Abstract
Hypoxia, a common factor ruling the microenvironment composition, leads to tumor progression. In this hypoxic context, cytokines and cells cooperate to favor cancer development and metastasis. Tumor hypoxia is heterogeneously distributed. Oxygen gradients depend on the vicinity, functionality of blood vessels, and oxygen ability to diffuse into surrounding tissues. Thus, the vasculature state modulates the microenvironment of the tumor cells. Cells sense and react to small variations in oxygen tension, which explains the lack of tumor cells’ unicity in their reaction to drugs. Ovarian cancers are highly hypoxia-dependent, ascites worsening the access to oxygen, in their reactions to both chemotherapy and new immunotherapy. Consequently, hypoxia affects the results of immunotherapy, and is thus, crucial for the design of treatments. Controlling key immunosuppressive factors and receptors, as well as immune checkpoint molecule expression on tumor, immune and stromal cells, hypoxia induces immunosuppression. Consequently, new approaches to alleviate hypoxia in the tumor microenvironment bring promises for ovarian cancer immunotherapeutic strategies. This review focuses on the effects of hypoxia in the microenvironment and its consequences on tumor treatments. This opens the way to innovative combined treatments to the advantage of immunotherapy outcome in ovarian cancers.
1. Ovarian Cancer to Date
Ovarian cancer (OC) is the most aggressive gynecological malignancy. It accounts for ci. 240,000 female cancer cases and is responsible for ci. 150,000 cancer-related deaths. More than three-fourths of patients suffer from disease recurrence within 24 months after initial treatment [1].
One of the main problems for OC treatment is its late diagnosis—ci. three-fourths of cancer cases are diagnosed at stage III/IV with disseminated disease. The early discovery of OC is rendered difficult due to the lack of specific symptoms and of effective diagnostic tools. Unfortunately, measurement of the serum CA-125 marker concentration, transvaginal ultrasound examination, or both have not provided clinically relevant evidence to detect OC in early stages, and the median age of diagnosis of OC is 63 years [2]. Abdominal pain and distension for 3–4 months are frequently attributed in women to irritable bowel syndrome and not a tumor mass present in the pelvis [3,4].
Based on physical examination and imaging, OC can be suspected, and an exploratory laparotomy is typically performed to obtain histopathological confirmation of the diagnosis, staging, and tumor debulking. To date, treatments are mainly based on aggressive cytoreductive surgery in combination with platinum-based and taxane-based chemotherapy. Other drugs (pegylated liposomal doxorubicin, topotecan, and gemcitabine) are used as the second-, third- and subsequent lines of treatment of recurrent OC [5]. Cytoreductive surgery usually offers successful removal at the level of more than 75% of the initial tumor mass, with residual tumor not larger than 2 cm further reduced to 1 cm. Resection methods, as well as the mode and duration of chemotherapy administration, have considerably evolved since the 1990s [4,6,7].
Lack of residual disease (R0) after primary debulking surgery (PDS) is considered the most important prognostic factor [8]. In advanced OC, two randomized phase III studies compared the effectiveness of first-line neoadjuvant chemotherapy (NACT) followed by surgery, to the standard therapeutic approach (i.e., PDS) followed by adjuvant chemotherapy. Both methods showed similar overall survival (OS) and progression-free survival (PFS). Significantly lower operational mortality was observed in the NACT first-line group [9,10]. However, both studies were criticized for low survival and R0 rates, and the choice between both modalities remains controversial [11,12].
The standard first-line chemotherapy regimen for OC is intravenous carboplatin (area under the curve 5–6) and paclitaxel (175 mg/m2) every three weeks [13]. Modifications of both carboplatin and paclitaxel administration advise a weekly dose-dense as an alternative [14,15,16].
Search for new drugs has resulted in a significant prolongation of the median PFS. This was mainly obtained when immunotherapy was introduced as anti-angiogenic monoclonal antibodies. Bevacizumab, an anti-vascular endothelial growth factor (VEGF) antibody, has been largely tested for its potential as an adjuvant strategy. It was finally observed that, despite the wide action of the monoclonal antibodies, the immunotherapy approach did not produce a noticeable improvement in OS. Indeed, bevacizumab did not improve OS when combined with standard paclitaxel/carboplatin chemotherapy [17,18]. The benefit of adding bevacizumab was observed to the greatest extent in FIGO III patients with residual disease larger than 1 cm after PDS or in FIGO IV patients [18]. These findings led to the reimbursement of bevacizumab with standard paclitaxel and carboplatin chemotherapy in high-risk patients in many countries.
Another issue is the administration of cisplatin and paclitaxel by intraperitoneal injection. Some benefit of this form of therapy was observed with a significant prolongation of median OS in patients with residual disease below 1 cm after PDS [19]. Despite controversial results, chemotherapy regimen has become the standard. Recently, after the GOG252 study questioning the effectiveness of intraperitoneal vs. intravenous chemotherapy combined with immunotherapy (bevacizumab), the enthusiasm for this treatment formula has significantly diminished [20].
Hyperthermic intraperitoneal chemotherapy (HIPEC) is one of the OC treatment optimizations. A Dutch randomized study using HIPEC during interval debulking surgery (IDS) after NACT showed significant prolongation of PFS and OS in the HIPEC-treated arm as compared to the IDS control [21]. However, those results were not confirmed in the Korean trial [22].
Recurrence of OC is incurable in about three-fourths of women with advanced-stage disease. For relapses susceptible to standard platinum, re-challenge with platinum doubled chemotherapy is recommended. Then, maintenance strategies have been developed to delay disease progression. Phase III studies maintaining bevacizumab have shown significant benefit in the disease control indexes in platinum-sensitive and platinum-refractory OC [23,24].
Non-platinum monotherapy is usually used to treat patients with platinum-resistant recurrent OC. This includes pegylated liposomes to deliver and release doxorubicin and weekly administration of topotecan, paclitaxel, gemcitabine, or docetaxel. However, this type of treatment is not very effective, as the overall response rate ranges from 10% to 35% with a relatively short response (less than eight months) [25].
Treatment with PARP inhibitors has been implemented as maintenance treatment, as well as in recurrent OC, exploiting the inherent disorders DNA repair in approximately 50% of OCs via BRCA1, BRCA2 mutations, or homologous recombination associated genes, as well as inactivation via methylation [6,26].
The mechanisms of chemoresistance are only partially elucidated. One of the most important factors affecting the resistance of cancer cells in the tumor microenvironment (TME), with its key modulator-oxygen level. This parameter was neither investigated nor taken into account when the drugs were approved for clinical applications.
Considering the new strategies that have been approached and that are leading the search for combined therapies, it appears that hypoxia alleviation is one of the main challenges.
As OCs typically display an acute hypoxic state, the need for hypoxia compensation appears in all treatment attempts, indicating that the combined treatments (although more promising) might be senseless if the TME is not considered as the first-line strategy, not allowing the second-line combinatorial attempts to gain any efficacy.
2. Hypoxia as a Key Microenvironment Modulator in Ovarian Cancer
Hypoxia, oxygen partial pressure lower than its physiological value, appears within the growing tumor and is one of the most important factors shaping the TME. Several studies show that it influences cellular processes, as angiogenesis and epithelial-to-mesenchymal transition (EMT), and cell characteristics (such as the acquisition of stem-like features) with deep consequences on the activity and effectiveness of anti-cancer drugs [27]. In cancer, as in other hypoxia-dependent diseases, low oxygen tension favors tumor growth and the development of immunosuppression. Hypoxia switches the tumor from benign to aggressive, and concomitantly, turns on the angiogenesis and its pathologic characteristics. The latter is a major factor in tumor development. Tumor angiogenesis is a condition that favors the growth and dissemination of the tumor cells for its lack of effectiveness in reestablishing the physiologic pO2, which is also responsible for immunosuppression and tolerization of anti-tumor cytotoxic immunocompetent cells [28].
Low oxygen partial pressure activates hypoxia-dependent signaling pathways mainly via stabilization of hypoxia-inducible factor-1α (HIF-1α), the presence and mechanism of which was first described by Gregg L. Semenza [29]. Under normoxia, two prolyl residues of HIF-1α are hydroxylated by prolyl hydroxylase 2 (PHD2), which was found by P. Kaelin and W. G. Ratcliffe to act as an oxygen sensor [30]. Hydroxylated HIF-1α can bind to Von Hippel Lindau protein (pVHL), as P. Radcliffe described [31], and such a complex is ubiquinylated before proteasomal degradation [32]. In hypoxic conditions, enzymes modifying HIF-1α are inactive, and unmodified HIF-1α translocates to the nucleus, where it binds to HIF-1β forming HIF-1 heterodimer. HIF-1complex functions as a transcription factor and locates to its downstream targets in the genome: The hypoxia-response elements (HREs) [29,31,33]. The other HIF mediating the hypoxic response is HIF-2 [34], which shares structural similarity with HIF-1α, but it regulates different downstream targets [35].
A consequence of intratumor hypoxia is a switch leading to the development of chaotic pathological vessels, which are inefficient in compensating hypoxia. This maintains the pro-angiogenic hypoxic state, which influences the physiology of the tumor cells and overall stroma. Typically, cancer cells in a hypoxic microenvironment may acquire a mesenchymal phenotype (via EMT), leading to increased cell mobility and the ability to metastasize [36]. It also significantly affects cell metabolism contributing to their chemoresistance [37]. Hypoxia causes acidosis via the production of high amounts of lactic acid in the cancer microenvironment [38].
Active migration of cancer cells into (intravasation) and out of (extravasation) the blood or the lymphatic vessels is also regulated by HIF-1 [39]. Its action in cancer cells affects cancer-endothelial and endothelial cell interactions [40,41,42]. Hypoxia-induced angiogenesis is essential for those processes as it produces pathological, “leaky” vessels. Vascular permeability is controlled by HIF-1-dependent genes, such as vascular endothelial growth factors (VEGFs), metalloproteinase 2 (MMP2), angiopoietin 2, and urokinase receptor (UPAR), that act on the disruption of vascular wall integrity and facilitate cancer cell migration [43]. HIF-1 signaling strongly modulates endothelium properties by modifying the characteristics of cell adhesion, coagulation, and endothelial permeability, as well as growth [43].
Prognostic and Predictive Value of HIF-1α
The exact prognostic and predictive values of HIF-1α expression remain to be fully understood, as clinical data are often inconsistent. High HIF-1α expressing tumors presented higher response rates to postoperative paclitaxel/carboplatin combined chemotherapy. Patients with such tumors after suboptimal resection (of stage III/IV tumors) and indicated for postoperative combined chemotherapy, showed significantly better survival [44]. HIF-1α was also strongly expressed in tumors of patients with longer PFS [45]. On the other hand, strong HIF-1α expression was a significant indicator of shorter OS and shorter median progression-free interval (PFI). Moreover, the overall PFI of patients with (1) tumors displaying strong HIF-1α expression and (2) suboptimal cytoreduction at primary surgery, was significantly worse [46]. A meta-analysis of 31 OC showed that the level of HIF-1α expression correlated with worse OS, worse disease-free survival (DFS), progression-free survival (PFS), cancer-specific survival (CSS), relapse-free survival (RFS), and worse metastasis-free survival (MFS). Such associations were found neither for HIF-2 expression nor other investigated parameters [47].
3. Chemotherapeutic Treatment of Ovarian Cancer in Hypoxia
3.1. Platinum-Based Chemotherapeutics
Low expression of HIF-1α protein in OC patients correlated with response to cisplatin treatment [48]. Treatment of some OCs with cisplatin resulted in elevated HIF-1α expression in cells in vitro [49]. Interestingly, cisplatin decreased the level of HIF-1α in cisplatin-sensitive OC but not in cisplatin-resistant ones. Both types of cells (resistant and sensitive ones) showed enhanced cisplatin sensitivity after HIF-1α knockdown or pharmacological promotion of HIF-1α degradation [50]. Cisplatin strongly reduced the protein levels of the HIF-1 co-activators p300 and CREB-binding protein (CBP) under hypoxia [51]. Hypoxia during treatment was the most important factor determining chemoresistance, as opposed to hypoxia exposure prior to treatment [52]. This effect occurred irrespectively of TP53 status [53]. The exact mechanisms as to how hypoxia contributes to chemoresistance is still a matter of intensive research. A hypoxic microenvironment was shown to affect the content of exosomes in a way that hypoxic exosomes (Hex) contain more oncogenic proteins than normoxic ones (Nex). When cultured in the presence of Hex, cancer cells displayed both higher survival and higher metastatic potential after cisplatin treatment [54]. Other processes involved in resistance to cisplatin might be HIF-1α-mediated decrease cisplatin-induced autophagy [55]. Knockdown of HIF-1α in SKOV3 and A2780 OC cells promoted autophagy and decreased the PI3K/AKT/mTOR signaling pathway [56]. HIF-1α with the histone deacetylase inhibitor 4 (HDAC4) possibly mediate p53-RAS crosstalk that actively regulates resistance to cisplatin via apoptosis and autophagy [57]. Although not fully elucidated, the mechanism of Emblica officinalis’ extract action, known to inhibit the growth of OC cells, was shown to inhibit HIF-1α and activate autophagy [58]. Moreover, the extract from the natural compound Chansu (bufalin) inhibited mTOR, and consequently, decreased HIF-1α, resulting in a lower rate of cancer cell growth and migration [48].
Taking into account the variability of the cells and pathways that are affected by the chemotherapeutic drugs and immunotherapeutic tools, as exemplified in Table 1, the need for a combined approach of treatments arises. It shows that the microenvironment might be strongly modified by immunomodulators designed to neutralize the immune checkpoints activity, lowering their barrier effect towards anti-cancer cells chemotherapeutics.

Table 1.
Molecular targets of drugs applied in ovarian cancer treatment.
In this line, HIF-1α emerges as an important target to improve cancer cells’ sensitivity to cisplatin, for its activation effect on PI3K/AKT and MAPK/ERK pathways [59]. SB202190, a MAPK inhibitor, helped to sensitize OC cells to glucose analogs (2-deoxy-glucose (2-DG) and D-allose) and further to cisplatin treatment. This effect involved a decrease in HIF-1α accumulation [60]. Blocking the Rho/ROCK pathway also increased the effectiveness of cisplatin treatment via HIF-1α inhibition in OC cells [61].
When CoCl2-induced resistant OC cells were treated with noscapine (a small opioid molecule and inhibitor of HIF-1α) upon cisplatin treatment, the level of apoptosis and proliferation inhibition increased. Noscapine-mediated inhibition of HIF-1α activity occurs by increased proteasomal degradation [62]. Sulforaphane decreases HIF-1α level promoting an anti-angiogenic response and elevates anti-cancer activity (p53, redox effector factor (ARE), interferon regulatory factor 1 (IRF-1), Pax-6, and X-responsive elements (XRE)). Moreover, it targets carbonyl anhydrase 9 (CA IX), an enzyme which is an important HIF-1α-downstream effector as it catalyzes the reversible hydration of carbon dioxide to bicarbonate ions and protons [63,64]. It protects cancer cells from hypoxia-induced pH imbalance, facilitating their migration and invasion [65].
MicroRNAs as noncoding RNAs are important modulators that can specifically target the gene coding for HIF-1α. As such, a low level of miR-199a expression was observed in OC tumors (as compared to normal tissues) and was associated with shorter survival. It downregulates HIF-1α, consequently, its lack affects the resistance level, as shown for cisplatin in OC cells [66].
Several proteins were identified as positive regulators of HIF-1α. SENP-1 upregulated HIF-1α expression by deSUMOylation and decreased hypoxic reaction to cisplatin treatment [67]. HIF-1α was also activated by exposure to recombinant human FSH (rhFSH) [68]. The Rab GTPase (Ras-related protein Rab25) was a positive regulator of HIF-1α in several OC cell lines, conferring increased resistance to platinum derivatives. This upregulation was based on de novo HIF-1α synthesis via ERRB2/ERK1 and p70S6K/mTOR pathways [69].
Targeting the formyl peptide receptor (FPR) and toll-like receptor 9 (TLR9) sensitized OC cells to cisplatin [70]. Indeed, hypoxia upregulated both molecules, and their blockage significantly reduced the cisplatin-induced inhibition.
Mitochondrial metabolism plays a key role in cancer cell adaptation to a hypoxic microenvironment. Hypoxia-induced reactive oxygen species (ROS) trigger mitochondrial fission, resulting in resistance to cisplatin by decreasing levels of p-Drp1 and Mnf1, which are key proteins regulating this process [53].
Resistance to carboplatin was shown to be cysteine-dependent. Some OC cells exhibited a strong dependency on their chemoresistance on cysteine levels in hypoxia [71,72].
Although mechanistically related to the hypoxic/physioxic balance, those treatments were hardly studied in instances that took into account the pO2 values in the microenvironment. Consequences on the degree of vascularization and on vessels functions are direct and contribute to modulate the composition of the microenvironment for its response to treatment.
3.2. Alkylating Agents
Alkylating agents prevent the proper formation of a DNA double helix by adding an alkyl group to guanine residues. Cyclophosphamide, one of the first drugs applied in OC treatment, is now rarely applied in clinics. It potentiates the cytotoxicity of genetically modified tumor-infiltrating macrophages (transduced with CYP2B6 gene under hypoxia-responsive promotor). This gene encodes a prodrug-activating enzyme, human cytochrome P450 2B6 [73]. Melphalan is an alkylating agent rarely applied in OC treatment. It increases the oxidative stress response mediated by VEGF/IL8-signaling, but it was not investigated in hypoxia in the context of OC [74].
3.3. Mitosis Inhibitors
Mitosis inhibitors are mostly compounds of plant origin. They inhibit cancer cell division by binding to tubulin and inhibiting its polymerization into microtubules. An initial study on the influence of paclitaxel and docetaxel on OC cells in hypoxia showed that they affect neither HIF-1α nor VEGF expression [51]. However, other studies show a possible role of HIF-1α in cancer cell resistance to paclitaxel. Huang and co-workers showed that HIF-1α expression induced by hypoxia contributes to chemoresistance via G0/G1 arrest [75]. Long-term exposure to the cytokine Epo, a glycoprotein secreted upon hypoxia that stimulates erythrocytes production, increased the OC cells’ resistance to paclitaxel. In hypoxia only, the treatment of Epo-stimulated OC cells increased the proangiogenic properties of A2780 cells [76], which indicates the involvement of HIF-1 activation. C-Src played an important role in hypoxia-mediated resistance to paclitaxel by decreasing the numbers of cells in G2/M, its inhibition blocked HIF-1α and reversed the resistance [77].
The treatment of OC, both in vivo and in vitro, with paclitaxel and sMEK1 helped overcome resistance to paclitaxel by inhibiting downstream target genes of the S6K/4E-BP pathway (i.e., HIF-1α and VEGF) [78].
Directly linked to the hypoxic status, albumin-bound paclitaxel (nab-paclitaxel) applied with topotecan in a metronomic schedule was able to significantly inhibit tube formation by endothelial cells [79].
Consequently, the mitosis inhibitors used in the treatments of OCs are clearly dependent on the hypoxic environment for their final activity through the modulation of their molecular targets.
3.4. Antibiotics
Antibiotics are compounds derived from microorganisms that affect DNA replication by several cytotoxic mechanisms. Doxorubicin interacting with DNA inhibits the hypoxia-mediated activation of HIF-1, although this drug has no significant effect on the expression levels of HIF-1α. The expression of p300 and CBP were only weakly reduced [51]. The resistance of cancer cells to doxorubicin could be induced by short exposure to rhFSH [68].
3.5. Miscellaneous Antineoplastic Agents
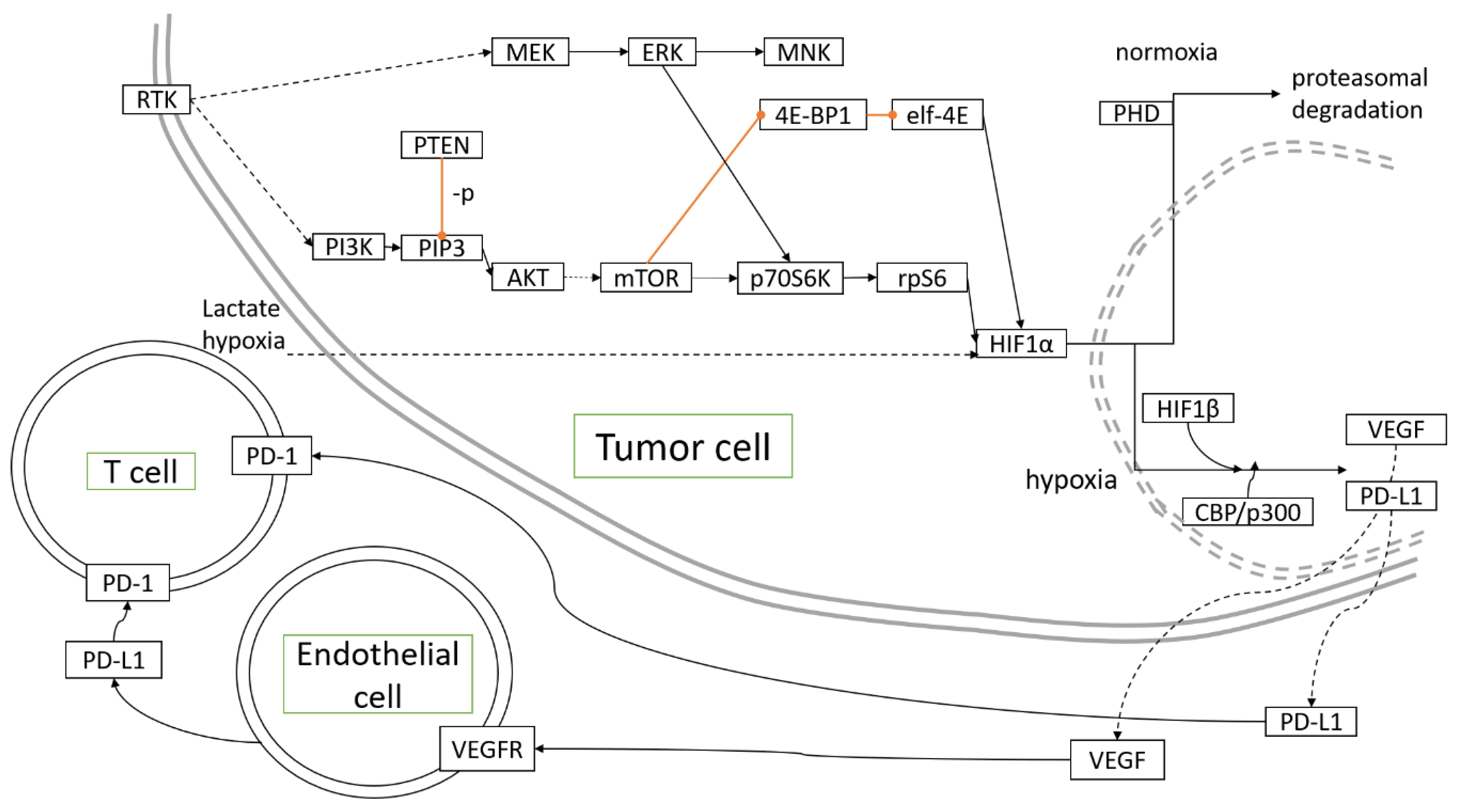
Miscellaneous antineoplastic agents are drugs that decrease cancer cells growth through diverse mechanisms. Topotecan, a topoisomerase I inhibitor, inhibited HIF-1α, and increased hypoxic areas in tumors developing in mouse models of OC [80]. Metronomic application of topotecan appeared to reduce HIF-1α and VEGF expression [81], thus, acting as an angiogenesis-directed treatment as hypothesized [82]. Upon treatment with topotecan, topoisomerase I binding to HIF-1α mRNA was essential to restore p53 transcriptional activity, which participated in reversing hypoxia-mediated resistance to cisplatin and paclitaxel [83]. Figure 1 depicts the molecular pathways associated with hypoxia in OC development.
Figure 1.
Molecular pathways activated and controlled by hypoxia in ovarian cancer development.  inhibition,
inhibition,  direct interaction/activation,
direct interaction/activation,  indirect interaction/activation.
indirect interaction/activation.
 inhibition,
inhibition,  direct interaction/activation,
direct interaction/activation,  indirect interaction/activation.
indirect interaction/activation.
Table 2 points to the remarkable variability of the tumor treatment effects and their dependency upon the microenvironmental oxygen tension, which may explain a large part of the cancer hallmarks molecular mechanisms and their consequences.

Table 2.
Summary of research on ovarian cancer (OC) cells reaction to chemotherapy treatment in hypoxia or related to the hypoxia pathway.
4. Targeted Therapies of Ovarian Cancer in Hypoxia
4.1. Anti-Angiogenic Therapies
Anti-angiogenic therapies aim at interfering with the development of blood vessels in the tumor site [88]. Withdrawal of anti-angiogenic treatment resulted in tumor rebound accompanied by platelet infiltration. Focal adhesion kinase (FAK) in platelets played an important role in their migration into the tumor site. Combined therapy with anti-angiogenic agents and FAK inhibitors did prevent tumor rebound [89].
The strong effect of HIF-1 modulators cannot be dissociated from their effects on endothelial cells in the process of angiogenesis. Tumor angiogenesis-directed treatments in OC are particularly significant with regards to the hypoxic regulation of the entire microenvironment.
4.2. PARP Inhibitors
Poly (ADP-ribose)polymerases (PARPs) are a family of enzymes involved in the synthesis of poly(ADP-ribose) (PAR) chains from NAD+. Three of them (PARP-1, -2, and -3) have defined roles in DNA damage repair. PARP inhibitors (PARPi) can sensitize cells to a variety of DNA damaging agents. This is particularly true in the case of cells incompetent for homologous recombination repair (HRR) (e.g., BRCA1 and BRCA2 mutants). Therefore, a combination with cytotoxic chemotherapy or radiotherapy has been proposed as an approach for the treatment of HRR incompetent tumors. However, PARPis used in combination therapies often lead to normal tissue toxicity [90]. PARPi with cediranib (a small molecule inhibitor of VEGFR-2, platelet-derived growth factor receptor (PDGFR), and c-kit) showed better clinical effects than PARPis alone, regardless of BRCA1/2 status [91]. Cediranib confers sensitivity to PARPis by decreasing homology-directed DNA repair (HDR). This effect was hypoxia-dependent as cediranib induced hypoxia-related suppression HDR elements (BRCA1/2 and RAD51). But hypoxia-independent effects occurred through PDGFR inhibition, protein phosphatase 2A (PP2A), and E2F transcription factor 4 (E2F4)/RB transcriptional corepressor like 2 (RB2/p130) [92].
5. Hypoxia in Tumor Immune Microenvironment and Its Role in Immunotherapy of Ovarian Carcinoma
The majority of early immune therapies focused on potentiating T lymphocyte-mediated anti-tumor adaptive immunity. Treatments applying interleukin (IL)-2 or based on autologous T lymphocytes resulted in little or no clinical benefit. Much more promising results were obtained with so-called immune checkpoint inhibitors (ICIs), whose goal is to block T cell immune activity, namely, cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) [93].
In hypoxic cells, HIF-1α binds the HRE located in the proximal PD-L1 promoter. The functional consequences of the overexpression of PD-L1 on the surface of myeloid-derived suppressor cells (MDSCs) include the increased production of IL-6 and IL-10 under hypoxic conditions and significantly decreased the proliferation of CD8+ T cells. Taken together, these phenotypic changes are indicative of enhanced immunosuppression when MDSCs are exposed to hypoxia. Treatment with an anti-PD-L1 antibody significantly decreased both the expression of IL-6 and IL-10 and CD8+ T cells [94].
The activity of hypoxia-induced factors affects cancer immunosuppression in patients with OC. In a study of 21 OC patients, the mRNA expression of selected hypoxia-related genes and tumor-infiltrating leucocytes was assessed by flow cytometry to identify regulatory T cells, MDSC, and type 2 macrophages. The number of tumor-infiltrating leucocytes varied from 2% to over 50% of the total cell population. The heterogeneous immunological microenvironment in OC patients was confirmed by the relative proportions of suppressors cells. This complicates immune checkpoint therapies and may contribute to the low response rate. Clustering at the mRNA level revealed a small group with high expression of HIF target genes and increased expression of HIF-1α and HIF-2α proteins, which may increase their susceptibility to immunotherapy [95].
The establishment of modern immunotherapy in OC comes from a study by Disis et al. [96]. In this phase 1B study, avelumab (anti-PD-L1 antibody) used as a single-agent in 125 patients with previously relapsed platinum-resistant OC showed encouraging anti-tumor activity, with an objective response rate (ORR) of 9.6% and an acceptable toxicity profile.
These results provided the basis for initiating the phase III JAVELIN Ovarian 200 study. It compared the activity of standard chemotherapy with pegylated liposomal doxorubicin (PLD) in patients with platinum-resistant OC vs. the combination of avelumab together with PLD or avelumab alone. Unfortunately, avelumab did not significantly increase the PFS or OS compared to standard PLD. The ORRs did not differ significantly between the arms and individual subgroups and were: 3.7%, 13.3%, and 4.2% for avelumab, avelumab plus PLD, and PLD monotherapy, respectively. PD-L1 status did not affect the median PFS [97].
The next randomized phase III JAVELIN Ovarian 100 study in previously untreated patients with locally advanced or metastatic (FIGO III/IV stage) OC compared the efficacy of avelumab with standard chemotherapy. Patients were randomized to three arms receiving: Carboplatin/paclitaxel (A); carboplatin/paclitaxel with the maintenance of avelumab in (B); or avelumab plus carboplatin/paclitaxel, followed by avelumab maintenance therapy (C). The hazard ratio, (HR with 95% CI) for PFS in the avelumab-treated patients was 1.43 (95% CI 1.051–1.946) and 1.14 (95% CI 0.832–1.565) for A and B, respectively. The median PFS was 16.8 months (95% CI 13.5-NE) and 18.1 months (95% CI 14.8-NE) in group B and C, respectively. In the control group, the medians (NE (18.2-NE)) were not reached. Subgroup analysis based on baseline characteristics and biomarkers (PD-L1, CD8, and BRCA mutations) failed to select subgroups that showed significant benefits. OS data were not published yet. The ORRs were: 30.4%, 36.0% and 30.4% for B, C and control, respectively. Avelumab treatment did not result in PFS prolongation (i.e., the primary endpoint was not reached compared to the control receiving standard chemotherapy) [98].
Those results had a direct impact on the decision of Merck and Pfizer, in March 2019, to discontinue another ongoing project [99], a randomized phase III trial of JAVELIN Ovarian PARP 100 [100] which planned to compare the efficacy and safety of avelumab in combination with standard paclitaxel/carboplatin chemotherapy or talazoparib, a PARPi.
Phase II study compared the efficacy of avelumab in combination with a class I selective HDAC—entinostat, to avelumab monotherapy in patients with advanced OC. Heavily pretreated patients (from 3–6 lines therapy) were randomly assigned in a 2:1 ratio to two arms—A: Avelumab (10 mg/kg, IV every 2 weeks) plus entinostat (5 mg, PO daily), or B: Avelumab plus placebo. Median PFS did not differ significantly between the groups, being 1.64 and 1.51 months (HR 0.90, 95% CI: 0.58–1.39; p = 0.31) for A and B, respectively. There were no significant differences in secondary endpoints (i.e., ORR (6% vs. 5%) and OS (median not reached vs. 11.3 months)) [101].
The activity of pembrolizumab, ICI, and anti-PD-1 antibody, was assessed in a phase II study, KEYNOTE-100, in patients with recurrent OC. Cohort A consisted of 285 patients who had previously received 1–3 treatment lines with a platinum-free interval or treatment-free interval (TFI) between 3 and 12 months. Cohort B included 91 patients who had previously received 4–6 previous lines with PFI/TFI ≥ 3 months. The ORRs were: 7.4% and 9.9% in cohorts A and B, respectively. The median response time was 8.2 months for cohort A and was not achieved for cohort B. The disease control rates (DCRs) were similar in both cohorts (37.2% and 37.4%). The median OS was not provided in cohort A, and in cohort B, was 17.6 months [102].
Preclinical studies data indicated a synergistic effect of ICIs with PARPis [103]. Patients with recurrent OC were enrolled in a single-armed phase II study evaluating the efficacy of ICI with PARPis. A total of 35 heavily pretreated patients, with a median of four prior lines of therapy, received olaparib 300 mg twice daily and durvalumab 1500 mg intravenously every four weeks. The ORR was 14% (5/35 patients). The DCR (PR + SD) was achieved in 71% (25/35 patients). The treatment increased the expression of IFNγ and CXCL9/CXCL10 in the tumor, as well as increased serum IFNγ/TNFα and expression of tumor-infiltrating lymphocytes, which may indicate the effectiveness of durvalumab/olaparib combination therapy in inducing an immune response in the tumor. Increased IFNγ expression was associated with improved PFS (HR 0.37 (95% CI 0.16–0.87); p = 0.023), while increased VEGFR3 levels were associated with worse PFS (HR 3.22 (95% CI 1.23–8.40); p = 0.017) [104].
The published results on the roles of avelumab, pembrolizumab and durvalumab are the first data from phase II and III studies to evaluate the role of ICIs in OC. No significant results of treatment improvement were achieved for ICIs, both in relapse and in the first-line treatment of OC. The combination of ICIs with classical cytostatics, such as paclitaxel/carboplatin, and PLD or with PARPis did not significantly improve the results of treatment.
The first results of studies with immunotherapy of OC patients have shown that without a better understanding of the mechanisms of tumor immune escape, better characterization of the TME, and mechanisms related to immunotherapy resistance, continuing research in this cancer issue may end up similarly to studies with avelumab.
Table 3 provides an overview of the ongoing phase III clinical trials with ICIs.

Table 3.
Immune checkpoint inhibitors in ongoing phase III clinical trials for ovarian cancer.
6. Hypoxia Alleviation as the Condition to Combined Treatments against Ovarian Cancer
In the TME, tumor cells represent the population that proliferates, moves, and escapes with the help of the TME conditions. This results from the cooperation with first, the blood vessels achieving the process of angiogenesis, together with the infiltrated immune/inflammatory cells [105] and various cells, such as cancer activated fibroblasts (CAFs) that compose, among others, “stromal cells” [106].
All cells associate in a cross-talk that dynamically participates in the evolution of the tumor in the TME context. This elaborated TME is consequently, determinant not only for the progression of a tumor but also for its aggressiveness. It exerts as a selection pressure to which tumor cells adapt through their heterogeneity [107] and similarly, heterogeneously react and resist when an anti-cancer treatment is applied. This is highly significant in the case of OCs. Indeed, besides the four main histological subtypes with low and high grades, OCs display a series of mutations and homologous recombination on germ lines, as well as epigenetic modifications. Such variability influences the stemness characters of cells, and consequently, the phenotype of ovary tumor initiating cells. In the case of OCs [108], in addition to the biological features of TME, the growing tumor mass generates hypoxia as a result of the physical lack of access to the oxygenated circulating blood. Hypoxia plays a maximal part as ovarian tumors develop and reside in a harsh hypoxic peritoneal environment, which additionally lowers the overall oxygen tension. Indeed, ascites aggravate hypoxia [109]. Consequently and more acutely in the development of OCs than other types of cancer, hypoxic stress is the most crucial factor, although still poorly taken into account in the design of treatment procedures.
It is remarkable that the main step determining the switch that turns a tumor from benign into an aggressive, uncontrolled growing tumor is the angiogenic switch (i.e., when the tumor goes from being physioxic to being hypoxic) [110]. This corresponds to signaling for angiogenesis, to bring in blood for reoxygenation, aiming to get back to the physioxic level of the normal ovarian tissue and transform the TME composition. In the tumor, angiogenesis never gets to be balanced because of the constant hypoxic state of the growing tumor mass [111]. Consequently, this maintains the need for neovascularization, which develops into a pathologic, non-efficient, and anarchic network. The resulting cellular and humoral components reflect an overall response, the origin of which is the hypoxic stress.
Thus, hypoxia of the TME is highly significant for the issue of cancer clinical treatment outcomes. More particularly, it determines the patients’ responses to treatments (especially to immunotherapy) because of its direct effect on metabolic changes, cell plasticity, and immunosuppression [112]. Indeed, cancer-linked immune resistance is a direct consequence of hypoxia-dependent shaping by the selection pressures exerted on the TME cells [113].
In that line, numerous immunoregulatory pathways render T cell-mediated tumor destruction inefficient. For example, the activity of killer immune cells in the immunological recognition of tumors is compromised by their concerted action with components of the tumor stroma, leading to immunosuppression [107].
This immunosuppressive conditioning of the TME affects the immune checkpoints orchestra. By their level of expression, distribution, and activity, the immune checkpoints molecules do cooperate to inactivate the tumor killer cells and tolerize tumor antigen-specific CD8 T cells [114]. Tregs are increased, and the phenotype of M1 macrophages is switched to M2 [115], actively increasing the overall immunosuppressive character of the tumor site.
As described above, very active blockers of immune checkpoints molecules are mainly antibodies directed to T cells and NK cells, such as anti-PD-1 [116] and anti-CTLA-4 [94,116], while anti-PD-L1 antibodies are mainly directed towards the tumor cells, endothelial cells, and macrophages in the TME. The modulation of their activity appears to be hypoxia-dependent. This may explain why using such blockers that improve survival in several types of metastatic cancers failed in a large proportion of patients with advanced cancer.
Those works are also pointing to the numerous and dynamic properties of the TME regulating the tumor cell response to immunotherapy. The unique-type of response is limited to the tumor-specific T cells. Thus, the tumor appears as a complex site composed as a whole “pseudo-organ” into which hypoxia regulates intercellular cooperation/recognition and dysregulates their effects comparatively to physioxia.
As a direct consequence, the hypoxic stress causes errors during the DNA repair process leading to mutations, and generally, to genomic instability that participates in the observed heterogeneity of the phenotypes displayed by cancer cells and their dynamic adaptation, described as plasticity. Because of such a permanent ability to adapt, plasticity is one of the features favoring the escape of tumor cells from susceptibility to treatment. This is particularly illustrated by the tumor cell’s property to undergo EMT. Distinct clones are produced during EMT with various degrees of ability to give rise to resistant and aggressive populations [117]. As a consequence, an effective treatment should not only take into account the tumor heterogeneity/plasticity to eliminate resistant cell selection but should also be devoted to avoiding sustained hypoxia.
Alleviating hypoxia is a challenging step and a crucial condition for treatment efficacy [118]. It is the criterion for adjuvant strategies designed to favor drug effects. Tumor neovascularization does not restore proper oxygenation despite the enhanced angiogenic activity. This makes vessel normalization approaches promising to enhance the potential of chemotherapy, as well as radiotherapy, and to overcome the main pitfalls met during immunotherapeutic treatments [111].
Normalized vessels allow for blood flow that mechanically helps treatments to reach the newly irrigated tumor cells. In addition to this physical role, the blood-borne oxyhaemoglobin dissociation in erythrocytes permits compensation of hypoxia, thus, breaking the chronic hypoxic stress [82,119,120].
The changes are effective on the whole microenvironment and indicate that combined treatments leading to elaborate adjuvant strategies are promising for immunotherapies [40,121,122]. Not only, but particularly illustrated, in the case of OCs, PD-1/PD-L1 recognition is very efficiently inactivating the immune response. This reaction is favored by hypoxia [123]. Indeed, PD-L1 expression has been shown to be induced by hypoxia in many cells of the TME and the tumor cells themselves. It was found in a circulating form and on key immune cells, such as dendritic cells (DCs) and macrophages [123,124].
In most cancers and particularly OC, the appearance of high-levels of PD-L1 corresponds to the angiogenic switch due to the establishment of hypoxia and occurs when the immunosuppression makes the treatments inefficient. In cancer and TME cells, the PD-L1 response to hypoxia is a final effect of the HIF-1-dependent induction of the PI3K/AKT/mTOR pathway [125]. Its activity is controlled upstream by the activity of the tumor suppressor PTEN, which is particularly important in OC [125].
Control of PD-L1 expression is directly exerted by the level of the local oxygen tension in the TME. In endothelial cells, PD-L1 induction by hypoxia is a hallmark of the pathologic angiogenesis on the endothelial cell membrane. Such overexpression correlates with the soluble form of PD-L1, which increases the PD-1 expression on the immunocompetent cells, such as NK cells and CD8T cells, making them both more susceptible to programmed death and blocking their passage into the tumor mass at the endothelial cell barrier level. Consequently, such a concordance of effects makes it necessary to design combinatorial treatments for OC. Strategies aim to modify the hypoxic situation by rendering the vessels functional to ensure the necessary blood flow to give the anti-PD-L1 antibodies access to tumor cells and other PD-L1+ cells of the TME. The latter compromise the action of PD-1+ immunocompetent cells, as NK and CD8T cells. As such, most of the combined treatment strategies are devoted to normalization of the vessels by using so-called anti-angiogenic monoclonal antibodies or molecules mostly directed to neutralize the excess of VEGF produced by the hypoxic tumor cells [121,122,126].
The challenge is the proper balance to avoid the deep anti-angiogenic effect leading to vessel destruction, anoxia, and selection of resistant cancer stem cells [127]. Indeed, in ovary cancer patients, PD-L1 expression indicates a bad prognosis and low treatment outcomes [128].
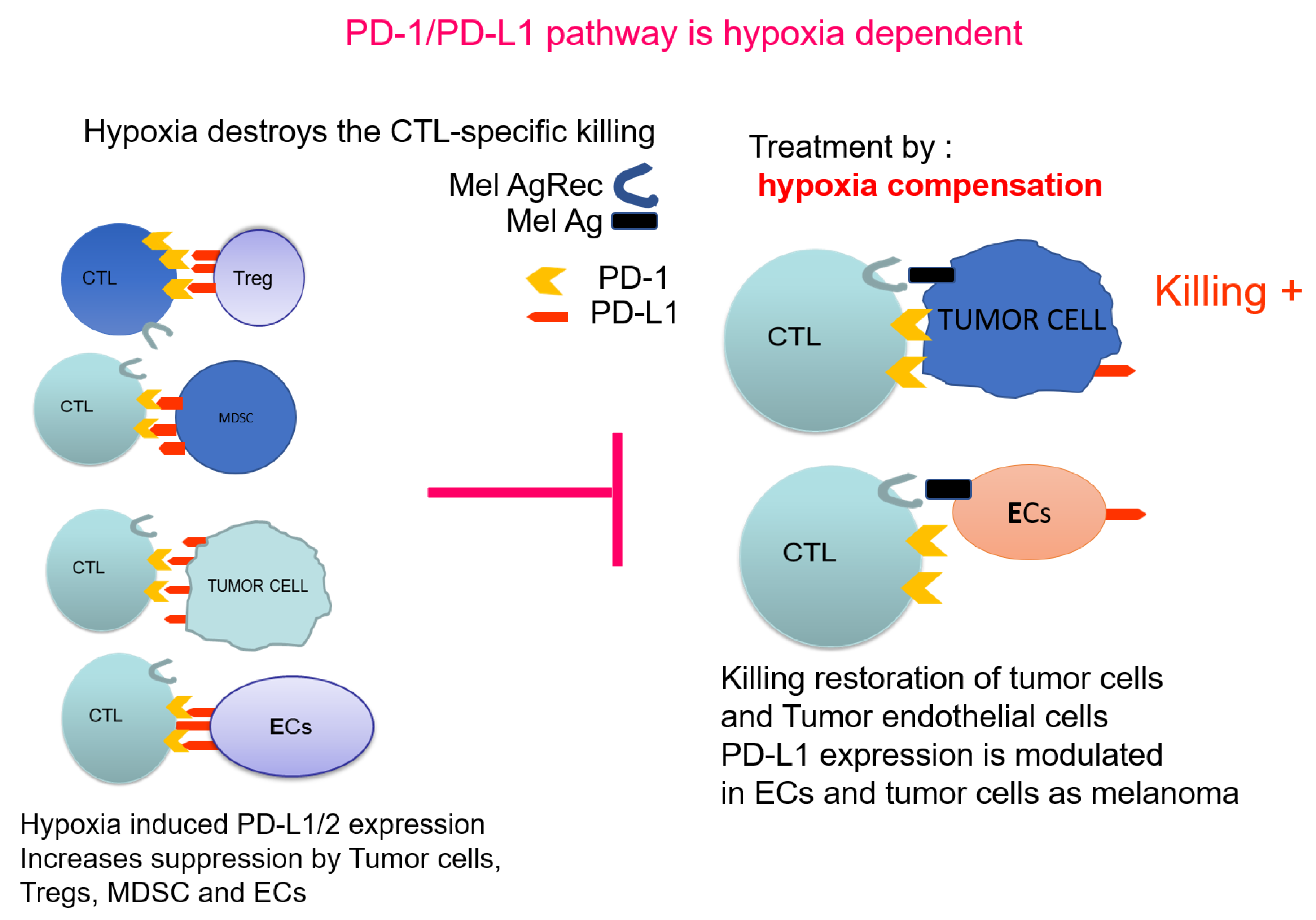
In conclusion, future approaches will aim to combine methods, which will first modify the TME by alleviating hypoxia to allow the further application of downstream combinations of anti-cancer therapies. Figure 2 illustrates PD-1/PD-L1 pathway in the context of hypoxia. The properties of the cancer endothelial cells and their response to hypoxia, indicate that PTEN-mediated regulation of PD-L1 is a most promising adjuvant approach. PTEN, as a controller of the angiogenic process, is a target to increase the chances of further immunotherapies.
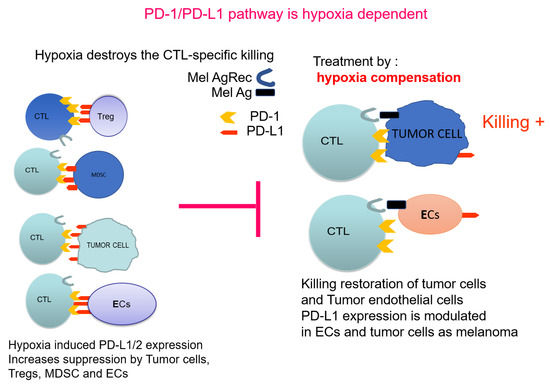
Figure 2.
PD-1/PD-L1 pathway as hypoxia dependent: Effect on immunosuppression.  indicates the blocking effect of hypoxia-induced PD-L1 towards normal killing.
indicates the blocking effect of hypoxia-induced PD-L1 towards normal killing.
 indicates the blocking effect of hypoxia-induced PD-L1 towards normal killing.
indicates the blocking effect of hypoxia-induced PD-L1 towards normal killing.
The mechanisms of resistance display a large spectrum of complexity in limiting the efficacy of checkpoint inhibitor monotherapy, combinatorial approaches are crucial. Monitoring angiogenesis is the most direct and non-avoidable condition to modify the properties of TME, so that tumor tolerance and resistance, can be overcome.
Again, hypoxia alleviation appears necessary for its influence on T cell function. Consequently, the combinatorial strategies focused on angiogenesis normalization are necessary to allow for efficient immunotherapy through its effect on both ICIs and induction of immune cells response. Such conditions, when fulfilled, will permit us to efficiently apply other therapies without facing failures that are actually encountered and that are attributed to the TME protection of the tumor expansion.
TME should be considered as an important target for anti-cancer therapy, allowing patients to ultimately benefit from drug combinations.
7. Conclusions
Hypoxia plays a crucial role in modulating the response of cells to various drug treatments. It is one of the key factors contributing to the observed chemoresistance. Targeting HIF-1 emerges as a tool for increasing the effectiveness of standard chemotherapy and helping to overcome chemoresistance. Thus, treatments directed at HIF, and consequently, controlling VEGFs, should help regulate angiogenesis and change the microenvironment.
It is to be noticed that, although treatments were devoted to tumor cells, their efficacy cannot be dissociated from their effect on the other cells of the microenvironment. Namely, cells that enter the tumor to kill it.
Immunotherapeutic strategies, having undoubtedly opened a new era in cancer research and treatment, are the best example of a need for the knowledge of the local conditions in which they have to operate. The typical example is provided by the anti-angiogenic therapies. Most of them aim at regulating the excessive growth of vessels, such as monoclonal antibodies against VEGFs or other stimulators of endothelial VEGF receptors [129]. Most often, they are designed to neutralize the excess VEGF produced in response to tumor hypoxia. Their highly successful efficacy leads to the destruction of newly formed vessels. Instead of making tumor cells apoptotic because of the lack of O2 and blood-borne nutrients, the whole process makes the cells resistant. Dedifferentiation is amplified, and cancer cells are selected to form a stem-like cell population (quiescent and senescent) but, at the same time, susceptible to move into an aggressive and highly invasive cell population. This raised the need for vessel normalization strategies, which appear to be the main present challenge that anti-cancer research has to face. Based on the cited knowledge of the local tumor conditions (i.e., the microenvironment), the normalization of the pathologic vessels is the main approach with the potential to reach a complete modification of the tumor environmental properties both in its molecular and cellular components [93,130]. The failure of recent clinical trials of ICIs naturally raises questions about the reason for such a phenomenon. One possible explanation emerges considering the hypoxia effect on TME. It was shown that HIF-1α directly increased PD-L1 gene expression in MDSCs, macrophages, dendritic cells, and tumor cells [124]. Lymphatic endothelial cells caused systemic peripheral tolerance through the expression of PD-L1. Observed tolerance occurred because of the lack of co-stimulation resulting in high-level PD-1 expression on CD8 T cells [131]. Therefore, concurrent PD-L1 and high-level PD-1 expression may provide a novel approach for immunotherapy in OC. Moreover, a better understanding of the mechanism involved in OC. Moreover, a better understanding of the mechanisms involved in OC hypoxia may provide biomarkers that would allow reliable monitoring and assessment of the efficacy of immunotherapy.
For efficient therapies against tumors, it appears that the reversal of hypoxic features brings promise to invert the immune suppression into active immune response. This type of approach should be understood, as an adjuvant mean to permit the application of treatments and provide a high added therapeutic value.
Author Contributions
Conceptualization: A.K., K.K.B., L.B., H.W., C.K.; manuscript writing: A.K., L.B., G.W., C.K.; supervision: C.K.; molecular consultation: K.K.B., H.W.; clinical consultation: L.B., G.W., C.A.S.; critical review: C.K., H.W. All authors have read and agreed to the published version of the manuscript.
Funding
A.K. was supported by the National Science Centre, Poland Grant No. 2012/07/N/NZ5/02052 and Military Institute of Medicine intramural grant no 1/8912(412). C.K. was supported by the National Scientific Centre, Poland Grants No. OPUS2016/23/B/NZ6/02542 and OPUS2016/23/B/NZ1/03211. H.W. was supported by the National Science Centre, Poland Sonata Bis Grant No. 2017/26/E/NZ3/00434.
Acknowledgments
Authors would like to acknowledge Kościuszko I funding as C.K., A.K. and K.K.B. were supported by the Ministry of Defence grant Kosciuszko I no 579/2016/DA.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PDS | primary deburring surgery |
| NACT | neoadjuvant chemotherapy |
| OS | overall survival |
| PFS | progression-free survival |
| FIGO | The International Federation of Gynecology and Obstetrics) |
| HIPEC | hyperthermic intraperitoneal chemotherapy |
| PARP | Poly(ADP-ribose)polymerases |
| BRCA1 | Breast cancer type 1 susceptibility protein |
| EMT | epithelial-to-mesenchymal transition |
| HIF-1α | hypoxia-inducible factor-1 alpha |
| pVHL | Von Hippel Lindau protein |
| VEGF | vascular endothelial growth factors |
| MMP2 | metalloproteinase 2 |
| UPAR | angiopoietin 2 and urokinase receptor |
| DSF | disease-free survival |
| CSS | cancer-specific survival |
| RFS | relapse-free survival |
| MFS | metastasis-free survival |
| CBP | CREB-binding protein |
| HEx | hypoxic exosomes |
| NEx | normoxic exosomes |
| PD-1 | programmed death protein |
| PD-L1 | programmed death ligand 1 |
| XRE | X-responsive elements |
| rhFSH | human recombinant follicule stimulating hormone |
| ROS | reactive oxygen species |
| IL8 | Interleukin 8 |
| Epo | erythropoietin |
| RTK | receptor tyrosine kinase |
| HRE | hypoxia-responsive elements |
| AE | amla extract |
| HRR | homologous recombination repair |
| FAK | focal adhesion kinase |
| PDGFR | platelet-derived growth factor receptor |
| PP2A | protein phosphatase 2A |
| CTLA-4 | cytotoxic T lymphocyte-associated protein 4 |
| DCR | disease control rate |
| CAF | cancer activated fibroblasts |
| TME | tumor microenvironment |
| PTEN | phosphatase and tensin homolog deleted on chromosome ten |
| NK | natural killers |
| MDSCs | myeloid-derived suppressor cells |
| ECs | endothelial cells |
References
- Markowska, A.; Rucinski, M.; Drews, K.; Malendowicz, L.K. Studies on leptin and leptin receptor gene expression in myometrium and uterine myomas of gnRH analogue-treated women. Eur. J. Gynaecol. Oncol. 2006, 27, 379–384. [Google Scholar] [PubMed]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Goff, B.A.; Mandel, L.S.; Melancon, C.H.; Muntz, H.G. Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA 2004, 291, 2705–2712. [Google Scholar] [CrossRef]
- Bankhead, C.R.; Collins, C.; Stokes-Lampard, H.; Rose, P.; Wilson, S.; Clements, A.; Mant, D.; Kehoe, S.T.; Austoker, J. Identifying symptoms of ovarian cancer: A qualitative and quantitative study. BJOG 2008, 115, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Jelovac, D.; Armstrong, D.K. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J. Clin. 2011, 61, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Schorge, J.O.; Bradford, L.S.; del Carmen, M.G. Primary cytoreductive surgery for advanced ovarian cancer: Is it the past, present, or future? Clin. Adv. Hematol. Oncol. 2011, 9, 912–918. [Google Scholar]
- du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.; Van Der Burg, M.E.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Wright, A.A.; Bohlke, K.; Armstrong, D.K.; Bookman, M.A.; Cliby, W.A.; Coleman, R.L.; Dizon, D.S.; Kash, J.J.; Meyer, L.A.; Moore, K.N.; et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 3460–3473. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Coens, C.; Nankivell, M.; Kristensen, G.B.; Parmar, M.K.B.; Ehlen, T.; Jayson, G.C.; Johnson, N.; Swart, A.M.; Verheijen, R.; et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: Pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018, 19, 1680–1687. [Google Scholar] [CrossRef]
- Karam, A.; Ledermann, J.A.; Kim, J.W.; Sehouli, J.; Lu, K.; Gourley, C.; Katsumata, N.; Burger, R.A.; Nam, B.H.; Bacon, M.; et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: First-line interventions. Ann. Oncol. 2017, 28, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, N.; Yasuda, M.; Isonishi, S.; Takahashi, F.; Michimae, H.; Kimura, E.; Aoki, D.; Jobo, T.; Kodama, S.; Terauchi, F.; et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): A randomised, controlled, open-label trial. Lancet Oncol. 2013, 14, 1020–1026. [Google Scholar] [CrossRef]
- Pignata, S.; Scambia, G.; Katsaros, D.; Gallo, C.; Pujade-Lauraine, E.; De Placido, S.; Bologna, A.; Weber, B.; Raspagliesi, F.; Panici, P.B.; et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 396–405. [Google Scholar] [CrossRef]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; DiSilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A.; et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N. Engl. J. Med. 2016, 374, 738–748. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Bundy, B.; Wenzel, L.; Huang, H.Q.; Baergen, R.; Lele, S.; Copeland, L.J.; Walker, J.L.; Burger, R.A.; Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med. 2006, 354, 34–43. [Google Scholar] [CrossRef]
- Monk, B.J.; Chan, J.K. Is intraperitoneal chemotherapy still an acceptable option in primary adjuvant chemotherapy for advanced ovarian cancer? Ann. Oncol. 2017, 28 (Suppl. 8), viii40–viii45. [Google Scholar] [CrossRef]
- van Driel, W.J.; Koole, S.N.; Sonke, G.S. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 1363–1364. [Google Scholar] [CrossRef]
- Lim, M.C.; Chang, S.-J.; Yoo, H.J. Randomized trial of hyperthermic intraperitoneal chemotherapy (HIPEC) in women with primary advanced peritoneal, ovarian, and tubal cancer. J. Clin. Oncol. 2017, 35, 5520. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012, 30, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Ray, C.M.; Spira, A.I.; Trepel, J.B.; Carter, C.A.; Cottrill, H.M. A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med. Oncol. 2017, 34, 103. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Crusz, S.M.; Ledermann, J.A. Olaparib maintenance for first-line treatment of ovarian cancer: Will SOLO1 reset the standard of care? Future Oncol. 2019, 15, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Azmi, A.S.; Ali, S.; Ahmad, A.; Li, Y.; Banerjee, S.; Kong, D.; Sarkar, F.H. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim. Biophys. Acta 2012, 1826, 272–296. [Google Scholar] [CrossRef]
- Chouaib, S.; Umansky, V.; Kieda, C. The role of hypoxia in shaping the recruitment of proangiogenic and immunosuppressive cells in the tumor microenvironment. Contemp. Oncol. (Pozn.) 2018, 22, 7–13. [Google Scholar] [CrossRef]
- Semenza, G.L.; Nejfelt, M.K.; Chi, S.M.; Antonarakis, S.E. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc. Natl. Acad. Sci. USA 1991, 88, 5680–5684. [Google Scholar] [CrossRef]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell. 2008, 30, 393–402. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.D.; Ebert, B.L.; Pugh, C.W.; Ratcliffe, P.J. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: Similarities with the erythropoietin 3’ enhancer. Proc. Natl. Acad. Sci. USA 1994, 91, 6496–6500. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; McKnight, S.L.; Russell, D.W. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997, 11, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Troy, H.; Leek, R.; Chung, Y.L.; Li, J.L.; Raval, R.R.; Turley, H.; Gatter, K.; Pezzella, F.; Griffiths, J.R.; et al. Effects of HIF-1alpha and HIF2alpha on Growth and Metabolism of Clear-Cell Renal Cell Carcinoma 786-0 Xenografts. J. Oncol. 2010, 2010, 757908. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.P.; Harishankar, M.K.; Pillai, A.A.; Devi, A. Hypoxia induced EMT: A review on the mechanism of tumor progression and metastasis in OSCC. Oral Oncol. 2018, 80, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl.) 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Chiche, J.; Brahimi-Horn, M.C.; Pouyssegur, J. Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J. Cell. Mol. Med. 2010, 14, 771–794. [Google Scholar] [CrossRef]
- Song, J.; Miermont, A.; Lim, C.T.; Kamm, R.D. A 3D microvascular network model to study the impact of hypoxia on the extravasation potential of breast cell lines. Sci. Rep. 2018, 8, 17949. [Google Scholar] [CrossRef]
- Klimkiewicz, K.; Weglarczyk, K.; Collet, G.; Paprocka, M.; Guichard, A.; Sarna, M.; Jozkowicz, A.; Dulak, J.; Sarna, T.; Grillon, C.; et al. A 3D model of tumour angiogenic microenvironment to monitor hypoxia effects on cell interactions and cancer stem cell selection. Cancer Lett. 2017, 396, 10–20. [Google Scholar] [CrossRef]
- Evans, C.E.; Branco-Price, C.; Johnson, R.S. HIF-mediated endothelial response during cancer progression. Int. J. Hematol. 2012, 95, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Arnould, T.; Remacle, J. Endothelial cell responses to hypoxia: Initiation of a cascade of cellular interactions. Biochim. Biophys. Acta 2000, 1497, 1–10. [Google Scholar] [CrossRef]
- Rankin, E.B.; Nam, J.M.; Giaccia, A.J. Hypoxia: Signaling the Metastatic Cascade. Trends Cancer 2016, 2, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Watanabe, Y.; Ueda, H.; Hoshiai, H. Hypoxia inducible factor 1-alpha expression as a factor predictive of efficacy of taxane/platinum chemotherapy in advanced primary epithelial ovarian cancer. Cancer Lett. 2007, 251, 164–167. [Google Scholar] [CrossRef]
- Karihtala, P.; Maenpaa, J.; Turpeenniemi-Hujanen, T.; Puistola, U. Front-line bevacizumab in serous epithelial ovarian cancer: Biomarker analysis of the FINAVAST trial. Anticancer Res. 2010, 30, 1001–1006. [Google Scholar]
- Daponte, A.; Ioannou, M.; Mylonis, I.; Simos, G.; Minas, M.; Messinis, I.E.; Koukoulis, G. Prognostic significance of Hypoxia-Inducible Factor 1 alpha(HIF-1 alpha) expression in serous ovarian cancer: An immunohistochemical study. BMC Cancer 2008, 8, 335. [Google Scholar] [CrossRef]
- Han, S.; Huang, T.; Hou, F.; Yao, L.; Wang, X.; Wu, X. The prognostic value of hypoxia-inducible factor-1alpha in advanced cancer survivors: A meta-analysis with trial sequential analysis. Ther. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef]
- Su, S.; Dou, H.; Wang, Z.; Zhang, Q. Bufalin inhibits ovarian carcinoma via targeting mTOR/HIF-alpha pathway. Basic Clin. Pharmacol. Toxicol. 2020. [Google Scholar] [CrossRef]
- Laatio, L.; Myllynen, P.; Serpi, R.; Rysa, J.; Ilves, M.; Lappi-Blanco, E.; Ruskoaho, H.; Vahakangas, K.; Puistola, U. BMP-4 expression has prognostic significance in advanced serous ovarian carcinoma and is affected by cisplatin in OVCAR-3 cells. Tumour Biol. 2011, 32, 985–995. [Google Scholar] [CrossRef]
- Ai, Z.; Lu, Y.; Qiu, S.; Fan, Z. Overcoming cisplatin resistance of ovarian cancer cells by targeting HIF-1-regulated cancer metabolism. Cancer Lett. 2016, 373, 36–44. [Google Scholar] [CrossRef]
- Duyndam, M.C.; van Berkel, M.P.; Dorsman, J.C.; Rockx, D.A.; Pinedo, H.M.; Boven, E. Cisplatin and doxorubicin repress Vascular Endothelial Growth Factor expression and differentially down-regulate Hypoxia-inducible Factor I activity in human ovarian cancer cells. Biochem. Pharmacol. 2007, 74, 191–201. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, L.M.; O’Toole, S.A.; Spillane, C.D.; Martin, C.M.; Gallagher, M.F.; Stordal, B.; Blackshields, G.; Sheils, O.; O’Leary, J.J. Identifying novel hypoxia-associated markers of chemoresistance in ovarian cancer. BMC Cancer 2015, 15, 547. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kim, B.; Cho, U.; Park, I.S.; Kim, S.I.; Dhanasekaran, D.N.; Tsang, B.K.; Song, Y.S. Mitochondrial fission causes cisplatin resistance under hypoxic conditions via ROS in ovarian cancer cells. Oncogene 2019. [Google Scholar] [CrossRef] [PubMed]
- Dorayappan, K.D.P.; Wanner, R.; Wallbillich, J.J.; Saini, U.; Zingarelli, R.; Suarez, A.A.; Cohn, D.E.; Selvendiran, K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: A novel mechanism linking STAT3/Rab proteins. Oncogene 2018, 37, 3806–3821. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Liu, W.; Jia, P.; Wang, H.; Jiang, G.; Wang, T. HIF-1alpha-induced autophagy contributes to cisplatin resistance in ovarian cancer cells. Pharmazie 2018, 73, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gao, L.; Li, B.; Liu, C.; Hong, S.; Min, J.; Hong, L. Knockdown of Hypoxia-Inducible Factor 1alpha (HIF-1alpha) Promotes Autophagy and Inhibits Phosphatidylinositol 3-Kinase (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Signaling Pathway in Ovarian Cancer Cells. Med. Sci. Monit. 2019, 25, 4250–4263. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Z.; Yin, H.; Yang, G. Interaction between p53 and Ras signaling controls cisplatin resistance via HDAC4- and HIF-1alpha-mediated regulation of apoptosis and autophagy. Theranostics 2019, 9, 1096–1114. [Google Scholar] [CrossRef]
- De, A.; De, A.; Papasian, C.; Hentges, S.; Banerjee, S.; Haque, I.; Banerjee, S.K. Emblica officinalis extract induces autophagy and inhibits human ovarian cancer cell proliferation, angiogenesis, growth of mouse xenograft tumors. PLoS ONE 2013, 8, e72748. [Google Scholar] [CrossRef]
- Hussain, I.; Waheed, S.; Ahmad, K.A.; Pirog, J.E.; Syed, V. Scutellaria baicalensis targets the hypoxia-inducible factor-1alpha and enhances cisplatin efficacy in ovarian cancer. J. Cell. Biochem. 2018, 119, 7515–7524. [Google Scholar] [CrossRef]
- Malm, S.W.; Hanke, N.T.; Gill, A.; Carbajal, L.; Baker, A.F. The anti-tumor efficacy of 2-deoxyglucose and D-allose are enhanced with p38 inhibition in pancreatic and ovarian cell lines. J. Exp. Clin. Cancer Res. 2015, 34, 31. [Google Scholar] [CrossRef]
- Ohta, T.; Takahashi, T.; Shibuya, T.; Amita, M.; Henmi, N.; Takahashi, K.; Kurachi, H. Inhibition of the Rho/ROCK pathway enhances the efficacy of cisplatin through the blockage of hypoxia-inducible factor-1alpha in human ovarian cancer cells. Cancer Biol. Ther. 2012, 13, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Huang, L.; Ao, Q.; Zhang, Q.; Tian, X.; Fang, Y.; Lu, Y. Noscapine sensitizes chemoresistant ovarian cancer cells to cisplatin through inhibition of HIF-1alpha. Cancer Lett. 2011, 305, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Pastorek, J.; Pastorekova, S.; Callebaut, I.; Mornon, J.P.; Zelnik, V.; Opavsky, R.; Zat’ovicova, M.; Liao, S.; Portetelle, D.; Stanbridge, E.J.; et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 1994, 9, 2877–2888. [Google Scholar] [PubMed]
- Pastorekova, S.; Gillies, R.J. The role of carbonic anhydrase IX in cancer development: Links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 2019, 38, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Pastorek, M.; Simko, V.; Takacova, M.; Barathova, M.; Bartosova, M.; Hunakova, L.; Sedlakova, O.; Hudecova, S.; Krizanova, O.; Dequiedt, F.; et al. Sulforaphane reduces molecular response to hypoxia in ovarian tumor cells independently of their resistance to chemotherapy. Int. J. Oncol. 2015, 47, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, N.; Deng, S.; Zhang, D.; Wang, K.; Lu, M. miR-199a modulates cisplatin resistance in ovarian cancer by targeting Hif1alpha. Onco Targets Ther. 2017, 10, 5899–5906. [Google Scholar] [CrossRef]
- Ao, Q.; Su, W.; Guo, S.; Cai, L.; Huang, L. SENP1 desensitizes hypoxic ovarian cancer cells to cisplatin by up-regulating HIF-1alpha. Sci. Rep. 2015, 5, 16396. [Google Scholar] [CrossRef]
- Bergandi, L.; Canosa, S.; Pittatore, G.; Silvagno, F.; Doublier, S.; Gennarelli, G.; Benedetto, C.; Revelli, A. Human recombinant FSH induces chemoresistance in human breast cancer cells via HIF-1alpha activationdagger. Biol. Reprod. 2019, 100, 1521–1535. [Google Scholar] [CrossRef]
- Gomez-Roman, N.; Sahasrabudhe, N.M.; McGregor, F.; Chalmers, A.J.; Cassidy, J.; Plumb, J. Hypoxia-inducible factor 1 alpha is required for the tumourigenic and aggressive phenotype associated with Rab25 expression in ovarian cancer. Oncotarget 2016, 7, 22650–22664. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, J.; Xing, H.; Li, B.; Li, L.; Wang, X.; Peng, D.; Chen, J. Contribution of FPR and TLR9 to hypoxia-induced chemoresistance of ovarian cancer cells. OncoTargets Ther. 2019, 12, 291–301. [Google Scholar] [CrossRef]
- Nunes, S.C.; Lopes-Coelho, F.; Gouveia-Fernandes, S.; Ramos, C.; Pereira, S.A.; Serpa, J. Cysteine boosters the evolutionary adaptation to CoCl2 mimicked hypoxia conditions, favouring carboplatin resistance in ovarian cancer. BMC Evol. Biol. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.C.; Ramos, C.; Lopes-Coelho, F.; Sequeira, C.O.; Silva, F.; Gouveia-Fernandes, S.; Rodrigues, A.; Guimaraes, A.; Silveira, M.; Abreu, S.; et al. Cysteine allows ovarian cancer cells to adapt to hypoxia and to escape from carboplatin cytotoxicity. Sci. Rep. 2018, 8, 9513. [Google Scholar] [CrossRef] [PubMed]
- Kan, O.; Day, D.; Iqball, S.; Burke, F.; Grimshaw, M.J.; Naylor, S.; Binley, K. Genetically modified macrophages expressing hypoxia regulated cytochrome P450 and P450 reductase for the treatment of cancer. Int. J. Mol. Med. 2011, 27, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Zub, K.A.; Sousa, M.M.; Sarno, A.; Sharma, A.; Demirovic, A.; Rao, S.; Young, C.; Aas, P.A.; Ericsson, I.; Sundan, A.; et al. Modulation of cell metabolic pathways and oxidative stress signaling contribute to acquired melphalan resistance in multiple myeloma cells. PLoS ONE 2015, 10, e0119857. [Google Scholar] [CrossRef]
- Huang, L.; Ao, Q.; Zhang, Q.; Yang, X.; Xing, H.; Li, F.; Chen, G.; Zhou, J.; Wang, S.; Xu, G.; et al. Hypoxia induced paclitaxel resistance in human ovarian cancers via hypoxia-inducible factor 1alpha. J. Cancer Res. Clin. Oncol. 2010, 136, 447–456. [Google Scholar] [CrossRef]
- Kriska, J.; Solar, P.; Varinska, L.; Solarova, Z.; Kimakova, P.; Mojzis, J.; Fedorocko, P.; Sytkowski, A.J. Human erythropoietin increases the pro-angiogenic potential of A2780 ovarian adenocarcinoma cells under hypoxic conditions. Oncol. Rep. 2013, 30, 1455–1462. [Google Scholar] [CrossRef]
- Guo, Q.; Lu, L.; Liao, Y.; Wang, X.; Zhang, Y.; Liu, Y.; Huang, S.; Sun, H.; Li, Z.; Zhao, L. Influence of c-Src on hypoxic resistance to paclitaxel in human ovarian cancer cells and reversal of FV-429. Cell Death Dis. 2018, 8, e3178. [Google Scholar] [CrossRef]
- Kim, B.R.; Yoon, K.; Byun, H.J.; Seo, S.H.; Lee, S.H.; Rho, S.B. The anti-tumor activator sMEK1 and paclitaxel additively decrease expression of HIF-1alpha and VEGF via mTORC1-S6K/4E-BP-dependent signaling pathways. Oncotarget 2014, 5, 6540–6551. [Google Scholar] [CrossRef]
- Previs, R.A.; Armaiz-Pena, G.N.; Lin, Y.G.; Davis, A.N.; Pradeep, S.; Dalton, H.J.; Hansen, J.M.; Merritt, W.M.; Nick, A.M.; Langley, R.R.; et al. Dual Metronomic Chemotherapy with Nab-Paclitaxel and Topotecan Has Potent Antiangiogenic Activity in Ovarian Cancer. Mol. Cancer Ther. 2015, 14, 2677–2686. [Google Scholar] [CrossRef]
- Kampan, N.C.; Madondo, M.T.; McNally, O.M.; Quinn, M.; Plebanski, M. Paclitaxel and Its Evolving Role in the Management of Ovarian Cancer. Biomed. Res. Int. 2015, 2015, 413076. [Google Scholar] [CrossRef]
- Merritt, W.M.; Danes, C.G.; Shahzad, M.M.; Lin, Y.G.; Kamat, A.A.; Han, L.Y.; Spannuth, W.A.; Nick, A.M.; Mangala, L.S.; Stone, R.L.; et al. Anti-angiogenic properties of metronomic topotecan in ovarian carcinoma. Cancer Biol. Ther. 2009, 8, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Kieda, C.; El Hafny-Rahbi, B.; Collet, G.; Lamerant-Fayel, N.; Grillon, C.; Guichard, A.; Dulak, J.; Jozkowicz, A.; Kotlinowski, J.; Fylaktakidou, K.C.; et al. Stable tumor vessel normalization with pO(2) increase and endothelial PTEN activation by inositol trispyrophosphate brings novel tumor treatment. J. Mol. Med. 2013, 91, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Parmakhtiar, B.; Burger, R.A.; Kim, J.H.; Fruehauf, J.P. HIF Inactivation of p53 in Ovarian Cancer Can Be Reversed by Topotecan, Restoring Cisplatin and Paclitaxel Sensitivity. Mol. Cancer Res. 2019, 17, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Mandai, M.; Yamaguchi, K.; Matsumura, N.; Kharma, B.; Baba, T.; Abiko, K.; Hamanishi, J.; Yoshioka, Y.; Konishi, I. Metabolic alterations caused by HNF1beta expression in ovarian clear cell carcinoma contribute to cell survival. Oncotarget 2015, 6, 26002–26017. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Bratasz, A.; Kuppusamy, M.L.; Tazi, M.F.; Rivera, B.K.; Kuppusamy, P. Hypoxia induces chemoresistance in ovarian cancer cells by activation of signal transducer and activator of transcription 3. Int. J. Cancer 2009, 125, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Mercado-Uribe, I.; Xing, Z.; Sun, B.; Kuang, J.; Liu, J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene 2014, 33, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Prislei, S.; Mariani, M.; Raspaglio, G.; Mozzetti, S.; Filippetti, F.; Ferrandina, G.; Scambia, G.; Ferlini, C. RON and cisplatin resistance in ovarian cancer cell lines. Oncol. Res. 2010, 19, 13–22. [Google Scholar] [CrossRef]
- Al-Abd, A.M.; Alamoudi, A.J.; Abdel-Naim, A.B.; Neamatallah, T.A.; Ashour, O.M. Anti-angiogenic agents for the treatment of solid tumors: Potential pathways, therapy and current strategies - A review. J. Adv. Res. 2016, 8, 591–605. [Google Scholar] [CrossRef]
- Haemmerle, M.; Bottsford-Miller, J.; Pradeep, S.; Taylor, M.L.; Choi, H.J.; Hansen, J.M.; Dalton, H.J.; Stone, R.L.; Cho, M.S.; Nick, A.M.; et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J. Clin. Investig. 2016, 126, 1885–1896. [Google Scholar] [CrossRef]
- Dickson, B.D.; Wong, W.W.; Wilson, W.R.; Hay, M.P. Studies Towards Hypoxia-Activated Prodrugs of PARP Inhibitors. Molecules 2019, 24, 1559. [Google Scholar] [CrossRef]
- Liu, J.F.; Barry, W.T.; Birrer, M.; Lee, J.M.; Buckanovich, R.J.; Fleming, G.F.; Rimel, B.J.; Buss, M.K.; Nattam, S.R.; Hurteau, J.; et al. Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer. Ann. Oncol. 2019, 30, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.R.; Gueble, S.E.; Liu, Y.; Oeck, S.; Kim, H.; Yun, Z.; Glazer, P.M. Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD51. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.Z.; Hasmim, M.; Lequeux, A.; Xiao, M.; Duhem, C.; Chouaib, S.; Berchem, G.; Janji, B. Improving Cancer Immunotherapy by Targeting the Hypoxic Tumor Microenvironment: New Opportunities and Challenges. Cells 2019, 8, 1083. [Google Scholar] [CrossRef] [PubMed]
- Lequeux, A.; Noman, M.Z.; Xiao, M.; Sauvage, D.; Van Moer, K.; Viry, E.; Bocci, I.; Hasmim, M.; Bosseler, M.; Berchem, G.; et al. Impact of hypoxic tumor microenvironment and tumor cell plasticity on the expression of immune checkpoints. Cancer Lett. 2019, 458, 13–20. [Google Scholar] [CrossRef]
- Duechler, M.; Peczek, L.; Szubert, M.; Suzin, J. Influence of hypoxia inducible factors on the immune microenvironment in ovarian cancer. Anticancer Res. 2014, 34, 2811–2819. [Google Scholar]
- Disis, M.L.; Taylor, M.H.; Kelly, K.; Beck, J.T.; Gordon, M.; Moore, K.M.; Patel, M.R.; Chaves, J.; Park, H.; Mita, A.C.; et al. Efficacy and Safety of Avelumab for Patients With Recurrent or Refractory Ovarian Cancer: Phase 1b Results From the JAVELIN Solid Tumor Trial. JAMA Oncol. 2019, 5, 393–401. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Banerjee, S.; Pignata, S. Management of Platinum-Resistant, Relapsed Epithelial Ovarian Cancer and New Drug Perspectives. J. Clin. Oncol. 2019, 37, 2437–2448. [Google Scholar] [CrossRef]
- Ledermann, J.; Colombo, N.; Oza, A.; Fujiwara, K.; Birrer, M.; Randall, L.; Poddubskaya, E.; Scambia, G.; Shparyk, Y.; Lim, M.; et al. Avelumab in combination with and/or following chemotherapy vs chemotherapy alone in patients with previously untreated epithelial ovarian cancer: Results from the phase 3 javelin ovarian 100 trial. Gynecol. Oncol. 2020, 159, 13–14. [Google Scholar] [CrossRef]
- KGaA, M. Merck KGaA, Darmstadt, Germany, and Pfizer Announce Discontinuation of Phase III JAVELIN Ovarian PARP 100 Trial in Previously Untreated Advanced Ovarian Cancer; Merck KGaA: Darmstadt, Germany, 2019. [Google Scholar]
- Avelumab and Talazoparib in Untreated Advanced Ovarian Cancer (JAVELIN OVARIAN PARP 100). Available online: https://clinicaltrials.gov/ct2/show/NCT03642132 (accessed on 30 September 2020).
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Shapira-Frommer, R.; Santin, A.D.; Lisyanskaya, A.S.; Pignata, S.; Vergote, I.; Raspagliesi, F.; Sonke, G.S.; Birrer, M.; Provencher, D.M.; et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: Results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019, 30, 1080–1087. [Google Scholar] [CrossRef]
- Stewart, R.A.; Pilie, P.G.; Yap, T.A. Development of PARP and Immune-Checkpoint Inhibitor Combinations. Cancer Res. 2018, 78, 6717–6725. [Google Scholar] [CrossRef] [PubMed]
- Lampert, E.J.; Zimmer, A.; Padget, M.; Cimino-Mathews, A.; Nair, J.R.; Liu, Y.; Swisher, E.M.; Hodge, J.W.; Nixon, A.B.; Nichols, E.; et al. Combination of PARP Inhibitor Olaparib, and PD-L1 Inhibitor Durvalumab, in Recurrent Ovarian Cancer: A Proof-of-Concept Phase II Study. Clin. Cancer Res. 2020, 26, 4268–4279. [Google Scholar] [CrossRef] [PubMed]
- Baci, D.; Bosi, A.; Gallazzi, M.; Rizzi, M.; Noonan, D.M.; Poggi, A.; Bruno, A.; Mortara, L. The Ovarian Cancer Tumor Immune Microenvironment (TIME) as Target for Therapy: A Focus on Innate Immunity Cells as Therapeutic Effectors. Int. J. Mol. Sci. 2020, 21, 3125. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Fu, S. Role of cancer-associated fibroblasts in tumor structure, composition and the microenvironment in ovarian cancer. Oncol. Lett. 2019, 18, 2173–2178. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, L.; Cai, G.; Xiong, X.; Wu, Y.; Ma, D.; Li, S.C.; Gao, Q. Heterogeneity of immune microenvironment in ovarian cancer and its clinical significance: A retrospective study. Oncoimmunology 2020, 9, 1760067. [Google Scholar] [CrossRef]
- Testa, U.; Petrucci, E.; Pasquini, L.; Castelli, G.; Pelosi, E. Ovarian Cancers: Genetic Abnormalities, Tumor Heterogeneity and Progression, Clonal Evolution and Cancer Stem Cells. Medicines 2018, 5, 16. [Google Scholar] [CrossRef]
- Runyon, B.A. Patients with deficient ascitic fluid opsonic activity are predisposed to spontaneous bacterial peritonitis. Hepatology 1988, 8, 632–635. [Google Scholar] [CrossRef]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Jain, R.K. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef]
- Lara, P.C.; Lloret, M.; Clavo, B.; Apolinario, R.M.; Henriquez-Hernandez, L.A.; Bordon, E.; Fontes, F.; Rey, A. Severe hypoxia induces chemo-resistance in clinical cervical tumors through MVP over-expression. Radiat. Oncol. 2009, 4, 29. [Google Scholar] [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Ovarian Tumor Tissue Analysis (OTTA) Consortium; Goode, E.L.; Block, M.S.; Kalli, K.R.; Vierkant, R.A.; Chen, W.; Fogarty, Z.C.; Gentry-Maharaj, A.; Toloczko, A.; Hein, A.; et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol. 2017, 3, e173290. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Chen, C.; Song, Y.; Cai, Q.; Li, J.; Tang, Y.; Han, X.; Qu, W.; Chen, A.; Wang, H.; et al. Hypoxia modifies the polarization of macrophages and their inflammatory microenvironment, and inhibits malignant behavior in cancer cells. Oncol. Lett. 2019, 18, 5871–5878. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.D.; Kang, N.; Choi, S.Y.; Kim, B.N.; Park, C.K.; Kim, J.W.; Kim, Y.K.; Kim, S.J. The role of hypoxia on the acquisition of epithelial-mesenchymal transition and cancer stemness: A possible link to epigenetic regulation. Korean J. Intern. Med. 2017, 32, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, H.; Zheng, Y.; Han, Y.; Wang, T.; Zhang, H.; Sun, Q.; Li, Z. Overcoming Radioresistance in Tumor Therapy by Alleviating Hypoxia and Using the HIF-1 Inhibitor. ACS Appl. Mater. Interfaces 2020, 12, 4231–4240. [Google Scholar] [CrossRef] [PubMed]
- Cully, M. Cancer: Tumour vessel normalization takes centre stage. Nat. Rev. Drug Discov. 2017, 16, 87. [Google Scholar] [CrossRef]
- Goel, S.; Fukumura, D.; Jain, R.K. Normalization of the tumor vasculature through oncogenic inhibition: An emerging paradigm in tumor biology. Proc. Natl. Acad. Sci. USA 2012, 109, E1214. [Google Scholar] [CrossRef]
- Collet, G.; Szade, K.; Nowak, W.; Klimkiewicz, K.; El Hafny-Rahbi, B.; Szczepanek, K.; Sugiyama, D.; Weglarczyk, K.; Foucault-Collet, A.; Guichard, A.; et al. Endothelial precursor cell-based therapy to target the pathologic angiogenesis and compensate tumor hypoxia. Cancer Lett. 2016, 370, 345–357. [Google Scholar] [CrossRef]
- Collet, G.; Lamerant-Fayel, N.; Tertil, M.; El Hafny-Rahbi, B.; Stepniewski, J.; Guichard, A.; Foucault-Collet, A.; Klimkiewicz, K.; Petoud, S.; Matejuk, A.; et al. Hypoxia-Regulated Overexpression of Soluble VEGFR2 Controls Angiogenesis and Inhibits Tumor Growth. Mol. Cancer Ther. 2014, 13, 165–178. [Google Scholar] [CrossRef]
- Tewalt, E.F.; Cohen, J.N.; Rouhani, S.J.; Engelhard, V.H. Lymphatic endothelial cells-key players in regulation of tolerance and immunity. Front. Immunol. 2012, 3, 305. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.Z.; Desantis, G.; Janji, B.; Hasmim, M.; Karray, S.; Dessen, P.; Bronte, V.; Chouaib, S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med. 2014, 211, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, W.; Xu, Z.P.; Gu, W. PD-L1 Distribution and Perspective for Cancer Immunotherapy-Blockade, Knockdown, or Inhibition. Front. Immunol. 2019, 10, 2022. [Google Scholar] [CrossRef] [PubMed]
- Collet, G.; El Hafny-Rahbi, B.; Nadim, M.; Tejchman, A.; Klimkiewicz, K.; Kieda, C. Hypoxia-shaped vascular niche for cancer stem cells. Contemp. Oncol. (Pozn.) 2015, 19, A39–A43. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bao, Q.; Renner, A.; Camaj, P.; Eichhorn, M.; Ischenko, I.; Angele, M.; Kleespies, A.; Jauch, K.W.; Bruns, C. Cancer stem cells and angiogenesis. Int. J. Dev. Biol. 2011, 55, 477–482. [Google Scholar] [CrossRef]
- Nhokaew, W.; Kleebkaow, P.; Chaisuriya, N.; Kietpeerakool, C. Programmed Death Ligand 1 (PD-L1) Expression in Epithelial Ovarian Cancer: A Comparison of Type I and Type II Tumors. Asian Pac. J. Cancer Prev. 2019, 20, 1161–1169. [Google Scholar] [CrossRef]
- Croci, D.O.; Cerliani, J.P.; Dalotto-Moreno, T.; Mendez-Huergo, S.P.; Mascanfroni, I.D.; Dergan-Dylon, S.; Toscano, M.A.; Caramelo, J.J.; Garcia-Vallejo, J.J.; Ouyang, J.; et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 2014, 156, 744–758. [Google Scholar] [CrossRef]
- Collet, G.; Skrzypek, K.; Grillon, C.; Matejuk, A.; El Hafni-Rahbi, B.; Lamerant-Fayel, N.; Kieda, C. Hypoxia control to normalize pathologic angiogenesis: Potential role for endothelial precursor cells and miRNAs regulation. Vascul Pharmacol 2012, 56, 252–261. [Google Scholar] [CrossRef]
- Tewalt, E.F.; Cohen, J.N.; Rouhani, S.J.; Guidi, C.J.; Qiao, H.; Fahl, S.P.; Conaway, M.R.; Bender, T.P.; Tung, K.S.; Vella, A.T.; et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 2012, 120, 4772–4782. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).