Potential of Bacterial Cellulose Chemisorbed with Anti-Metabolites, 3-Bromopyruvate or Sertraline, to Fight against Helicobacter pylori Lawn Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions and Bacterial Strains Used
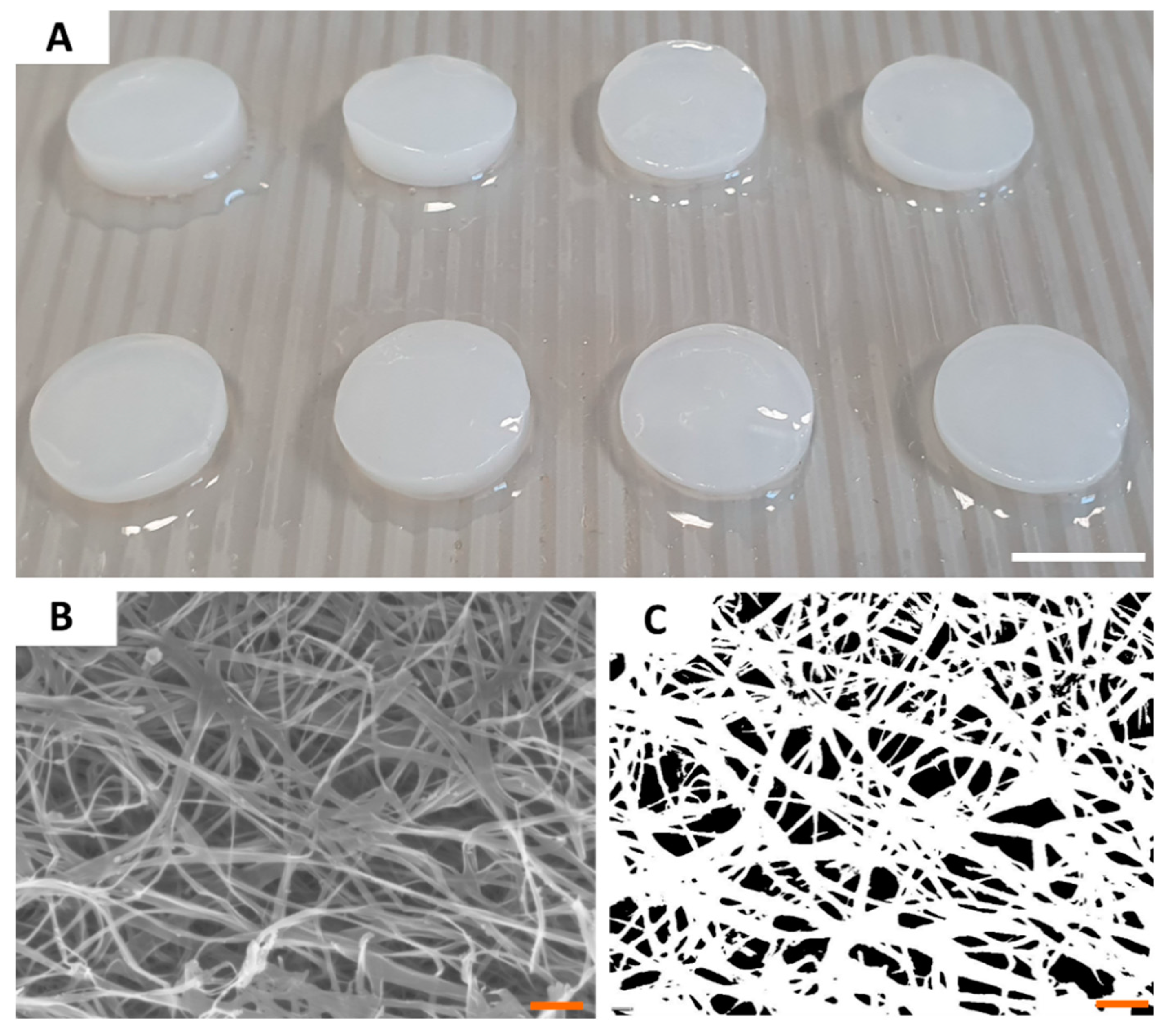
2.2. Carrier Characteristics
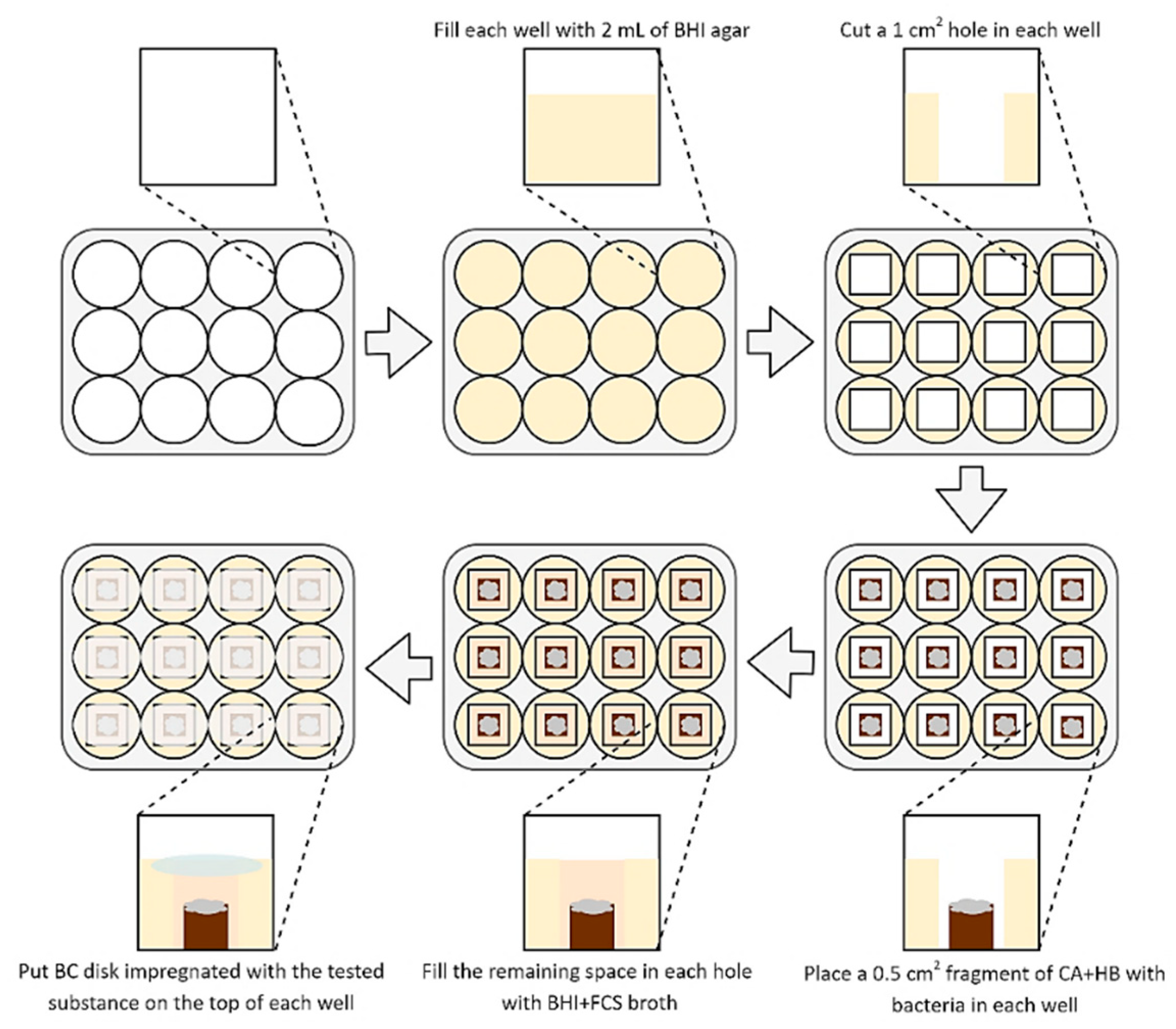
2.3. Determination of Antimicrobial Activity of Tested Compounds
2.4. Statistical Analysis
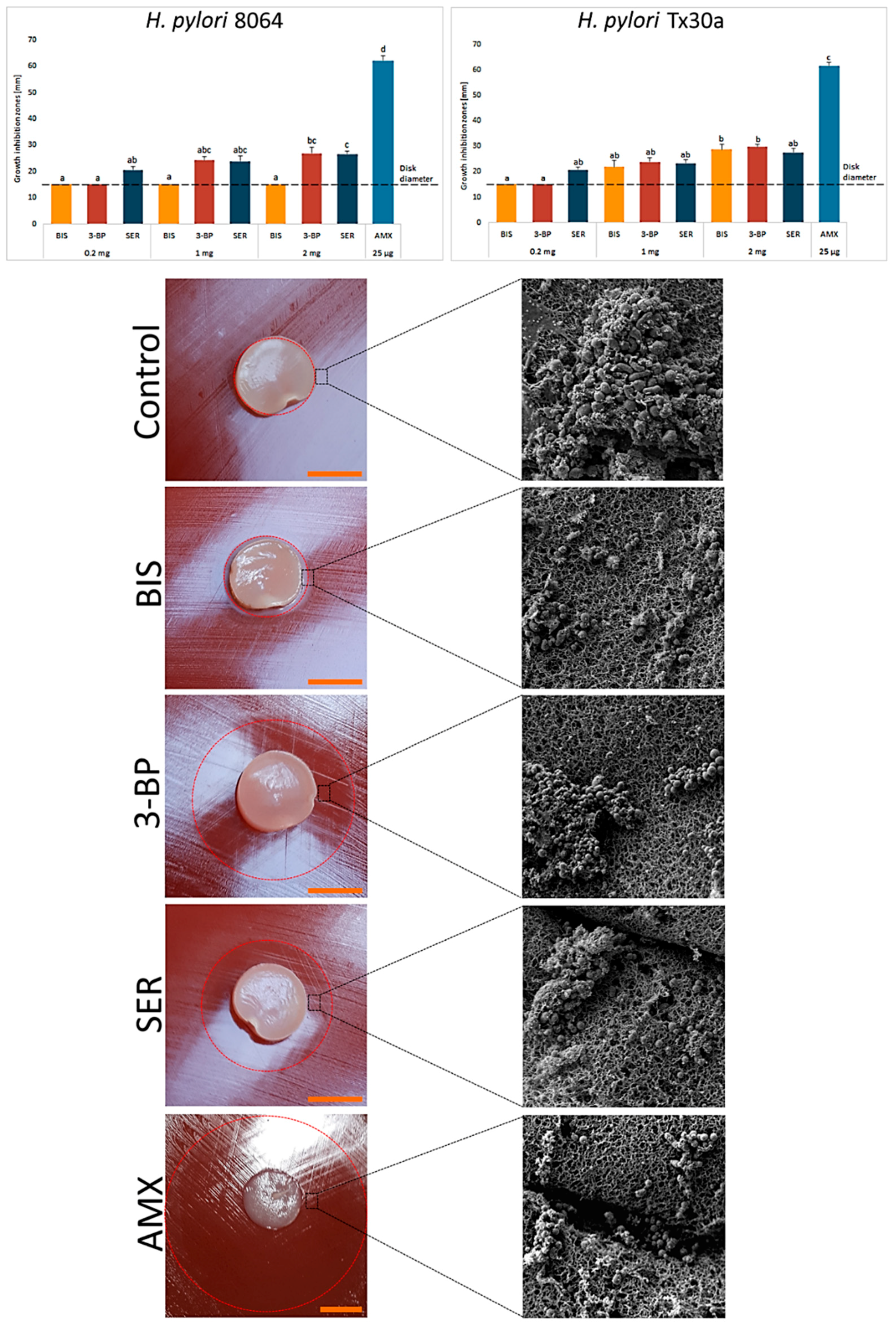
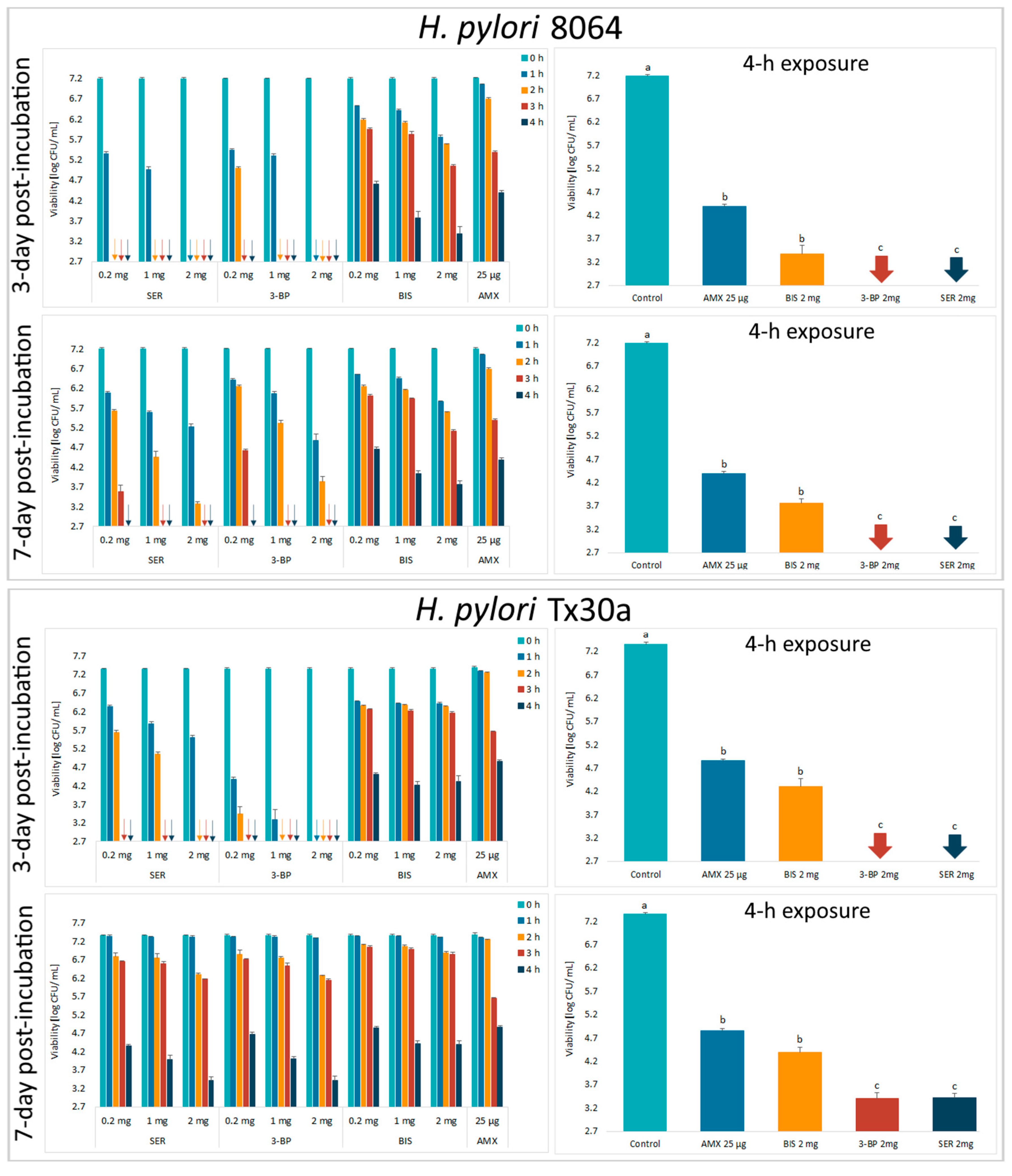
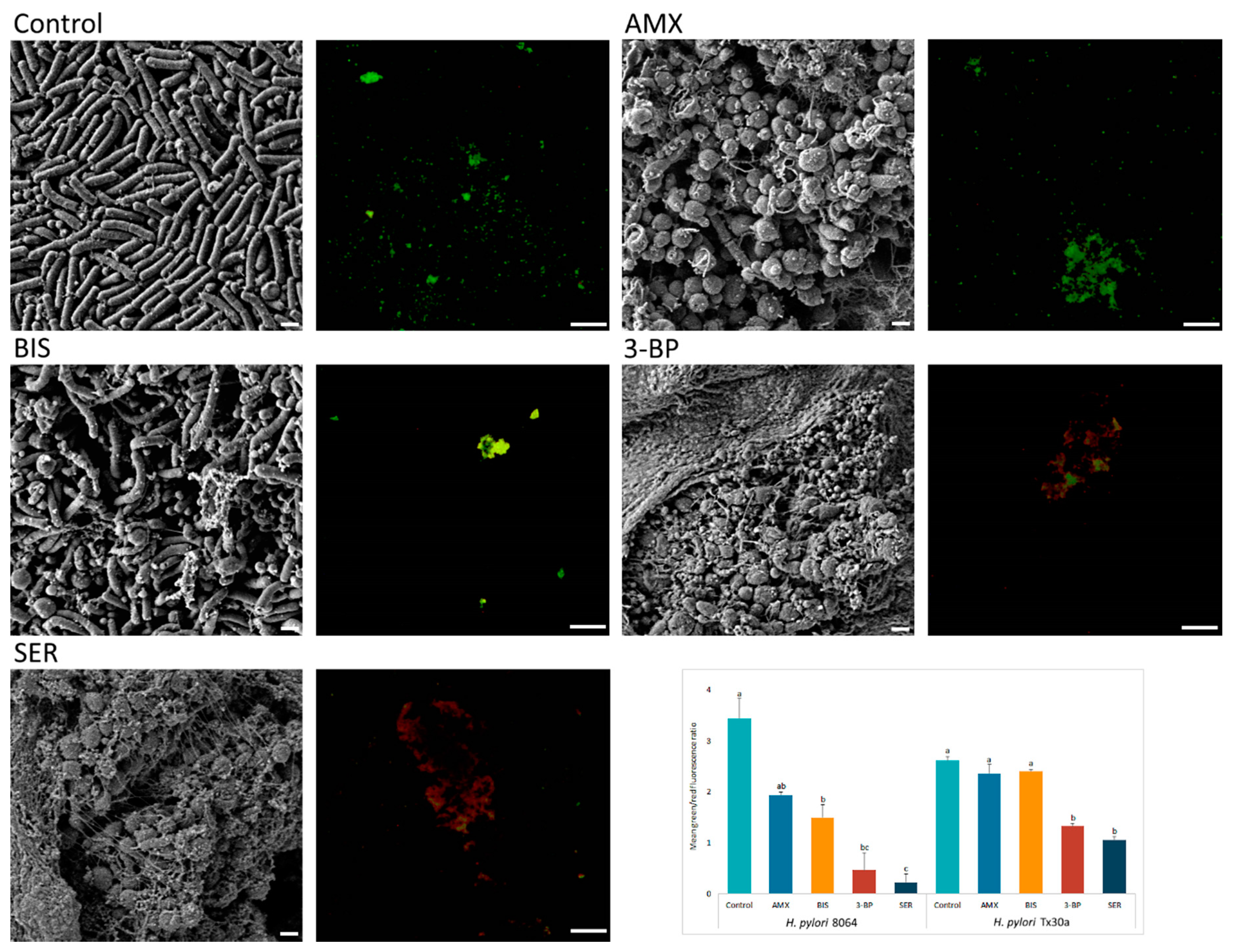
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Y.; Wang, Q.-L.; Cheng, D.-D.; Xu, W.-T.; Lu, N.-H. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front. Cell. Infect. Microbiol. 2016, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.S.; Ottemann, K.M. Colonization, Localization, and Inflammation: The Roles of H. pylori Chemotaxis In Vivo. Curr. Opin. Microbiol. 2018, 41, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and Its Pathogenicity. Toxins (Basel) 2019, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori Infection—The Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Boyanova, L.; Hadzhiyski, P.; Kandilarov, N.; Markovska, R.; Mitov, I. Multidrug Resistance in Helicobacter pylori: Current State and Future Directions. Expert Rev. Clin. Pharmacol. 2019, 12, 909–915. [Google Scholar] [CrossRef]
- Debraekeleer, A.; Remaut, H. Future Perspective for Potential Helicobacter pylori Eradication Therapies. Future Microbiol. 2018, 13, 671–687. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Fallone, C.A.; Moss, S.F.; Malfertheiner, P. Reconciliation of Recent Helicobacter pylori Treatment Guidelines in a Time of Increasing Resistance to Antibiotics. Gastroenterology 2019, 157, 44–53. [Google Scholar] [CrossRef]
- Tsay, F.W.; Wu, D.C.; Yu, H.C.; Kao, S.S.; Lin, K.H.; Cheng, J.S.; Wang, H.M.; Chen, W.C.; Sun, W.C.; Tsai, K.W.; et al. A Randomized Controlled Trial Shows that both 14-Day Hybrid and Bismuth Quadruple Therapies Cure Most Patients with Helicobacter pylori Infection in Populations with Moderate Antibiotic Resistance. Antimicrob. Agents Chemother. 2017, 61, e00140-17. [Google Scholar] [CrossRef]
- Liou, J.M.; Fang, Y.J.; Chen, C.C.; Bair, M.J.; Chang, C.Y.; Lee, Y.C.; Chen, M.J.; Chen, C.C.; Tseng, C.H.; Hsu, Y.C.; et al. Concomitant, Bismuth Quadruple, and 14-day Triple Therapy in the First-Line Treatment of Helicobacter pylori: A Multicentre, Open-Label, Randomised Trial. Lancet 2016, 388, 2355–2365. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.X.; Han, S.X.; Gao, C.P. High Dose Dual Therapy versus Bismuth Quadruple Therapy for Helicobacter pylori Eradication Treatment: A Systematic Review and Meta-Analysis. Medicine 2019, 98, e14396. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-I.; Pan, C.-Y.; Kao, J.Y.; Tsay, F.-W.; Peng, N.-J.; Kao, S.-S.; Wang, H.-M.; Tsai, T.-J.; Wu, D.-C.; Chen, C.-L.; et al. Helicobacter pylori Eradication with Bismuth Quadruple Therapy Leads to Dysbiosis of Gut Microbiota with an Increased Relative Abundance of Proteobacteria and Decreased Relative Abundances of Bacteroidetes and Actinobacteria. Helicobacter 2018, 23, e12498. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.W.C.; Gan, H.M.; Lee, Y.P.; Leow, A.H.R.; Azmi, A.N.; Francois, F.; Perez-Perez, G.I.; Loke, M.F.; Goh, K.L.; Vadivelu, J. Helicobacter pylori Eradication Causes Perturbation of the Human Gut Microbiome in Young Adults. PLoS ONE 2016, 11, e0151893. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, W.; Lee, A.; He, J.; Huang, B.; Zheng, W.; Su, T.; Lai, S.; Long, Y.; Chu, H.; et al. The Impact of Helicobacter pylori Infection, Eradication Therapy and Probiotic Supplementation on Gut Microenvironment Homeostasis: An Open-Label, Randomized Clinical Trial. EBioMedicine 2018, 35, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Olekhnovich, E.I.; Manolov, A.I.; Samoilov, A.E.; Prianichnikov, N.A.; Malakhova, M.V.; Tyakht, A.V.; Pavlenko, A.V.; Babenko, V.V.; Larin, A.K.; Kovarsky, B.A.; et al. Shifts in the Human Gut Microbiota Structure Caused by Quadruple Helicobacter pylori Eradication Therapy. Front. Microbiol. 2019, 10, 1902. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Lee, S.Y. How to Effectively Use Bismuth Quadruple Therapy: The Good, the Bad, and the Ugly. Gastroenterol. Clin. N. Am. 2015, 44, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Yuan, M.; Li, H.; Song, X.; Lu, J.; Jing, T. In Vitro and In Vivo Effects of 3-Bromopyruvate against Echinococcus metacestodes. Vet. Res. 2019, 50, 96. [Google Scholar] [CrossRef]
- Milanes, J.E.; Suryadi, J.; Abendroth, J.; Van Voorhis, W.C.; Barrett, K.F.; Dranow, D.M.; Phan, I.Q.; Patrick, S.L.; Rozema, S.D.; Khalifa, M.M.; et al. Enzymatic and Structural Characterization of the Naegleria fowleri Glucokinase. Antimicrob. Agents Chemother. 2019, 63, e02410-18. [Google Scholar] [CrossRef]
- Kumar, A.; Boradia, V.M.; Thakare, R.; Singh, A.K.; Gani, Z.; Das, S.; Patidar, A.; Dasgupta, A.; Chopra, S.; Raje, M.; et al. Repurposing Ethyl Bromopyruvate as a Broad-Spectrum Antibacterial. J. Antimicrob. Chemother. 2019, 74, 912–920. [Google Scholar] [CrossRef]
- Ferreira, D.D.; Mesquita, J.T.; da Costa Silva, T.A.; Romanelli, M.M.; da Gama Jaen Batista, D.; da Silva, C.F.; da Gama, A.N.S.; Neves, B.J.; Melo-Filho, C.C.; de Soeiro, M.C.; et al. Efficacy of Sertraline Against Trypanosoma cruzi: An In Vitro and In Silico Study. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 30. [Google Scholar] [CrossRef]
- Lima, M.L.; Abengózar, M.A.; Nácher-Vázquez, M.; Martínez-Alcázar, M.P.; Barbas, C.; Tempone, A.G.; López-Gonzálvez, Á.; Rivas, L. Molecular Basis of the Leishmanicidal Activity of the Antidepressant Sertraline as a Drug Repurposing Candidate. Antimicrob. Agents Chemother. 2018, 62, e01928-18. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Franiczek, R.; Krzyżanowska, B.; Łaczmański, Ł.; Migdał, P.; Gościniak, G. In Vitro Activity of 3-Bromopyruvate, an Anti-Cancer Compound, Against Antibiotic-Susceptible and Antibiotic-Resistant Helicobacter pylori Strains. Cancers (Basel) 2019, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Krzyżek, P.; Franiczek, R.; Krzyżanowska, B.; Łaczmański, Ł.; Migdał, P.; Gościniak, G. In Vitro Activity of Sertraline, an Antidepressant, Against Antibiotic-Susceptible and Antibiotic-Resistant Helicobacter pylori Strains. Pathogens 2019, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Vali, M.; Liapi, E.; Kowalski, J.; Hong, K.; Khwaja, A.; Torbenson, M.S.; Georgiades, C.; Geschwind, J.-F.H. Intraarterial Therapy with a New Potent Inhibitor of Tumor Metabolism (3-Bromopyruvate): Identification of Therapeutic Dose and Method of Injection in an Animal Model of Liver Cancer. J. Vasc. Interv. Radiol. 2007, 18, 95–101. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L.; Raggi, M.A. Evaluation of the Pharmacokinetics, Safety and Clinical Efficacy of Sertraline Used to Treat Social Anxiety. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1495–1505. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Della Pepa, C.; Dosio, F.; Serpe, L. Recent Developments in Antibacterial Therapy: Focus on Stimuli-Responsive Drug-Delivery Systems and Therapeutic Nanoparticles. Molecules 2019, 24, 1991. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 11. [Google Scholar] [CrossRef]
- Van Giau, V.; An, S.S.A.; Hulme, J. Recent Advances in the Treatment of Pathogenic Infections using Antibiotics and Nano-Drug Delivery Vehicles. Drug Des. Devel. Ther. 2019, 13, 327–343. [Google Scholar] [CrossRef]
- Fijałkowski, K.; Żywicka, A.; Drozd, R.; Junka, A.F.; Peitler, D.; Kordas, M.; Konopacki, M.; Szymczyk, P.; El Fray, M.; Rakoczy, R. Increased Yield and Selected Properties of Bacterial Cellulose Exposed to Different Modes of a Rotating Magnetic Field. Eng. Life Sci. 2016, 16, 483–493. [Google Scholar] [CrossRef]
- Champ, M. Carbohydrate Digestion in Monogastric Animals. Reprod. Nutr. Dev. 1985, 25, 819–842. [Google Scholar] [CrossRef]
- Żywicka, A.; Fijałkowski, K.; Junka, A.F.; Grzesiak, J.; El Fray, M. Modification of Bacterial Cellulose with Quaternary Ammonium Compounds Based on Fatty Acids and Amino Acids and the Effect on Antimicrobial Activity. Biomacromolecules 2018, 19, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Junka, A.; Bartoszewicz, M.; Dziadas, M.; Szymczyk, P.; Dydak, K.; Żywicka, A.; Owczarek, A.; Bil-Lula, I.; Czajkowska, J.; Fijałkowski, K. Application of Bacterial Cellulose Experimental Dressings Saturated with Gentamycin for Management of Bone Biofilm In Vitro and Ex Vivo. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2020, 108, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Junka, A.; Żywicka, A.; Chodaczek, G.; Dziadas, M.; Czajkowska, J.; Duda-Madej, A.; Bartoszewicz, M.; Mikołajewicz, K.; Krasowski, G.; Szymczyk, P.; et al. Potential of Biocellulose Carrier Impregnated with Essential Oils to Fight Against Biofilms Formed on Hydroxyapatite. Sci. Rep. 2019, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Dydak, K.; Junka, A.; Szymczyk, P.; Chodaczek, G.; Toporkiewicz, M.; Fijałkowski, K.; Dudek, B.; Bartoszewicz, M. Development and Biological Evaluation of Ti6Al7Nb Scaffold Implants Coated with Gentamycin-Saturated Bacterial Cellulose Biomaterial. PLoS ONE 2018, 13, e0205205. [Google Scholar] [CrossRef] [PubMed]
- Krasowski, G.; Wicher-Dudek, R.; Paleczny, J.; Bil-Lula, I.; Fijałkowski, K.; Sedghizadeh, P.P.; Szymczyk, P.; Dudek, B.; Bartoszewicz, M.; Junka, A. Potential of Novel Bacterial Cellulose Dressings Chemisorbed with Antiseptics for the Treatment of Oral Biofilm Infections. Appl. Sci. 2019, 9, 5321. [Google Scholar] [CrossRef]
- EUCAST The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 9.0; 2019. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 11 November 2019).
- Junka, A.F.; Żywicka, A.; Szymczyk, P.; Dziadas, M.; Bartoszewicz, M.; Fijałkowski, K. Test (Antibiofilm Dressing’s Activity Measurement)—Simple Method for Evaluating Anti-Biofilm Activity of Drug-Saturated Dressings against Wound Pathogens. J. Microbiol. Methods 2017, 143, 6–12. [Google Scholar] [CrossRef]
- Lochab, V.; Jones, T.H.; Dusane, D.H.; Peters, C.W.; Stoodley, P.; Wozniak, D.J.; Subramaniam, V.V.; Prakash, S. Ultrastructure Imaging of Pseudomonas aeruginosa Lawn Biofilms and Eradication of the Tobramycin-resistant Variants under In Vitro Electroceutical Treatment. Sci. Rep. 2020, 10, 9879. [Google Scholar] [CrossRef]
- Dusane, D.H.; Brooks, J.R.; Sindeldecker, D.; Peters, C.W.; Li, A.; Farrar, N.R.; Diamond, S.M.; Knecht, C.S.; Plaut, R.D.; Delury, C.; et al. Complete Killing of Agar Lawn Biofilms by Systematic Spacing of Antibiotic-Loaded Calcium Sulfate Beads. Materials (Basel) 2019, 12, 4052. [Google Scholar] [CrossRef]
- Cooke, A.C.; Nello, A.V.; Ernst, R.K.; Schertzer, J.W. Analysis of Pseudomonas aeruginosa Biofilm Membrane Vesicles Supports Multiple Mechanisms of Biogenesis. PLoS ONE 2019, 14, e0212275. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, Y.; Xiao, Q.; Zheng, W.; Long, G.; Chen, B.; Shu, X.; Jiang, M. Mutations in the Antibiotic Target Genes Related to Clarithromycin, Metronidazole and Levofloxacin Resistance in Helicobacter pylori Strains from Children in China. Infect. Drug Resist. 2020, 13, 311–322. [Google Scholar] [CrossRef]
- Lauener, F.N.; Imkamp, F.; Lehours, P.; Buissonnière, A.; Benejat, L.; Zbinden, R.; Keller, P.M.; Wagner, K. Genetic Determinants and Prediction of Antibiotic Resistance Phenotypes in Helicobacter pylori. J. Clin. Med. 2019, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Bloom, H.K. Global Brain: The Evolution of Mass Mind from the Big Bang to the 21st Century; Wiley: Hoboken, NJ, USA, 2000; ISBN 9780471295846. [Google Scholar]
- Drozd, R.; Rakoczy, R.; Wasak, A.; Junka, A.; Fijałkowski, K. The Application of Magnetically Modified Bacterial Cellulose for Immobilization of Laccase. Int. J. Biol. Macromol. 2018, 108, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.F.; Pereira, T.; Silva, N.H.C.S.; Gomes, F.P.; Silvestre, A.J.D.; Freire, C.S.R.; Sousa Lobo, J.M.; Costa, P.C. Bacterial Cellulose Membranes as Drug Delivery Systems: An In Vivo Skin Compatibility Study. Eur. J. Pharm. Biopharm. 2014, 86, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Justo, J.A.; Bookstaver, P.B. Antibiotic Lock Therapy: Review of Technique and Logistical Challenges. Infect. Drug Resist. 2014, 7, 343–363. [Google Scholar] [PubMed]
- Singh, R.; Kumari, A.; Kaur, K.; Sethi, P.; Chakrabarti, A. Relevance of Antifungal Penetration in Biofilm-Associated Resistance of Candida albicans and non-albicans Candida Species. J. Med. Microbiol. 2018, 67, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, J.; Tan, N.J.H.J.; But, K.P.; Cohen, Y.; Rice, S.A.; Wohland, T. Single Microcolony Diffusion Analysis in Pseudomonas aeruginosa Biofilms. npj Biofilms Microbiomes 2019, 5, 35. [Google Scholar] [CrossRef]
- Hathroubi, S.; Zerebinski, J.; Ottemann, K.M. Helicobacter pylori Biofilm Involves a Multigene Stress-Biased Response, Including a Structural Role for Flagella. MBio 2018, 9, e01973-18. [Google Scholar] [CrossRef]
- Bordeleau, E.; Mazinani, S.A.; Nguyen, D.; Betancourt, F.; Yan, H. Abrasive Treatment of Microtiter Plates Improves the Reproducibility of Bacterial Biofilm Assays. RSC Adv. 2018, 8, 32434–32439. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Tasse, J.; Cara, A.; Saglio, M.; Villet, R.; Laurent, F. A Steam-Based Method to Investigate Biofilm. Sci. Rep. 2018, 8, 13040. [Google Scholar] [CrossRef]
- He, X.; Liao, X.; Li, H.; Xia, W.; Sun, H. Bismuth-Induced Inactivation of Ferric Uptake Regulator from Helicobacter pylori. Inorg. Chem. 2017, 56, 15041–15048. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guan, C.; Wan, H.; Zhang, Z.; Li, H.; Sun, H.; Xia, W. Inactivation of NikR from Helicobacter pylori by a Bismuth Drug. J. Inorg. Biochem. 2019, 196, 110685. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, L.; Xu, F.; Quan, Q.; Lai, Y.T.; Xia, W.; Yang, Y.; Chang, Y.Y.; Yang, X.; Chai, Z.; et al. Integrative Approach for the Analysis of the Proteome-Wide Response to Bismuth Drugs in: Helicobacter pylori. Chem. Sci. 2017, 8, 4626–4633. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, J. Helicobacter pylori Recrudescence and Its Influencing Factors. J. Cell. Mol. Med. 2019, 23, 7919–7925. [Google Scholar] [CrossRef]
- Krzyżek, P.; Grande, R. Transformation of Helicobacter pylori into Coccoid Forms as a Challenge for Research Determining Activity of Antimicrobial Substances. Pathogens 2020, 9, 184. [Google Scholar] [CrossRef]
- Hathroubi, S.; Zerebinski, J.; Clarke, A.; Ottemann, K.M. Helicobacter pylori Biofilm Confers Antibiotic Tolerance in Part via a Protein-Dependent Mechanism. Antibiotics 2020, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, P.; Shen, Y.; Zou, Y.; Yuan, G.; Hu, H. Rhamnolipid-involved Antibiotics Combinations Improve the Eradication of Helicobacter pylori Biofilm In Vitro: A Comparison with Conventional Triple Therapy. Microb. Pathog. 2019, 131, 112–119. [Google Scholar] [CrossRef]
- Turner, L.; Bitto, N.J.; Steer, D.L.; Lo, C.; D’Costa, K.; Ramm, G.; Shambrook, M.; Hill, A.F.; Ferrero, R.L.; Kaparakis-Liaskos, M. Helicobacter pylori Outer Membrane Vesicle Size Determines their Mechanisms of Host Cell Entry and Protein Content. Front. Immunol. 2018, 9, 1466. [Google Scholar] [CrossRef] [PubMed]
- Zavan, L.; Bitto, N.J.; Johnston, E.L.; Greening, D.W.; Kaparakis-Liaskos, M. Helicobacter pylori Growth Stage Determines the Size, Protein Composition, and Preferential Cargo Packaging of Outer Membrane Vesicles. Proteomics 2019, 19, e1800209. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Di Giulio, M.; Bessa, L.J.; Di Campli, E.; Baffoni, M.; Guarnieri, S.; Cellini, L. Extracellular DNA in Helicobacter pylori Biofilm: A Backstairs Rumour. J. Appl. Microbiol. 2011, 110, 490–498. [Google Scholar] [CrossRef]
- Grande, R.; Di Marcantonio, M.C.; Robuffo, I.; Pompilio, A.; Celia, C.; Di Marzio, L.; Paolino, D.; Codagnone, M.; Muraro, R.; Stoodley, P.; et al. Helicobacter pylori ATCC 43629/NCTC 11639 Outer Membrane Vesicles (OMVs) from Biofilm and Planktonic Phase Associated with Extracellular DNA (eDNA). Front. Microbiol. 2015, 6, 1369. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA Chelates Cations and Induces Antibiotic Resistance in Pseudomonas aeruginosa Biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Lewenza, S.; Johnson, L.; Charron-Mazenod, L.; Hong, M.; Mulcahy-O’Grady, H. Extracellular DNA Controls Expression of Pseudomonas aeruginosa Genes Involved in Nutrient Utilization, Metal Homeostasis, Acid pH Tolerance and Virulence. J. Med. Microbiol. 2020, 69, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, K.; Markow, Z.E.; Fishell, A.K.; Sherafati, A.; Burns-Yocum, T.M.; Schroeder, M.L.; Svoboda, A.M.; Eggebrecht, A.T.; Anastasio, M.A.; Schlaggar, B.L.; et al. Decoding Visual Information from High-Density Diffuse Optical Tomography Neuroimaging Data. Neuroimage 2020, 226, 117516. [Google Scholar] [CrossRef] [PubMed]
| H. pylori Strains | SER | 3-BP | AMX | BIS | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Tx30a | 4 | 8 | 128 | 128 | 0.12 | 0.48 | 2 | 2 |
| 8064 | 2 | 2 | 128 | 128 | 0.06 | 0.12 | 2 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzyżek, P.; Gościniak, G.; Fijałkowski, K.; Migdał, P.; Dziadas, M.; Owczarek, A.; Czajkowska, J.; Aniołek, O.; Junka, A. Potential of Bacterial Cellulose Chemisorbed with Anti-Metabolites, 3-Bromopyruvate or Sertraline, to Fight against Helicobacter pylori Lawn Biofilm. Int. J. Mol. Sci. 2020, 21, 9507. https://doi.org/10.3390/ijms21249507
Krzyżek P, Gościniak G, Fijałkowski K, Migdał P, Dziadas M, Owczarek A, Czajkowska J, Aniołek O, Junka A. Potential of Bacterial Cellulose Chemisorbed with Anti-Metabolites, 3-Bromopyruvate or Sertraline, to Fight against Helicobacter pylori Lawn Biofilm. International Journal of Molecular Sciences. 2020; 21(24):9507. https://doi.org/10.3390/ijms21249507
Chicago/Turabian StyleKrzyżek, Paweł, Grażyna Gościniak, Karol Fijałkowski, Paweł Migdał, Mariusz Dziadas, Artur Owczarek, Joanna Czajkowska, Olga Aniołek, and Adam Junka. 2020. "Potential of Bacterial Cellulose Chemisorbed with Anti-Metabolites, 3-Bromopyruvate or Sertraline, to Fight against Helicobacter pylori Lawn Biofilm" International Journal of Molecular Sciences 21, no. 24: 9507. https://doi.org/10.3390/ijms21249507
APA StyleKrzyżek, P., Gościniak, G., Fijałkowski, K., Migdał, P., Dziadas, M., Owczarek, A., Czajkowska, J., Aniołek, O., & Junka, A. (2020). Potential of Bacterial Cellulose Chemisorbed with Anti-Metabolites, 3-Bromopyruvate or Sertraline, to Fight against Helicobacter pylori Lawn Biofilm. International Journal of Molecular Sciences, 21(24), 9507. https://doi.org/10.3390/ijms21249507








