rev. 2000, 64, 461–488. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Jung, Y.; Han, J.; Yun, J.-H.; Chang, I.; Lee, W. Probing the folding-unfolding transition of a thermophilic protein, MTH1880. PLoS ONE 2016, 11, e0145853. [Google Scholar] [CrossRef]
- Daggett, V.; Fersht, A.R. Is there a unifying mechanism for protein folding? Trends Biochem. Sci. 2003, 28, 18–25. [Google Scholar] [CrossRef]
- Fersht, A.R.; Sato, S. Phi-value analysis and the nature of protein-folding transition states. Proc. Natl. Acad. Sci. USA 2004, 101, 7976–7981. [Google Scholar] [CrossRef] [PubMed]
- Gianni, S.; Ivarsson, Y.; Jemth, P.; Brunori, M.; Travaglini-Allocatelli, C. Identification and characterization of protein folding intermediates. Biophys. Chem. 2007, 128, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.; Musiyenko, A.; Kumar, R.; Barik, S. A novel class of dual-family immunophilins. J. Biol. Chem. 2005, 280, 24308–24314. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Raveendran, A.; Nagotu, S. Chaperones and proteostasis: Role in Parkinson’s Disease. Diseases 2020, 8, 24. [Google Scholar] [CrossRef]
- Mallik, S.; Kundu, S. A comparison of structural and evolutionary attributes of Escherichia coli and Thermus thermophilus small ribosomal subunits: Signatures of thermal adaptation. PLoS ONE 2013, 8, e69898. [Google Scholar] [CrossRef]
- Polley, S.; Jana, B.; Chakrabarti, G.; Sau, S. Inhibitor-induced conformational stabilization and structural alteration of a mip-like peptidyl prolyl cis-trans isomerase and its C-terminal domain. PLoS ONE 2014, 9, e102891. [Google Scholar] [CrossRef][Green Version]
- Isticato, R.; Lanzilli, M.; Petrillo, C.; Donadio, G.; Baccigalupi, L.; Ricca, E. Bacillus subtilis builds structurally and functionally different spores in response to the temperature of growth. Environ. Microbiol. 2020, 22, 170–182. [Google Scholar] [CrossRef]
- Vieille, C.; Burdette, D.S.; Zeikus, J.G. Thermozymes. Biotechnol. Annu. Rev. 1996, 2, 1–83. [Google Scholar] [CrossRef]
- Grättinger, M.; Dankesreiter, A.; Schurig, H.; Jaenicke, R. Recombinant phosphoglycerate kinase from the hyperthermophilic bacterium Thermotoga maritima: Catalytic, spectral and thermodynamic properties. J. Mol. Biol. 1998, 280, 525–533. [Google Scholar] [CrossRef]
- Bauer, M.W.; Kelly, R.M. The family 1b-glucosidases from Pyrococcus furiosus and Agrobacterium faecalis share a common catalytic mechanism. Biochemistry 1998, 37, 17170–17178. [Google Scholar] [CrossRef]
- Vieille, C.; Hess, J.M.; Kelly, R.M.; Zeikus, J.G. xylA cloning and sequencing and biochemical characterization of xylose from Thermotoga neapolitana. Appl. Environ. Microbiol. 1995, 61, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Zwickl, P.; Fabry, S.; Bogedain, C.; Hass, A.; Hensel, R. Glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic archaebacterium Pyrococcus woesei: Characterization of the enzyme, cloning and sequencing of the gene, and expression in Escherichia coli. J. Bacteriol. 1990, 172, 4329–4338. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, G.; Ostendorp, R.; Prade, L.; Korndörfer, L.; Dams, T.; Huber, R.; Jaenicke, R. Lactate dehydrogenase from the hyperthermophilic bacterium Thermotoga maritima: The crystal structure at 2.1 A resolution reveals strategies for intrinsic protein stabilization. Structure 1998, 6, 769–781. [Google Scholar] [CrossRef]
- Chi, Y.I.; Martinez-Cruz, L.A.; Jancarik, J.; Swanson, R.V.; Robertson, D.E.; Kim, S.H. Crystal structure of the beta-glycosidase from the hyperthermophile Thermosphaera aggregans: Insights into its activity and thermostability. FEBS Lett. 1999, 445, 375–383. [Google Scholar] [CrossRef]
- Tahirov, T.H.; Oki, H.; Tsukihara, T.; Ogasahara, K.; Yutani, K.; Ogata, K.; Izu, Y.; Tsunasawa, S.; Kato, I. Crystal structure of methionine aminopeptidase from hyperthermophile, Pyrococcus furiosus. J. Mol. Biol. 1998, 284, 101–124. [Google Scholar] [CrossRef]
- Russell, R.J.; Ferguson, J.M.; Hough, D.W.; Danson, M.J.; Taylor, G.L. The crystal structure of citrate synthase from the hyperthermophilic archaeon Pyrococcus furiosus at 1.9A resolution. Biochemistry 1997, 36, 9983–9994. [Google Scholar] [CrossRef]
- Morimoto, R.I. Cells in stress: Transcriptional activation of heat shock genes. Science 1993, 259, 1409–1410. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337. [Google Scholar] [CrossRef]
- Gould, S.J.; Eldredge, N. Punctuated equilibrium comes of age. Nature 1993, 366, 223–227. [Google Scholar] [CrossRef]
- Bloom, J.D.; Labthavikul, S.T.; Otey, C.R.; Arnold, F.H. Protein stability promotes evolvability. Proc. Natl. Acad. Sci. USA 2006, 103, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Shoichet, B.K.; Baase, W.A.; Kuroki, R.; Matthews, B.W. A relationship between protein stability and protein function. Proc. Natl. Acad. Sci. USA 1995, 92, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, N.; Stricher, F.; Serrano, L.; Tawfik, D.S. How protein stability and new functions trade off. PLoS Comp. Biol. 2008, 4, e1000002. [Google Scholar] [CrossRef] [PubMed]
- Slatkina, M.; Racimoa, F. Ancient DNA and human history. Proc. Natl. Acad. Sci. USA 2016, 113, 6380–6387. [Google Scholar] [CrossRef]
- Harms, M.J.; Thornton, J.W. Analyzing protein structure and function using ancestral gene reconstruction. Curr. Opin. Struct. Biol. 2010, 20, 360–366. [Google Scholar] [CrossRef]
- Arenas, M.; Sánchez-Cobos, A.; Bastolla, U. Maximum-likelihood phylogenetic inference with selection on protein folding stability. Mol. Biol. Evol. 2015, 32, 2195–2207. [Google Scholar] [CrossRef]
- Akanuma, S. Characterization of reconstructed ancestral proteins suggests a change in temperature of the ancient biosphere. Life 2017, 7, 33. [Google Scholar] [CrossRef]
- Arenas, M.; Weber, C.C.; Liberles, D.A.; Bastolla, U. ProtASR: An evolutionary framework for ancestral protein reconstruction with selection on folding stability. Syst. Biol. 2017, 66, 1054–1064. [Google Scholar] [CrossRef]
- Garcia, A.K.; Kaçar, B. How to resurrect ancestral proteins as proxies for ancient biogeochemistry. Free Radic. Biol. Med. 2019, 140, 260–269. [Google Scholar] [CrossRef]
- Akanuma, S.; Nakajima, Y.; Yokobori, S.-I.; Kimura, M.; Nemoto, N.; Mase, T.; Miyazono, K.-I.; Tanokura, M.; Yamagishi, A. Experimental evidence for the thermophilicity of ancestral life. Proc. Natl. Acad. Sci. USA 2013, 110, 11067–11072. [Google Scholar] [CrossRef]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W.F. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Wilson, C.; Hoemberger, M.; Stiller, J.B.; Agafonov, R.V.; Kutter, S.; English, J.; Theobald, D.L.; Kern, D. Evolutionary drivers of thermoadaptation in enzyme catalysis. Science 2017, 355, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Fralick, P.; Carter, J.E. Neoarchean deep marine paleotemperature: Evidence from turbidite successions. Precambrian Res. 2011, 191, 78–84. [Google Scholar] [CrossRef]
- Achenbach-Richter, L.; Gupta, R.; Zillig, W.; Woese, C.R. Rooting the archaebacterial tree: The pivotal role of Thermococcus celer in archaebacterial evolution. Syst. Appl. Microbiol. 1988, 10, 231–240. [Google Scholar] [CrossRef]
- Ciccarelli, F.D.; Doerks, T.; von Mering, C.; Creevey, C.J.; Snel, B.; Bork, P. Toward automatic reconstruction of a highly resolved tree of life. Science 2006, 311, 1283–1287. [Google Scholar] [CrossRef]
- Barik, S. On the role, ecology, phylogeny, and structure of dual-family immunophilins. Cell Stress Chaperones 2017, 22, 833–845. [Google Scholar] [CrossRef]
- Semba, Y.; Ishida, M.; Yokobori, S.-I.; Yamagishi, A. Ancestral amino acid substitution improves the thermal stability of recombinant lignin-peroxidase from white-rot fungi, Phanerochaete chrysosporium strain UAMH 3641. Protein Eng. Des. Sel. 2015, 28, 221–230. [Google Scholar] [CrossRef]
- Iwabata, H.; Watanabe, K.; Ohkuri, T.; Yokobori, S.-I.; Yamagishi, A. Thermostability of ancestral mutants of Caldococcus noboribetus isocitrate dehydrogenase. FEMS Microbiol. Lett. 2005, 243, 393–398. [Google Scholar] [CrossRef]
- Miyazaki, J.; Nakaya, S.; Suzuki, T.; Tamakoshi, M.; Oshima, T.; Yamagishi, A. Ancestral residues stabilizing 3-isopropylmalate dehydrogenase of an extreme thermophile: Experimental evidence supporting the thermophilic common ancestor hypothesis. J. Biochem. 2001, 129, 777–782. [Google Scholar] [CrossRef]
- Gaucher, E.A.; Govindarajan, S.; Ganesh, O.K. Palaeotemperature trend for Precambrian life inferred from resurrected proteins. Nature 2008, 451, 704–707. [Google Scholar] [CrossRef]
- Perez-Jimenez, R.; Inglés-Prieto, A.; Zhao, Z.M.; Sanchez-Romero, I.; Alegre-Cebollada, J.; Kosuri, P.; Garcia-Manyes, S.; Kappock, T.J.; Tanokura, M.; Holmgren, A.; et al. Single-molecule paleoenzymology probes the chemistry of resurrected enzymes. Nat. Struct. Mol. Biol. 2011, 18, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Abe, A.; Tamura, T.; Kishimoto, T.; Sogabe, A.; Akanuma, S.; Yokobori, S.-I.; Yamagishi, A.; Imada, K.; Inagaki, K. Epistasis effects of multiple ancestral-consensus amino acid substitutions on the thermal stability of glycerol kinase from Cellulomonas sp. NT3060. J. Biosci. Bioeng. 2016, 121, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, R.; Toma, W.; Yamazaki, K.; Akanuma, S. Ancestral sequence reconstruction produces thermally stable enzymes with mesophilic enzyme-like catalytic properties. Sci. Rep. 2020, 10, 15493. [Google Scholar] [CrossRef] [PubMed]
- Vieille, C.; Zeikus, G.J. Hyperthermophilic enzymes: Sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 2001, 65, 1–43. [Google Scholar] [CrossRef]
- Watters, A.L.; Baker, D. Searching for folded proteins in vitro and in silico. Eur. J. Biochem. 2004, 271, 1615–1622. [Google Scholar] [CrossRef]
- Lesk, A.M.; Chothia, C. Solvent accessibility, protein surfaces, and protein folding. Biophys. J. 1980, 32, 35–47. [Google Scholar] [CrossRef]
- Chothia, C.; Levitt, M.; Richardson, D. Helix to helix packing in proteins. J. Mol. Biol. 1981, 145, 215–250. [Google Scholar] [CrossRef]
- Gerstein, M.; Lesk, A.M.; Chothia, C. Structural mechanisms for domain movements in proteins. Biochemistry 1994, 33, 6739–6749. [Google Scholar] [CrossRef]
- Baker, D. What has de novo protein design taught us about protein folding and biophysics? Protein Sci. 2019, 28, 678–683. [Google Scholar] [CrossRef]
- Hait, S.; Mallik, S.; Basu, S.; Kundu, S. Finding the generalized molecular principles of protein thermal stability. Proteins 2020, 88, 788–808. [Google Scholar] [CrossRef]
- Zeldovich, K.B.; Berezovsky, I.N.; Shakhnovich, E.I. Protein and DNA sequence determinants of thermophilic adaptation. PLoS Comput. Biol. 2007, 3, e5. [Google Scholar] [CrossRef] [PubMed]
- Miralles, F. Compositional and structural features related to thermal stability in the archaea SRP19 and SRP54 signal recognition particle proteins. J. Mol. Evol. 2011, 72, 450–465. [Google Scholar] [CrossRef] [PubMed]
- Pack, S.P.; Yoo, Y.J. Protein thermostability: Structure-based difference of amino acid between thermophilic and mesophilic proteins. J. Biotechnol. 2004, 111, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tsai, C.-J.; Nussinov, R. Factors enhancing protein thermostability. Protein Eng. Des. Sel. 2000, 13, 179–191. [Google Scholar] [CrossRef]
- Mizuguchi, K.; Sele, M.; Cubellis, M.V. Environment specific substitution tables for thermophilic proteins. BMC Bioinform. 2007, 8 (Suppl. 1), S15. [Google Scholar] [CrossRef]
- Khan, M.F.; Patra, S. Deciphering the rationale behind specific codon usage pattern in extremophiles. Sci. Rep. 2018, 8, 15548. [Google Scholar] [CrossRef]
- Petukhov, M.; Kil, Y.; Kuramitsu, S.; Lanzov, V. Insights into thermal resistance of proteins from the intrinsic stability of their α-helices. Proteins 1997, 29, 309–320. [Google Scholar] [CrossRef]
- Szilágyi, A.; Závodszky, P. Structural differences between mesophilic, moderately thermophilic and extremely thermophilic protein subunits: Results of a comprehensive survey. Structure 2000, 8, 493–504. [Google Scholar] [CrossRef]
- Bowie, J.U. Solving the membrane protein folding problem. Nature 2005, 438, 581–589. [Google Scholar] [CrossRef]
- Minetti, C.A.S.; Remeta, D.P. Energetics of membrane protein folding and stability. Arch. Biochem. Biophys. 2006, 453, 32–53. [Google Scholar] [CrossRef]
- Matthews, B.W.; Nicholson, H.; Becktel, W.J. Enhanced protein thermostability from site-directed mutations that decrease the entropy of unfolding. Proc. Natl. Acad. Sci. USA 1987, 84, 6663–6667. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Naderi-Manesh, H.; Zarrabi, M.; Ranjbar, B. Effective factors in thermostability of thermophilic proteins. Biophys. Chem. 2006, 119, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-X.; Wang, Y.-B.; Pan, Y.-J.; Li, W.-F. Differences in amino acids composition and coupling patterns between mesophilic and thermophilic proteins. Amino Acids 2008, 34, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Greaves, R.B.; Warwicker, J. Stability and solubility of proteins from extremophiles. Biochem. Biophys. Res. Commun. 2009, 380, 581–585. [Google Scholar] [CrossRef]
- Haney, P.J.; Badger, J.H.; Buldak, G.L.; Reich, C.I.; Woese, C.R.; Olsen, G.J. Thermal adaptation analyzed by comparison of protein sequences from mesophilic and extremely thermophilic Methanococcus species. Proc. Natl. Acad. Sci. USA 1999, 96, 3578–3583. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Merritt, M.; Schaefferkoetter, D.; Evans, T.G. High-throughput quantification of protein structural change reveals potential mechanisms of temperature adaptation in Mytilus mussels. BMC Evol. Biol. 2020, 20, 28. [Google Scholar] [CrossRef]
- Taylor, T.J.; Vaisman, I.I. Discrimination of thermophilic and mesophilic proteins. BMC Struct. Biol. 2010, 10 (Suppl. 1), S5. [Google Scholar] [CrossRef]
- Sterpone, F.; Bertonati, C.; Briganti, G.; Melchionna, S. Key role of proximal water in regulating thermostable proteins. J. Phys. Chem. B 2009, 113, 131–137. [Google Scholar] [CrossRef]
- Mou, Z.; Ding, Y.; Wang, X.; Cai, Y. Comparison of protein-water interactions in psychrophilic, mesophilic, and thermophilic Fe-SOD. Protein Pept. Lett. 2014, 21, 578–583. [Google Scholar] [CrossRef]
- Saunders, N.F.W.; Thomas, T.; Curmi, P.M.G.; Mattick, J.S.; Kuczek, E.; Slade, R.; Davis, J.; Franzmann, P.D.; Boone, D.; Rusterholtz, K.; et al. Mechanisms of thermal adaptation revealed from the genomes of the Antarctic archaea Methanogenium frigidum and Methanococcoides burtonii. Genome Res. 2003, 13, 1580–1588. [Google Scholar] [CrossRef]
- Karshikoff, A.; Ladenstein, R. Ion pairs and the thermotolerance of proteins from hyperthermophiles: A “Traffic Rule” for hot roads. Trends Biochem. Sci. 2001, 26, 550–556. [Google Scholar] [CrossRef]
- Robinson-Rechavi, M.; Alibés, A.; Godzik, A. Contribution of electrostatic interactions, compactness and quaternary structure to protein thermostability: Lessons from structural genomics of Thermotoga maritima. J. Mol. Biol. 2006, 356, 547–557. [Google Scholar] [CrossRef]
- Panja, A.S.; Maiti, S.; Bandyopadhyay, B. Protein stability governed by its structural plasticity is inferred by physicochemical factors and salt bridges. Sci. Rep. 2020, 10, 1822. [Google Scholar] [CrossRef]
- Sternke, M.; Tripp, K.W.; Barrick, D. Consensus sequence design as a general strategy to create hyperstable, biologically active proteins. Proc. Natl. Acad. Sci. USA 2019, 116, 11275–11284. [Google Scholar] [CrossRef] [PubMed]
- Okada, J.; Okamoto, T.; Mukaiyama, A.; Tadokoro, T.; You, D.-J.; Chon, H.; Koga, Y.; Takano, K.; Kanaya, S. Evolution and thermodynamics of the slow unfolding of hyperstable monomeric proteins. BMC Evol. Biol. 2010, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Gromiha, M.M.; Pathak, M.C.D.; Saraboji, K.; Ortlund, E.A.; Gaucher, E.A. Hydrophobic environment is a key factor for the stability of thermophilic proteins. Proteins 2013, 81, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Aoi, A.; Koga, Y.; Kanaya, S. Evolvability of thermophilic proteins from archaea and bacteria. Biochemistry 2013, 52, 4774–4780. [Google Scholar] [CrossRef]
- Pace, A.L.; Wong, R.L.; Zhang, Y.T.; Kao, Y.H.; Wang, Y.J. Asparagine deamidation dependence on buffer type, pH, and temperature. J. Pharm. Sci. 2013, 102, 1712–1723. [Google Scholar] [CrossRef]
- Saelensminde, G.; Halskau, Ø., Jr.; Jonassen, I. Amino acid contacts in proteins adapted to different temperatures: Hydrophobic interactions and surface charges play a key role. Extremophiles 2009, 13, 11–20. [Google Scholar] [CrossRef][Green Version]
- Barik, S. The uniqueness of tryptophan in biology: Properties, metabolism, interactions and localization in proteins. Int. J. Mol. Sci. 2020, 21, 8776. [Google Scholar] [CrossRef]
- Main, E.R.G.; Xiong, Y.; Cocco, M.J.; D’Andrea, L.; Regan, L. Design of stable alpha-helical arrays from an idealized TPR motif. Structure 2003, 11, 497–508. [Google Scholar] [CrossRef]
- Barik, S. Protein tetratricopeptide repeat and the companion non-tetratricopeptide repeat helices: Bioinformatic analysis of interhelical interactions. Bioinform. Biol. Insights 2019, 13, 1177932219863363. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. The nature and arrangement of pentatricopeptide domains and the linker sequences between them. Bioinform. Biol. Insights 2020, 14, 1177932220906434. [Google Scholar] [CrossRef] [PubMed]
- Kajava, A.V. Tandem repeats in proteins: From sequence to structure. J. Struct. Biol. 2012, 179, 279–288. [Google Scholar] [CrossRef]
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant. Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef]
- Perez-Riba, A.; Itzhaki, L.S. The tetratricopeptide-repeat motif is a versatile platform that enables diverse modes of molecular recognition. Curr. Opin. Struct. Biol. 2019, 54, 43–49. [Google Scholar] [CrossRef]
- Sawyer, N.; Chen, J.; Regan, L. All repeats are not equal: A module-based approach to guide repeat protein design. J. Mol. Biol. 2013, 425, 1826–1838. [Google Scholar] [CrossRef]
- Tomazic, S.J.; Klibanov, A.M. Mechanisms of irreversible thermal inactivation of Bacillus alpha-amylases. J. Biol. Chem. 1988, 263, 3086–3091. [Google Scholar] [PubMed]
- Flaugh, S.L.; Mills, I.A.; King, J. Glutamine deamidation destabilizes human gammaD-crystallin and lowers the kinetic barrier to unfolding. J. Biol. Chem. 2006, 281, 30782–30793. [Google Scholar] [CrossRef]
- Soulby, A.J.; Heal, J.W.; Barrow, M.P.; Roemer, R.A.; O’Connor, P.B. Does deamidation cause protein unfolding? A top-down tandem mass spectrometry study. Protein Sci. 2015, 24, 850–860. [Google Scholar] [CrossRef]
- Chen, H.M.; Ford, C.; Reilly, P.J. Substitution of asparagine residues in Aspergillus awamori glucoamylase by site-directed mutagenesis to eliminate N-glycosylation and inactivation by deamidation. Biochem. J. 1994, 301, 275–281. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sriprapundh, D.; Vieille, C.; Zeikus, J.G. Molecular determinants of xylose isomerase thermal stability and activity: Analysis of thermozymes by site-directed mutagenesis. Protein Eng. 2000, 13, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Porcelli, M.; Cacciapuoti, G.; Ealick, S.E. The crystal structure of 5′-deoxy-5′-methylthioadenosine phosphorylase II from Sulfolobus solfataricus, a thermophilic enzyme stabilized by intramolecular disulfide bonds. J. Mol. Biol. 2006, 357, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Hsu, C.-H.; Wu, Y.-M.; Luo, Y.-C.; Tu, M.-H.; Chang, W.; Cheng, R.H.; Lin, C.-S. Roles of cysteines Cys115 and Cys201 in the assembly and thermostability of grouper betanodavirus particles. Virus Genes 2010, 41, 73–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pecher, P.; Arnold, U. The effect of additional disulfide bonds on the stability and folding of ribonuclease A. Biophys. Chem. 2009, 141, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, Z.; Yang, H.; Li, J.; Shin, H.-d.; Chen, R.R.; Du, G.; Chen, J. In silico rational design and systems engineering of disulfide bridges in the catalytic domain of an alkaline α-amylase from Alkalimonas amylolytica to improve thermostability. Appl. Environ. Microbiol. 2014, 80, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Finch, A.J.; Kim, J.R. Thermophilic proteins as versatile scaffolds for protein engineering. Microorganisms 2018, 6, 97. [Google Scholar] [CrossRef]
- Petsko, G.A. Structural basis of thermostability in hyperthermophilic proteins, or “there’s more than one way to skin a cat”. Methods Enzymol. 2001, 334, 469–478. [Google Scholar] [CrossRef]
- Camps, M.; Herman, A.; Loh, E.; Loeb, L.A. Genetic constraints on protein evolution. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 313–326. [Google Scholar] [CrossRef]
- Razvi, A.; Scholtz, J.M. Lessons in stability from thermophilic proteins. Protein Sci. 2006, 15, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- DePristo, M.A.; Weinreich, D.M.; Hartl, D.L. Missense meanderings in sequence space: A biophysical view of protein evolution. Nat. Rev. Genet. 2005, 6, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Glyakina, A.V.; Galzitskaya, O.V. How quickly do proteins fold and unfold, and what structural parameters correlate with these values? Biomolecules 2020, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.H. How proteins adapt: Lessons from directed evolution. Cold Spring Harb. Symp. Quant. Biol. 2009, 74, 41–46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karshikoff, A.; Nilsson, L.; Ladenstein, R. Rigidity versus flexibility: The dilemma of understanding protein thermal stability. FEBS J. 2015, 282, 3899–3917. [Google Scholar] [CrossRef]
- Riehle, M.M.; Bennett, A.F.; Long, A.D. Genetic architecture of thermal adaptation in Escherichia coli. Proc. Natl. Acad. Sci. USA 2001, 98, 525–530. [Google Scholar] [CrossRef]
- Shi, B.; Xia, X. Genetic variation in clones of Pseudomonas pseudoalcaligenes after ten months of selection in different thermal environments in the laboratory. Curr. Microbiol. 2005, 50, 238–245. [Google Scholar] [CrossRef]
- Tamakoshi, M.; Yamagishi, A.; Oshima, T. Screening of stable proteins in an extreme thermophile, Thermus thermophilus. Mol. Microbiol. 1995, 16, 1031–1036. [Google Scholar] [CrossRef]
- Perl, D.; Mueller, U.; Heinemann, U.; Schmid, F.X. Two exposed amino acid residues confer thermostability on a cold shock protein. Nat. Struct. Biol. 2000, 7, 380–383. [Google Scholar] [CrossRef]
- Akanuma, S.; Yamagishi, A.; Tanaka, N.; Oshima, T. Serial increase in the thermal stability of 3-isopropylmalate dehydrogenase from Bacillus subtilis by experimental evolution. Protein Sci. 1998, 7, 698–705. [Google Scholar] [CrossRef]
- Blaby, I.K.; Lyons, B.J.; Wroclawska-Hughes, E.; Phillips, G.C.F.; Pyle, T.P.; Chamberlin, S.G.; Benner, S.A.; Lyons, T.J.; de Crécy-Lagard, V.; de Crécy, E. Experimental evolution of a facultative thermophile from a mesophilic ancestor. Appl. Environ. Microbiol. 2012, 78, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.C.; et al. Trophic downgrading of planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Lee, J.-W.; Garcia, S.; Trinh, C.T. Single mutation at a highly conserved region of chloramphenicol acetyltransferase enables isobutyl acetate production directly from cellulose by Clostridium thermocellum at elevated temperatures. Biotechnol. Biofuels 2019, 12, 245. [Google Scholar] [CrossRef]
- Yan, F.; Wei, R.; Cui, Q.; Bornscheuer, U.T.; Liu, Y.-J. Thermophilic whole-cell degradation of polyethylene terephthalate using engineered Clostridium thermocellum. Microb. Biotechnol. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Williams, T.J.; Walsh, J.C.; Ji, M.; Poljak, A.; Curmi, P.M.G.; Duggin, I.G.; Cavicchioli, R. Developing a genetic manipulation system for the Antarctic archaeon, Halorubrum lacusprofundi: Investigating acetamidase gene function. Sci. Rep. 2016, 6, 34639. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wei, R.; Luo, W.; Hu, L.; Li, B.; Di, Y.; Shi, H. Microplastic pollution in water and sediment in a textile industrial area. Environ. Pollut. 2020, 258, 113658. [Google Scholar] [CrossRef] [PubMed]
- Moritz, M.; Behnke, R.; Beitl, C.M.; Bird, R.B.; Chiaravalloti, R.M.; Clark, J.K.; Crabtree, S.A.; Downey, S.S.; Hamilton, I.M.; Phang, S.C.; et al. Emergent sustainability in open property regimes. Proc. Natl. Acad. Sci. USA 2018, 115, 12859–12867. [Google Scholar] [CrossRef]
- Rane, R.V.; Pearce, S.L.; Li, F.; Coppin, C.; Schiffer, M.; Shirriffs, J.; Sgrò, C.M.; Griffin, P.C.; Zhang, G.; Lee, S.F.; et al. Genomic changes associated with adaptation to arid environments in cactophilic Drosophila species. BMC Genom. 2019, 20, 52. [Google Scholar] [CrossRef]
- Baudier, K.; O’Donnell, S. Complex body size differences in thermal tolerance among army ant workers (Eciton burchellii parvispinum). J. Therm. Biol. 2018, 78, 277–280. [Google Scholar] [CrossRef]
- Oms, C.S.; Cerdá, X.; Boulay, R. Is phenotypic plasticity a key mechanism for responding to thermal stress in ants? Naturwissenschaften 2017, 104, 42. [Google Scholar] [CrossRef]
- Otte, T.; Hilker, M.; Geiselhardt, S. Phenotypic plasticity of cuticular hydrocarbon profiles in insects. J. Chem. Ecol. 2018, 44, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Duarte, B.F.; Michelutti, K.B.; Antonialli-Junior, W.F.; Cardoso, C.A.L. Effect of temperature on survival and cuticular composition of three different ant species. J. Therm. Biol. 2019, 80, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Goff, G.L.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef] [PubMed]
- Denic, V.; Weissman, J.S. A molecular caliper mechanism for determining very long-chain fatty acid length. Cell 2007, 130, 663–677. [Google Scholar] [CrossRef]
- Helmuth, B.; Kingsolver, J.G.; Carrington, E. Biophysics, physiological ecology, and climate change: Does mechanism matter? Annu. Rev. Physiol. 2005, 67, 177–201. [Google Scholar] [CrossRef]
- Calosi, P.; Bilton, D.T.; Spicer, J.I. Thermal tolerance, acclimatory capacity and vulnerability to global climate change. Biol. Lett. 2008, 4, 99–102. [Google Scholar] [CrossRef]
- Mazzucco, R.; Nolte, V.; Vijayan, T.; Schlötterer, C. Long-term dynamics among Wolbachia strains during thermal adaptation of their Drosophila melanogaster hosts. Front. Genet. 2020, 11, 482. [Google Scholar] [CrossRef]
- Pikuta, E.V.; Hoover, R.B.; Tang, J. Microbial extremophiles at the limits of life. Crit. Rev. Microbiol. 2007, 33, 183–209. [Google Scholar] [CrossRef]
- Schwieterman, E.W.; Kiang, N.Y.; Parenteau, M.N.; Harman, C.E.; DasSarma, S.; Fisher, T.M.; Arney, G.N.; Hartnett, H.E.; Reinhard, C.T.; Olson, S.L.; et al. Exoplanet biosignatures: A review of remotely detectable signs of life. Astrobiology 2018, 18, 663–708. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Airo, A.; Schirmack, J. The adaptability of life on Earth and the diversity of planetary habitats. Front. Microbiol. 2017, 8, 2011. [Google Scholar] [CrossRef]
- Arenas, M.; Ray, N.; Currat, M.; Excoffier, L. Consequences of range contractions and range shifts on molecular diversity. Mol. Biol. Evol. 2012, 29, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Sheng, X.; Murray-Nerger, L.A.; Cristea, I.M. Temporal dynamics of protein complex formation and dissociation during human cytomegalovirus infection. Nat. Commun. 2020, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Marine viruses–major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Mihindukulasuriya, K.A.; Wu, G.; St Leger, J.; Nordhausen, R.W.; Wang, D. Identification of a novel coronavirus from a beluga whale by using a panviral microarray. J. Virol. 2008, 82, 5084–5088. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.; Lam, C.S.; Tsang, A.K.; Hui, S.W.; Fan, R.Y.; Martelli, P.; Yuen, K.Y. Discovery of a novel bottlenose dolphin coronavirus reveals a distinct species of marine mammal coronavirus in Gammacoronavirus. J. Virol. 2014, 88, 1318–1331. [Google Scholar] [CrossRef] [PubMed]
- Barik, S. Genus-specific pattern of intrinsically disordered central regions in the nucleocapsid protein of coronaviruses. Comput. Struct. Biotechnol. J. 2020, 18, 1884–1890. [Google Scholar] [CrossRef]
- Kendrick, B.J.; DiTullio, G.R.; Cyronak, T.J.; Fulton, J.M.; Van Mooy, B.A.S.; Bidle, K.D. Temperature-induced viral resistance in Emiliania huxleyi (Prymnesiophyceae). PLoS ONE 2014, 9, e112134. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate warming and disease risks for terrestrial and marine biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef]
- Piedade, G.J.; Wesdorp, E.M.; Montenegro-Borbolla, E.; Maat, D.S.; Brussaard, C.P.D. Influence of irradiance and temperature on the virus MpoV-45T infecting the arctic picophytoplankter Micromonas polaris. Viruses 2018, 10, 676. [Google Scholar] [CrossRef]
- Barik, S. Molecular interactions between pathogens and the circadian clock. Int. J. Mol. Sci. 2019, 20, 5824. [Google Scholar] [CrossRef]
- Urnowey, S.; Ansai, T.; Bitko, V.; Barik, S. Regulation of NF-kappa B and cell death by bacterial gingipains. BioRxiv 2020. [Google Scholar] [CrossRef]
- Dziuba, M.K.; Herdegen-Radwan, M.; Pluta, E.; Wejnerowski, Ł.; Szczuciński, W.; Cerbin, S. Temperature increase altered Daphnia community structure in artificially heated lakes: A potential scenario for a warmer future. Sci. Rep. 2020, 10, 13956. [Google Scholar] [CrossRef] [PubMed]
- Bryan, P.N.; Orban, J. Proteins that switch folds. Curr. Opin. Struct. Biol. 2010, 20, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, R.L.; Peterson, F.C.; Kutlesa, S.; Elgin, E.S.; Kron, M.A.; Volkman, B.F. Interconversion between two unrelated protein folds in the lymphotactin native state. Proc. Natl. Acad. Sci. USA 2008, 105, 5057–5062. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barik, S. Evolution of Protein Structure and Stability in Global Warming. Int. J. Mol. Sci. 2020, 21, 9662. https://doi.org/10.3390/ijms21249662
Barik S. Evolution of Protein Structure and Stability in Global Warming. International Journal of Molecular Sciences. 2020; 21(24):9662. https://doi.org/10.3390/ijms21249662
Chicago/Turabian StyleBarik, Sailen. 2020. "Evolution of Protein Structure and Stability in Global Warming" International Journal of Molecular Sciences 21, no. 24: 9662. https://doi.org/10.3390/ijms21249662
APA StyleBarik, S. (2020). Evolution of Protein Structure and Stability in Global Warming. International Journal of Molecular Sciences, 21(24), 9662. https://doi.org/10.3390/ijms21249662



