Structural Characterization of Glycerol Kinase from the Thermophilic Fungus Chaetomium thermophilum
Abstract
1. Introduction
2. Results
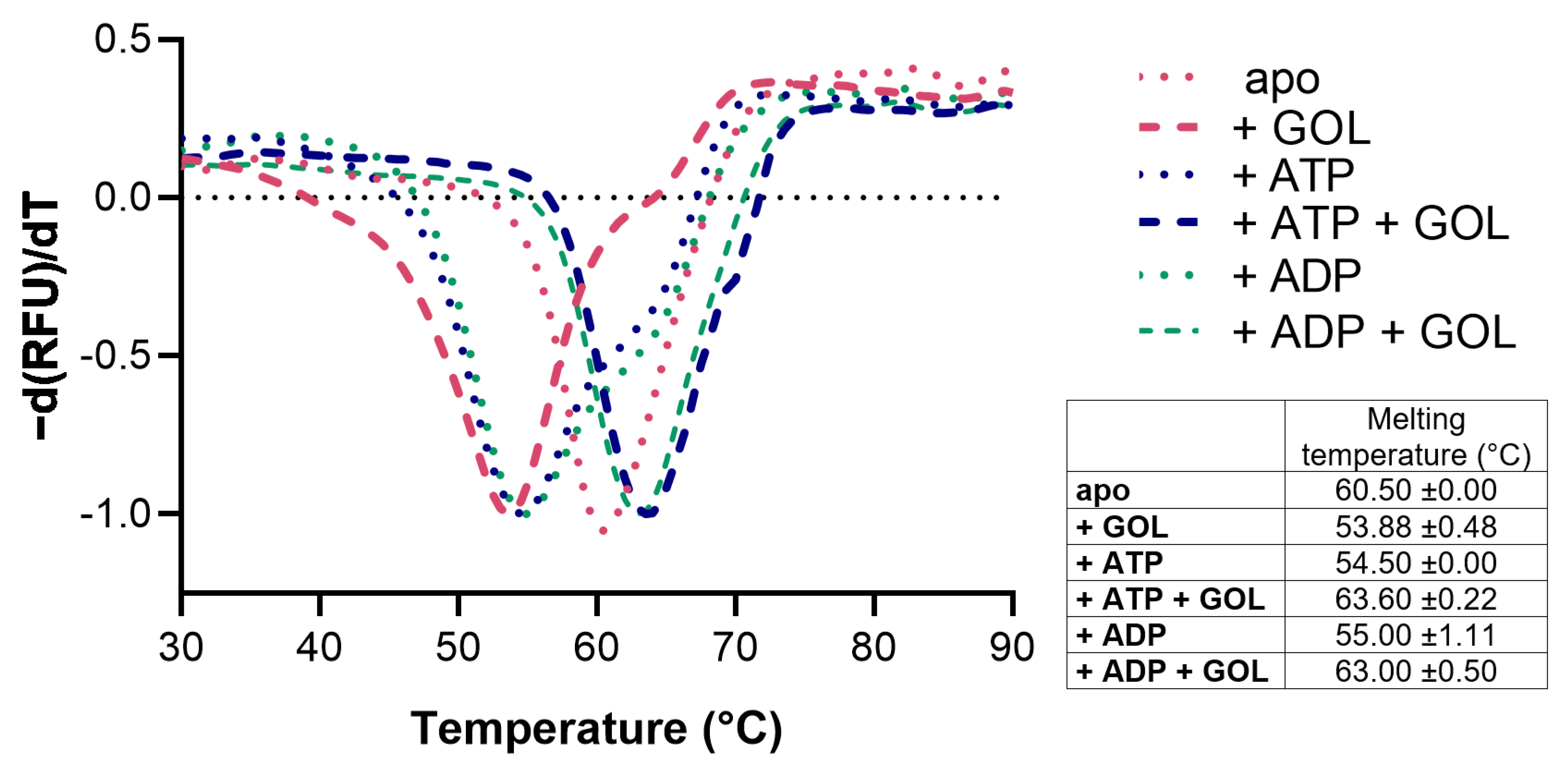
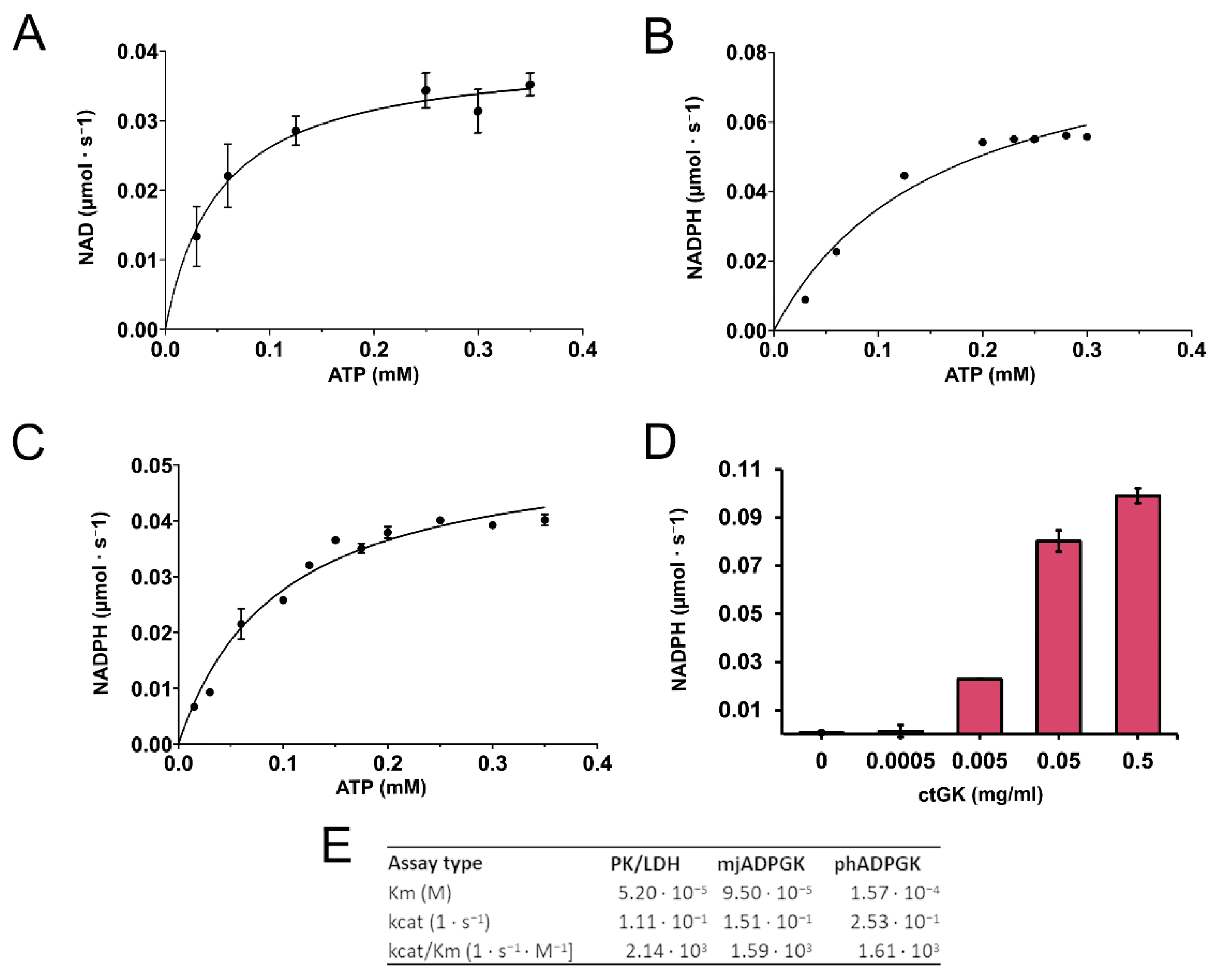
2.1. Expression, Purification, Stability, and Activity Measurements
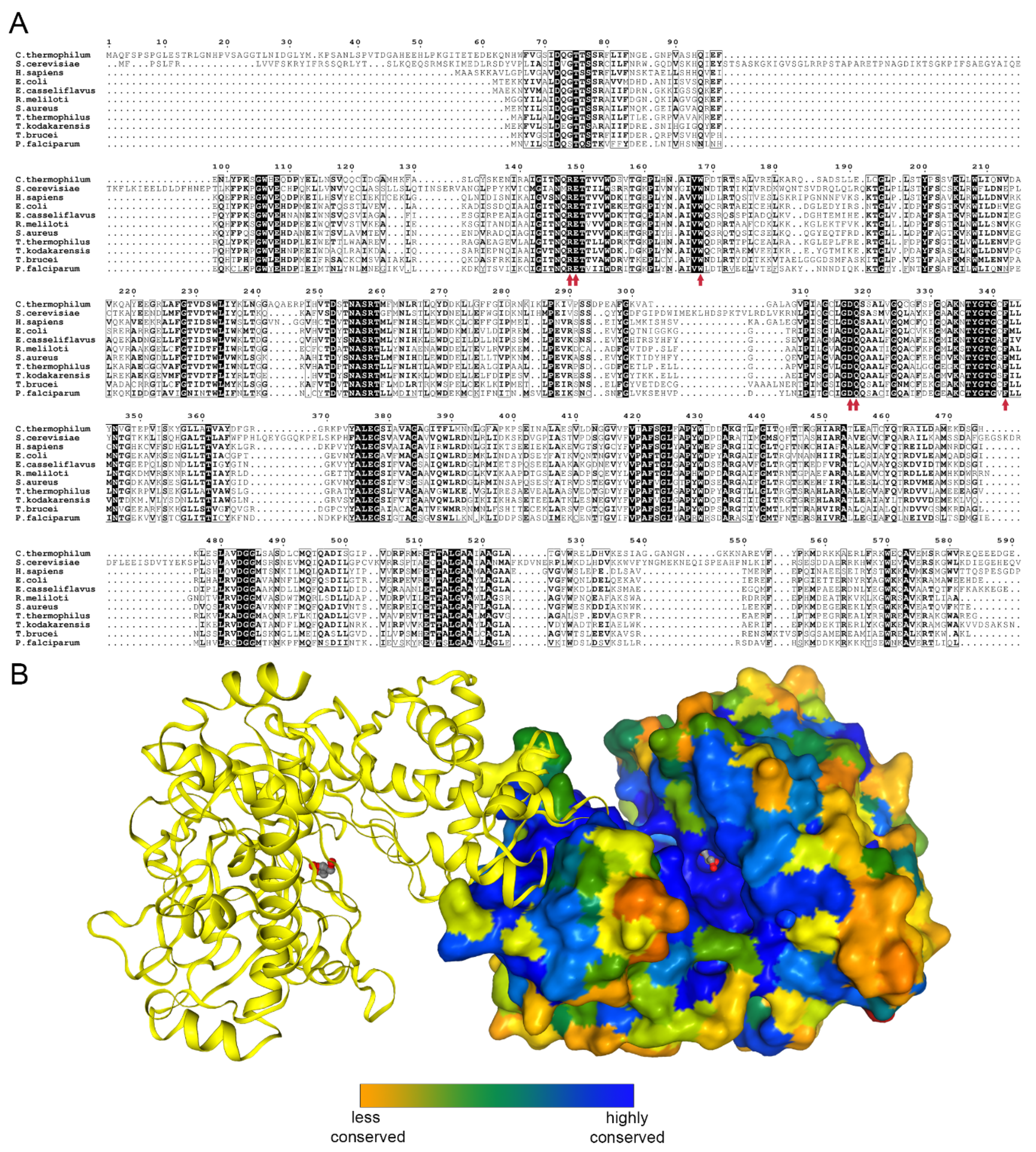
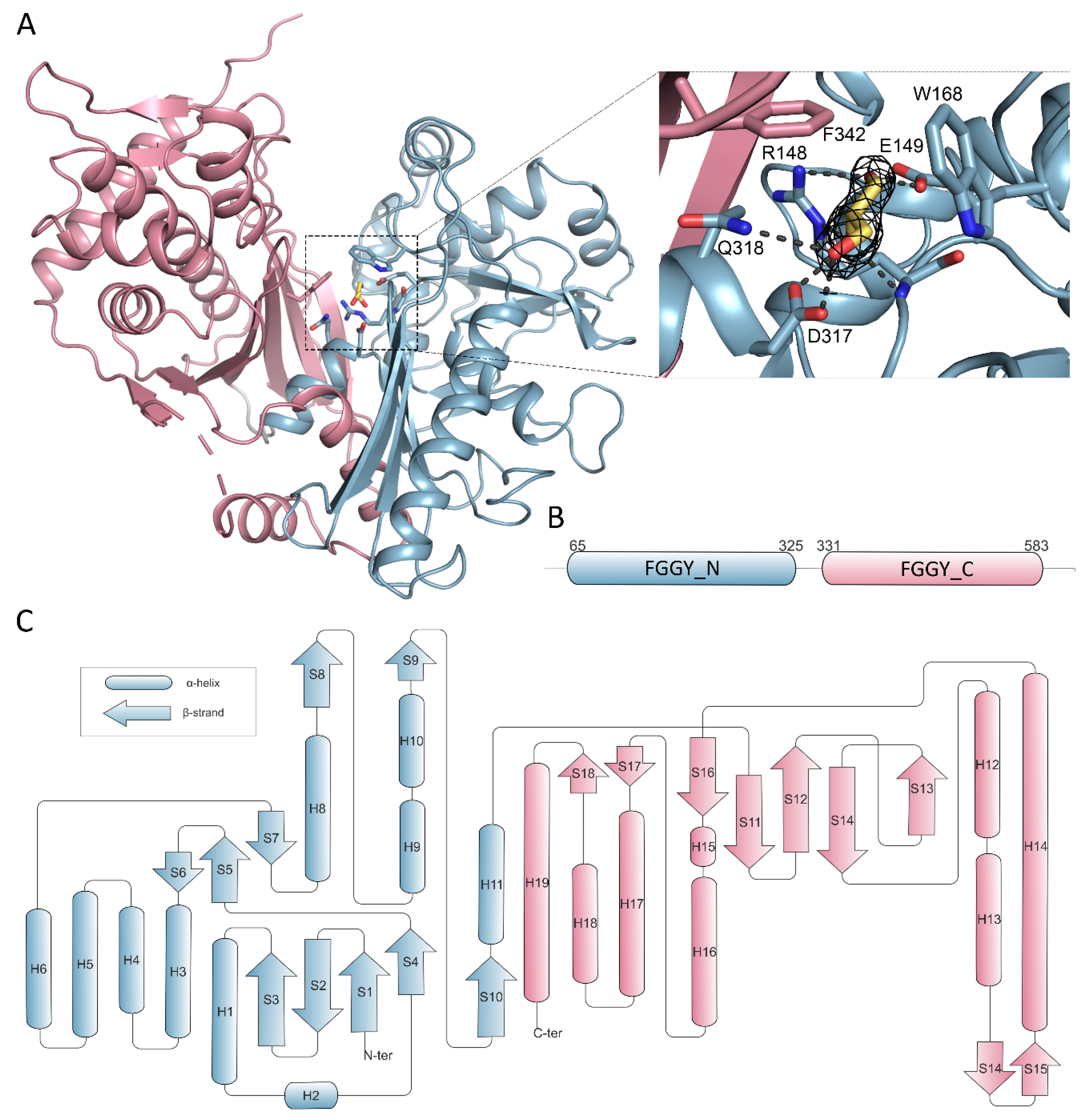
2.2. Overall Structure and Domain Organization
2.3. Glycerol Binding
2.4. Nucleotide Binding
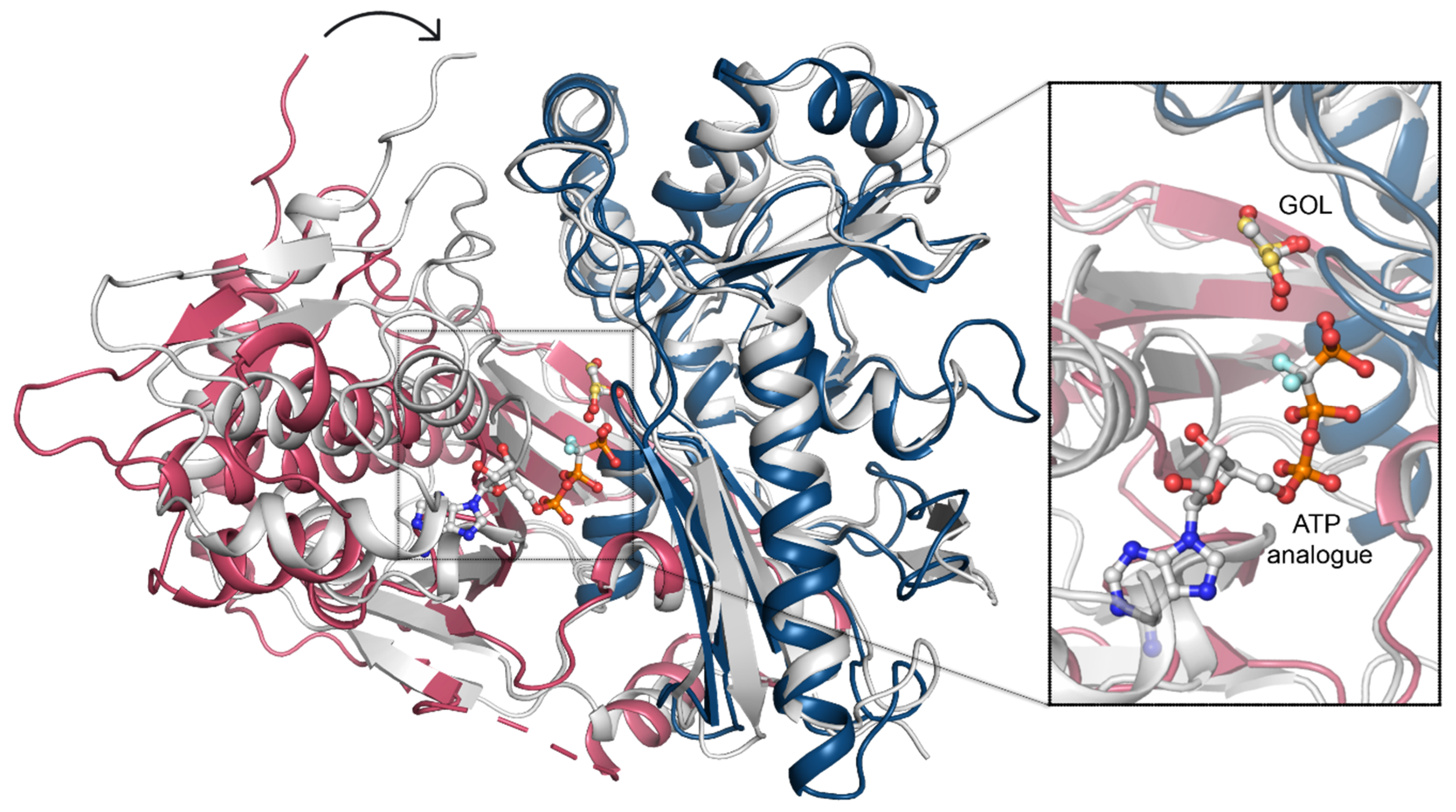
2.5. Domain Motion
3. Discussion
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. Thermal Stability Assay
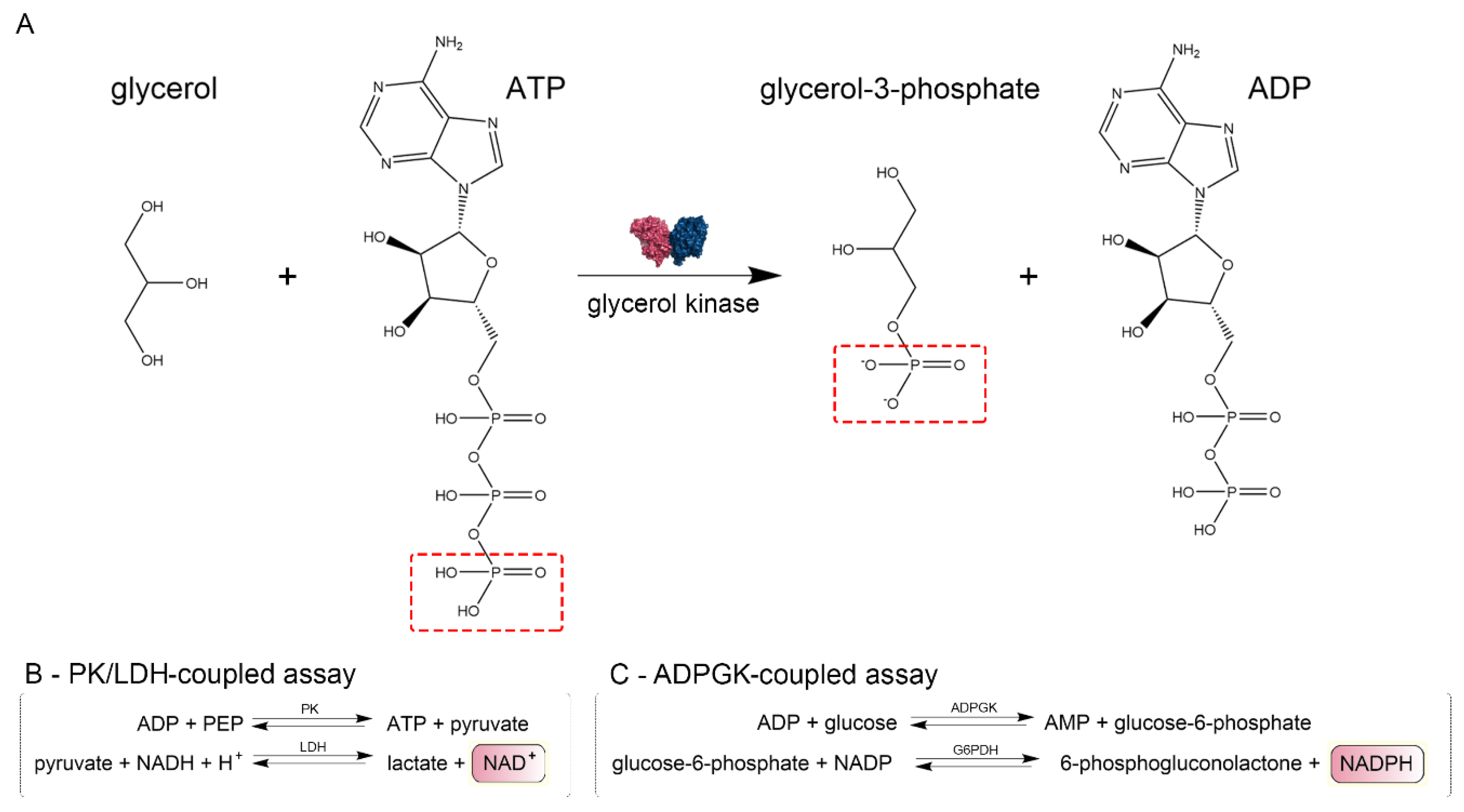
4.3. CtGK Activity Assays
4.3.1. Pyruvate Kinase/Lactate Dehydrogenase (PK/LDH)-Coupled Activity Assay
4.3.2. ADP-Dependent Glucokinase/Glucose-6-Phosphate Dehydrogenase (ADPGK/G6PD)-Coupled Activity Assay
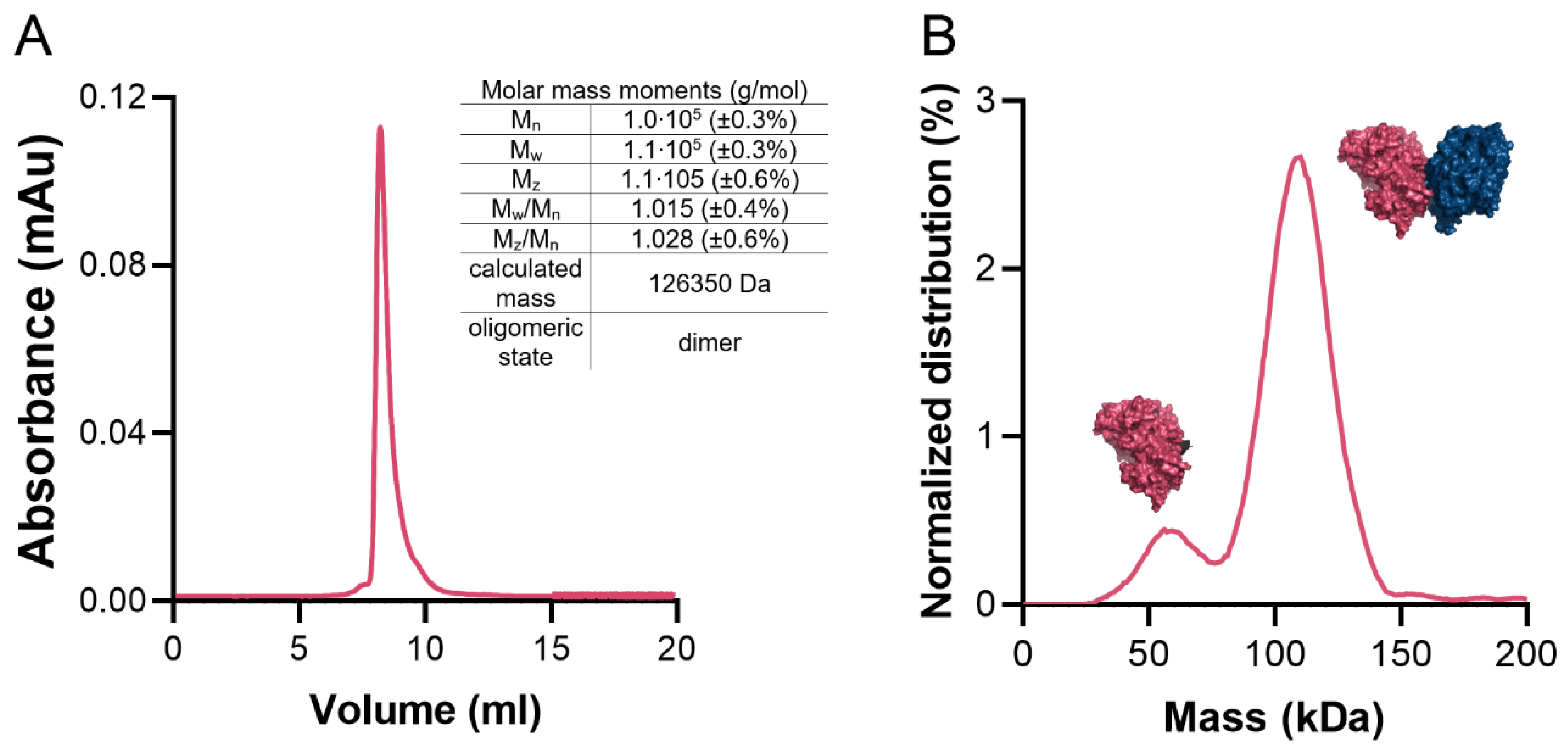
4.4. Determination of Oligomeric State in Solution
4.5. Crystallization, Data Collection, and Structure Determination
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-ITU | 5-Iodotubercidin |
| 8-Br-AMP | 8-Bromoadenosine 5′-monophosphate |
| AMPNP | Adenosine-5′-[(α,β)-imido]diphosphate |
| ADPGK | ADP-dependent glucokinase |
| ADPGK/PFK | ADP-dependent gluco/phosphofructokinase |
| ASU | Asymmetric unit |
| CtGK | Chaetomium thermophilum glycerol kinase |
| FAD | Flavin adenine dinucleotide |
| FBP | Fructose-1,6-bisphoshpate |
| G3P | Glycerol 3-phosphate |
| G6PD | Glucose-6-phosphate dehydrogenase |
| GOL | Glycerol |
| GK | Glycerol kinase |
| LDH | Lactate dehydrogenase |
| MALS | Multi-angle light scattering |
| NAD/NADH | Nicotinamide adenine dinucleotide |
| NADP/NADPH | Nicotinamide adenine dinucleotide phosphate |
| PDB | Protein Data Bank |
| PfGK | Plasmodium falciparum glycerol kinase |
| PK | Pyruvate kinase |
| SEC | Size-exclusion chromatography |
References
- Xue, L.-L.; Chen, H.-H.; Jiang, J.-G. Implications of glycerol metabolism for lipid production. Prog. Lipid Res. 2017, 68, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Swinnen, S.; Thevelein, J.M.; Nevoigt, E. Glycerol metabolism and transport in yeast and fungi: Established knowledge and ambiguities. Environ. Microbiol. 2017, 19, 878–893. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.I.; Chinte, U.; Du, S. Structure of glycerol-3-phosphate dehydrogenase, an essential monotopic membrane enzyme involved in respiration and metabolism. Proc. Natl. Acad. Sci. USA 2008, 105, 3280–3285. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.E.; Pichaud, N.; Darveau, C.-A. “Alternative” fuels contributing to mitochondrial electron transport: Importance of non-classical pathways in the diversity of animal metabolism. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2018, 224, 185–194. [Google Scholar] [CrossRef]
- Rahib, L.; MacLennan, N.K.; Horvath, S.; Liao, J.C.; Dipple, K.M. Glycerol kinase deficiency alters expression of genes involved in lipid metabolism, carbohydrate metabolism, and insulin signaling. Eur. J. Hum. Genet. 2007, 15, 646–657. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Bliven, S.; Lafita, A.; Parker, A.; Capitani, G.; Duarte, J.M. Automated evaluation of quaternary structures from protein crystals. PLoS Comput. Biol. 2018, 14, e1006104. [Google Scholar] [CrossRef]
- Roy, S.; Vivoli Vega, M.; Harmer, N.J. Carbohydrate Kinases: A Conserved Mechanism Across Differing Folds. Catalysts 2019, 9, 29. [Google Scholar] [CrossRef]
- Zak, K.M.; Kalińska, M.; Wator, E.; Kuśka, K.; Krutyhołowa, R.; Dubin, G.; Popowicz, G.M.; Grudnik, P. Crystal Structure of Kluyveromyces lactis Glucokinase (KlGlk1). Int. J. Mol. Sci. 2019, 20, 4821. [Google Scholar] [CrossRef]
- Sugahara, M.; Asada, Y.; Morikawa, Y.; Kageyama, Y.; Kunishima, N. Nucleant-mediated protein crystallization with the application of microporous synthetic zeolites. Acta Crystallogr. Sect. D Biol. Crystallogr. 2008, 64, 686–695. [Google Scholar] [CrossRef]
- Bystrom, C.E.; Pettigrew, D.W.; Branchaud, B.P.; O’Brien, P.; Remington, S.J. Crystal structures of Escherichia coli glycerol kinase variant S58-->W in complex with nonhydrolyzable ATP analogues reveal a putative active conformation of the enzyme as a result of domain motion. Biochemistry 1999, 38, 3508–3518. [Google Scholar] [CrossRef]
- Anderson, M.J.; DeLaBarre, B.; Raghunathan, A.; Palsson, B.O.; Brunger, A.T.; Quake, S.R. Crystal Structure of a Hyperactive Escherichia coli Glycerol Kinase Mutant Gly230→Asp Obtained Using Microfluidic Crystallization Devices †, ‡. Biochemistry 2007, 46, 5722–5731. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.I.; Kettering, R.; Saxl, R.; Bourand, A.; Darbon, E.; Joly, N.; Briozzo, P.; Deutscher, J. Structural Characterizations of Glycerol Kinase: Unraveling Phosphorylation-Induced Long-Range Activation †. Biochemistry 2009, 48, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Katsumi, R.; You, D.-J.; Matsumura, H.; Takano, K.; Kanaya, S. Crystal structure of highly thermostable glycerol kinase from a hyperthermophilic archaeon in a dimeric form. FEBS J. 2008, 275, 2632–2643. [Google Scholar] [CrossRef] [PubMed]
- Balogun, E.O.; Inaoka, D.K.; Shiba, T.; Tsuge, C.; May, B.; Sato, T.; Kido, Y.; Nara, T.; Aoki, T.; Honma, T.; et al. Discovery of trypanocidal coumarins with dual inhibition of both the glycerol kinase and alternative oxidase of Trypanosoma brucei brucei. FASEB J. 2019, 33, 13002–13013. [Google Scholar] [CrossRef] [PubMed]
- Schnick, C.; Polley, S.D.; Fivelman, Q.L.; Ranford-Cartwright, L.C.; Wilkinson, S.R.; Brannigan, J.A.; Wilkinson, A.J.; Baker, D.A. Structure and non-essential function of glycerol kinase in Plasmodium falciparum blood stages. Mol. Microbiol. 2009, 71, 533–545. [Google Scholar] [CrossRef]
- Reinhard, L.; Mayerhofer, H.; Geerlof, A.; Mueller-Dieckmann, J.; Weiss, M.S. Optimization of protein buffer cocktails using Thermofluor. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013, 69, 209–214. [Google Scholar] [CrossRef]
- Tokarz, P.; Wiśniewska, M.; Kamiński, M.M.; Dubin, G.; Grudnik, P. Crystal structure of ADP-dependent glucokinase from Methanocaldococcus jannaschii in complex with 5-iodotubercidin reveals phosphoryl transfer mechanism. Protein Sci. 2018, 27, 790–797. [Google Scholar] [CrossRef]
- Ueda, S.; Sakasegawa, S. Enzymatic cycling method using creatine kinase to measure creatine by real-time detection. Anal. Biochem. 2016, 506, 8–12. [Google Scholar] [CrossRef]
- Kengen, S.W.M.; Tuininga, J.E.; de Bok, F.A.M.; Stams, A.J.M.; de Vos, W.M. Purification and Characterization of a Novel ADP-dependent Glucokinase from the Hyperthermophilic Archaeon Pyrococcus furiosus. J. Biol. Chem. 1995, 270, 30453–30457. [Google Scholar] [CrossRef]
- Tuininga, J.E.; Verhees, C.H.; van der Oost, J.; Kengen, S.W.M.; Stams, A.J.M.; de Vos, W.M. Molecular and Biochemical Characterization of the ADP-dependent Phosphofructokinase from the Hyperthermophilic Archaeon Pyrococcus furiosus. J. Biol. Chem. 1999, 274, 21023–21028. [Google Scholar] [CrossRef] [PubMed]
- Ormö, M.; Bystrom, C.E.; Remington, S.J. Crystal Structure of a Complex of Escherichia c oli Glycerol Kinase and an Allosteric Effector Fructose 1,6-Bisphosphate †, ‡. Biochemistry 1998, 37, 16565–16572. [Google Scholar] [CrossRef] [PubMed]
- Jurica, M.S.; Mesecar, A.; Heath, P.J.; Shi, W.; Nowak, T.; Stoddard, B.L. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure 1998, 6, 195–210. [Google Scholar] [CrossRef]
- Vagin, A.; Lebedev, A. MoRDa, an automatic molecular replacement pipeline. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, s19. [Google Scholar] [CrossRef]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Krissinel, E. Crystal contacts as nature’s docking solutions. J. Comput. Chem. 2010, 31, 133–143. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- Grudnik, P.; Kamiński, M.M.; Rembacz, K.P.; Kuśka, K.; Madej, M.; Potempa, J.; Dawidowski, M.; Dubin, G. Structural basis for ADP-dependent glucokinase inhibition by 8-bromo–substituted adenosine nucleotide. J. Biol. Chem. 2018, 293, 11088–11099. [Google Scholar] [CrossRef]
- Zebisch, M.; Krauss, M.; Schäfer, P.; Lauble, P.; Sträter, N. Crystallographic Snapshots along the Reaction Pathway of Nucleoside Triphosphate Diphosphohydrolases. Structure 2013, 21, 1460–1475. [Google Scholar] [CrossRef][Green Version]
- Balogun, E.O.; Inaoka, D.K.; Shiba, T.; Kido, Y.; Tsuge, C.; Nara, T.; Aoki, T.; Honma, T.; Tanaka, A.; Inoue, M.; et al. Molecular basis for the reverse reaction of African human trypanosomes glycerol kinase. Mol. Microbiol. 2014, 94, 1315–1329. [Google Scholar] [CrossRef]
- Holtman, C.K.; Pawlyk, A.C.; Meadow, N.D.; Pettigrew, D.W. Reverse Genetics of Escherichia coliGlycerol Kinase Allosteric Regulation and Glucose Control of Glycerol Utilization In Vivo. J. Bacteriol. 2001, 183, 3336–3344. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Pardo, J.A.; Herrera-Morande, A.; Castro-Fernandez, V.; Fernandez, F.J.; Vega, M.C.; Guixé, V. Crystal Structure, SAXS and Kinetic Mechanism of Hyperthermophilic ADP-Dependent Glucokinase from Thermococcus litoralis Reveal a Conserved Mechanism for Catalysis. PLoS ONE 2013, 8, e66687. [Google Scholar] [CrossRef]
- Cook, P.F.; Neville, M.E.; Vrana, K.E.; Hartl, F.T.; Roskoski, R. Adenosine cyclic 3’,5’-monophosphate dependent protein kinase: Kinetic mechanism for the bovine skeletal muscle catalytic subunit. Biochemistry 1982, 21, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
- Wątor, E.; Wilk, P.; Grudnik, P. Half Way to Hypusine-Structural Basis for Substrate Recognition by Human Deoxyhypusine Synthase. Biomolecules 2020, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Sonn-Segev, A.; Belacic, K.; Bodrug, T.; Young, G.; VanderLinden, R.T.; Schulman, B.A.; Schimpf, J.; Friedrich, T.; Dip, P.V.; Schwartz, T.U.; et al. Quantifying the heterogeneity of macromolecular machines by mass photometry. Nat. Commun. 2020, 11, 1772. [Google Scholar] [CrossRef]
- Mueller, U.; Darowski, N.; Fuchs, M.R.; Förster, R.; Hellmig, M.; Paithankar, K.S.; Pühringer, S.; Steffien, M.; Zocher, G.; Weiss, M.S. Facilities for macromolecular crystallography at the Helmholtz-Zentrum Berlin. J. Synchrotron Radiat. 2012, 19, 442–449. [Google Scholar] [CrossRef]
- Basu, S.; Kaminski, J.W.; Panepucci, E.; Huang, C.Y.; Warshamanage, R.; Wang, M.; Wojdyla, J.A. Automated data collection and real-time data analysis suite for serial synchrotron crystallography. J. Synchrotron Radiat. 2019, 26, 244–252. [Google Scholar] [CrossRef]
- Burkhardt, A.; Pakendorf, T.; Reime, B.; Meyer, J.; Fischer, P.; Stübe, N.; Panneerselvam, S.; Lorbeer, O.; Stachnik, K.; Warmer, M.; et al. Status of the crystallography beamlines at PETRA III. Eur. Phys. J. Plus 2016, 131, 56. [Google Scholar] [CrossRef]
- Sparta, K.M.; Krug, M.; Heinemann, U.; Mueller, U.; Weiss, M.S. XDSAPP2.0. J. Appl. Crystallogr. 2016, 49, 1085–1092. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef] [PubMed]
| PDB ID | 6ZQ4 | 6ZQ5 | 6ZQ6 | 6ZQ7 | 6ZQ8 |
|---|---|---|---|---|---|
| Light Source | BESSY 14.1 | BESSY 14.1 | BESSY 14.1 | SLS PXI | DESY P11 |
| Wavelength (Å) | 0.918 | 0.918 | 0.918 | 1.00 | 1.033 |
| Resolution range (Å) | 23.01–2.02 (2.07–2.02) | 46.71–2.14 (2.27–2.14) | 48.6–2.30 (2.44–2.30) | 44.72–2.42 (2.51–2.42) | 47.04–2.38 (2.53–2.38) |
| Space group | P1 | P2221 | P21212 | I222 | P3221 |
| Unit cell (Å, °) | 61.38 112.82 179.54 85.77 90.04 85.45 | 63.57 111.47 171.25 90.0 90.0 90.0 | 170.73 222.03 61.33 90.0 90.0 90.0 | 88.60 89.44 131.29 90.0 90.0 90.0 | 92.14 92.14 232.95 90.0 90.0 120.0 |
| Total reflections | 1906807 (71895) | 374918 (38178) | 383976 (62534) | 40292 (3843) | 888970 (135787) |
| Unique reflections | 307534 (19548) | 61278 (8088) | 103148 (16723) | 20156 (1926) | 46200 (6995) |
| Multiplicity | 6.2 (3.68) | 6.12 (4.72) | 3.72 (3.74) | 2.0 (2.0) | 19.24 (19.41) |
| Completeness (%) | 97.4 (83.6) | 89.9 (74.4) | 98.5 (98.1) | 99.25 (96.28) | 98.8 (94.3) |
| Mean I/sigma(I) | 4.85 (0.74) | 11.59 (0.67) | 6.80 (0.77) | 17.17 (1.44) | 17.95 (1.49) |
| Wilson B-factor | 36.82 | 47.97 | 47.10 | 75.60 | 66.89 |
| R-merge (%) | 21.9 (147.4) | 11.9 (219.7) | 17.7 (159.1) | 1.76 (36.24) | 12.4 (196.4) |
| R-meas (%) | 23.7 (171.7) | 13.0 (243.5) | 20.7 (185.0) | 2.48 (51.25) | 12.7 (201.7) |
| CC1/2 (%) | 99.1 (26.9) | 99.8 (27.6) | 99.3 (33.1) | 100 (80.6) | 99.9 (63.3) |
| Reflections used in refinement | 306624 (28330) | 61158 (4981) | 103082 (10153) | 20131 (1931) | 46193 (4159) |
| Reflections used for R-free | 3221 (298) | 2096 (171) | 2099 (207) | 1008 (96) | 2099 (189) |
| R-work (%) | 20.49 | 21.55 | 21.47 | 18.64 | 24.36 |
| R-free (%) | 23.87 | 24.95 | 24.91 | 25.13 | 28.66 |
| Number of protein chains in ASU | 8 | 2 | 4 | 1 | 2 |
| Number of non-hydrogen atoms | 33032 | 8167 | 16060 | 3995 | 7887 |
| macromolecules | 31248 | 7801 | 15603 | 3912 | 7809 |
| ligands | 78 | 44 | 46 | 10 | 4 |
| solvent | 1706 | 322 | 411 | 73 | 74 |
| Protein residues | 4085 | 1021 | 2041 | 510 | 1021 |
| RMS (bonds) | 0.009 | 0.004 | 0.006 | 0.008 | 0.004 |
| RMS (angles) | 1.05 | 0.68 | 0.87 | 1.03 | 0.66 |
| Ramachandran favored (%) | 97.16 | 97.24 | 96.64 | 95.45 | 96.05 |
| Ramachandran allowed (%) | 2.76 | 2.76 | 3.06 | 4.36 | 3.95 |
| Ramachandran outliers (%) | 0.07 | 0 | 0.30 | 0.2 | 0 |
| Rotamer outliers (%) | 0.40 | 1.34 | 0.85 | 0.49 | 0.49 |
| Clashscore | 6.42 | 3.71 | 3.95 | 12.65 | 9.07 |
| Average B-factor | 51.66 | 53.36 | 51.92 | 93.07 | 79.91 |
| macromolecules | 51.84 | 53.42 | 51.98 | 93.29 | 80.12 |
| ligands | 42.64 | 62.71 | 52.98 | 75.4 | 53.78 |
| solvent | 48.90 | 50.69 | 49.33 | 83.77 | 59.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilk, P.; Kuśka, K.; Wątor, E.; Małecki, P.H.; Woś, K.; Tokarz, P.; Dubin, G.; Grudnik, P. Structural Characterization of Glycerol Kinase from the Thermophilic Fungus Chaetomium thermophilum. Int. J. Mol. Sci. 2020, 21, 9570. https://doi.org/10.3390/ijms21249570
Wilk P, Kuśka K, Wątor E, Małecki PH, Woś K, Tokarz P, Dubin G, Grudnik P. Structural Characterization of Glycerol Kinase from the Thermophilic Fungus Chaetomium thermophilum. International Journal of Molecular Sciences. 2020; 21(24):9570. https://doi.org/10.3390/ijms21249570
Chicago/Turabian StyleWilk, Piotr, Katarzyna Kuśka, Elżbieta Wątor, Piotr H. Małecki, Klaudia Woś, Piotr Tokarz, Grzegorz Dubin, and Przemysław Grudnik. 2020. "Structural Characterization of Glycerol Kinase from the Thermophilic Fungus Chaetomium thermophilum" International Journal of Molecular Sciences 21, no. 24: 9570. https://doi.org/10.3390/ijms21249570
APA StyleWilk, P., Kuśka, K., Wątor, E., Małecki, P. H., Woś, K., Tokarz, P., Dubin, G., & Grudnik, P. (2020). Structural Characterization of Glycerol Kinase from the Thermophilic Fungus Chaetomium thermophilum. International Journal of Molecular Sciences, 21(24), 9570. https://doi.org/10.3390/ijms21249570





