Experimental Pulmonary Tuberculosis in the Absence of Detectable Brain Infection Induces Neuroinflammation and Behavioural Abnormalities in Male BALB/c Mice
Abstract
1. Introduction
2. Results
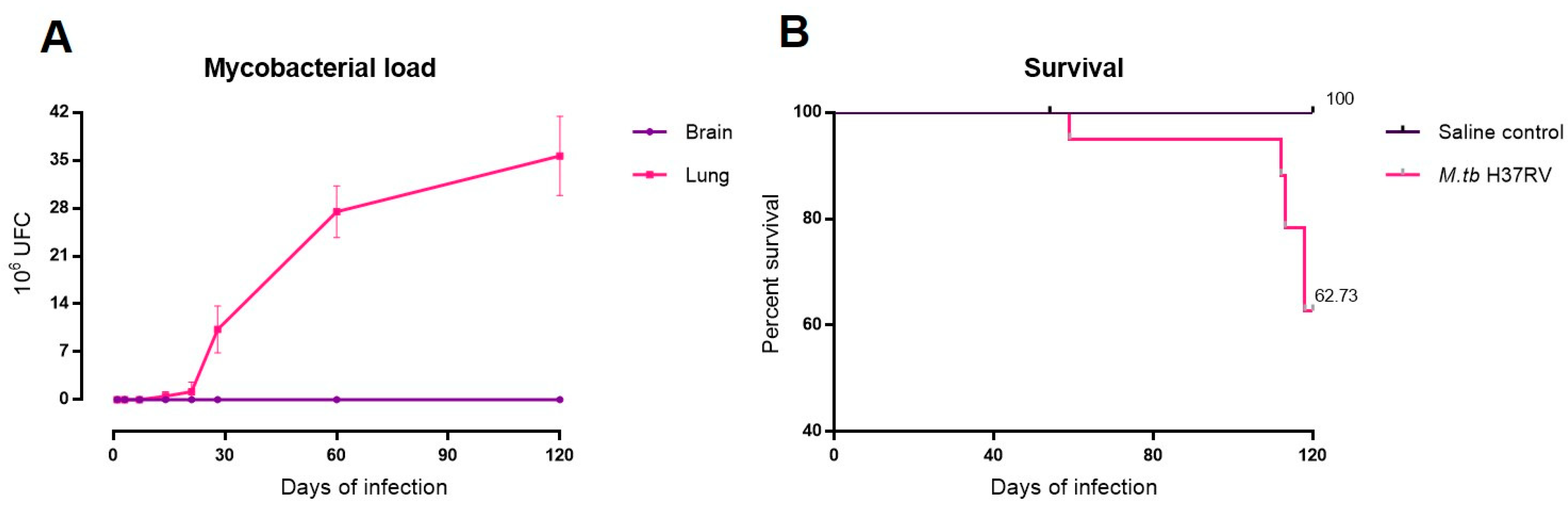
2.1. There Is No Brain Infection during Progressive Pulmonary TB
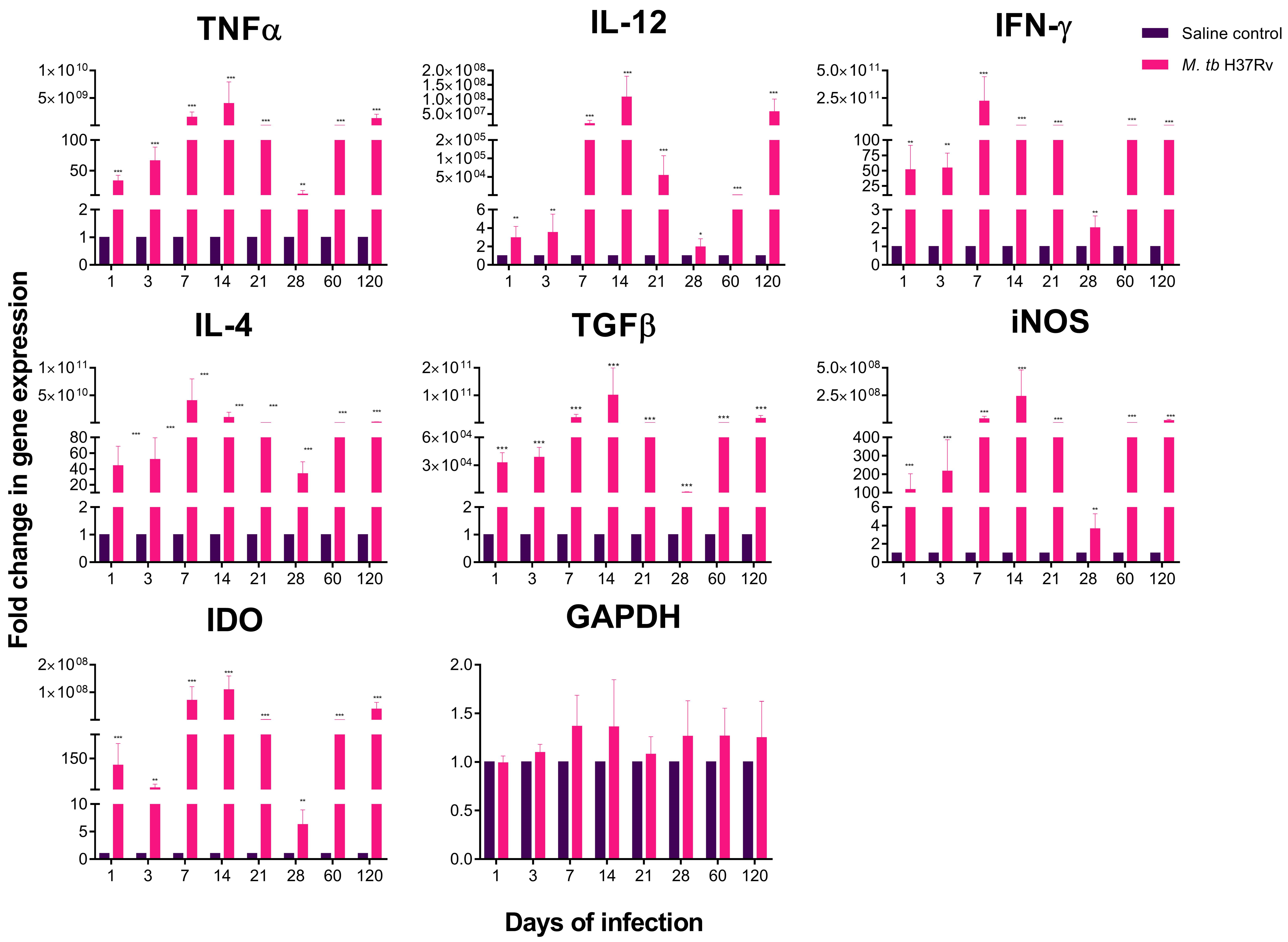
2.2. There Is a High Expression of Cytokines in Selected Areas of the Brain during Pulmonary TB
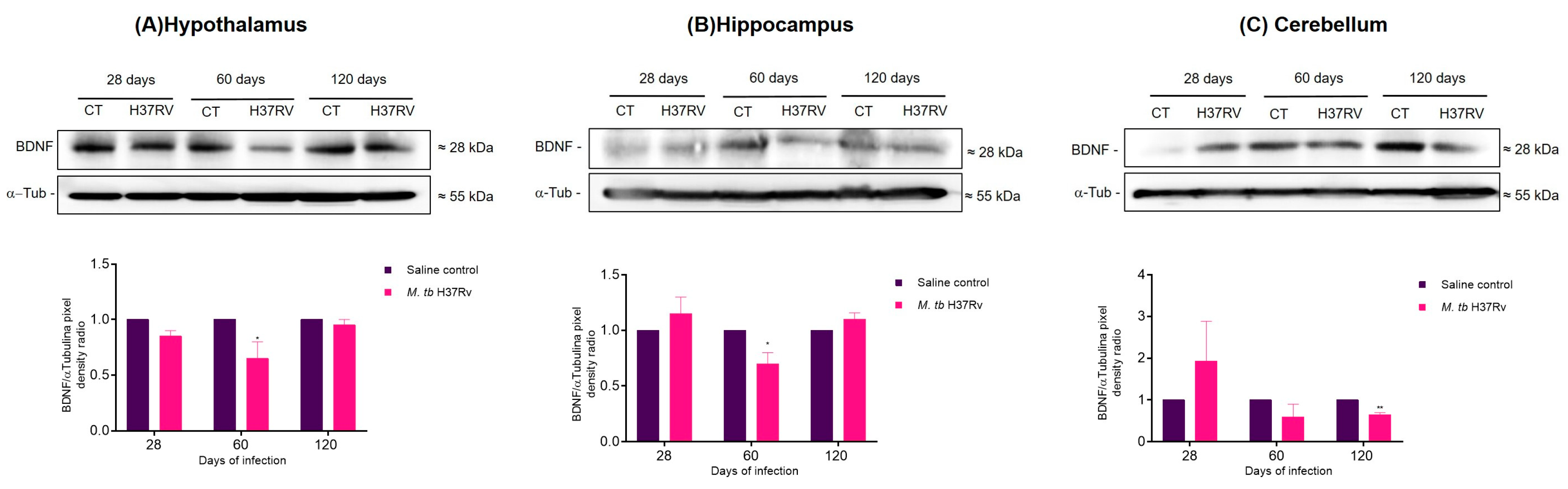
2.3. Pulmonary TB Affected in the Brain Components of the MAPK Pathway, Increasing the Activation of p38 and JNK and Decreasing BNDF Production
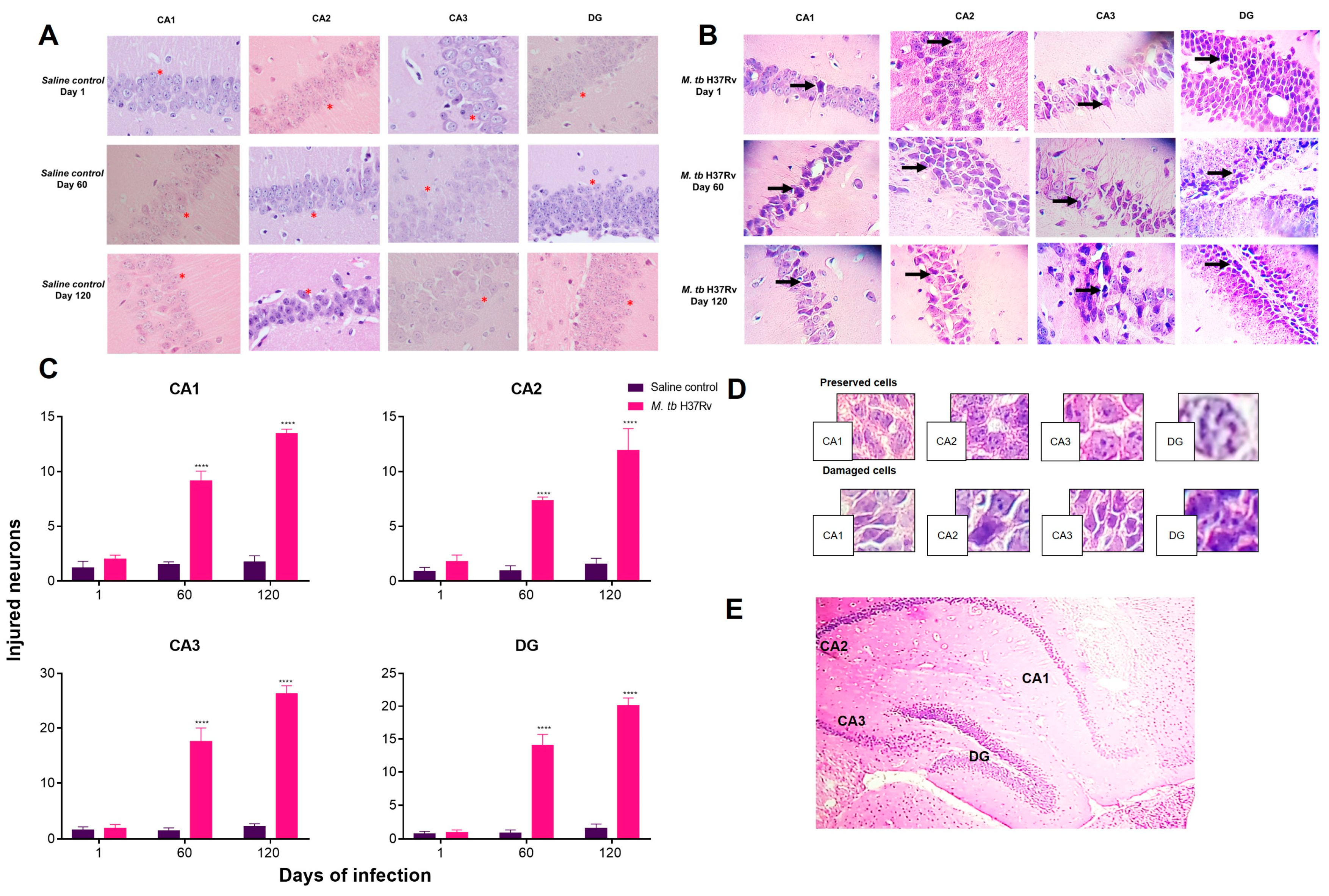
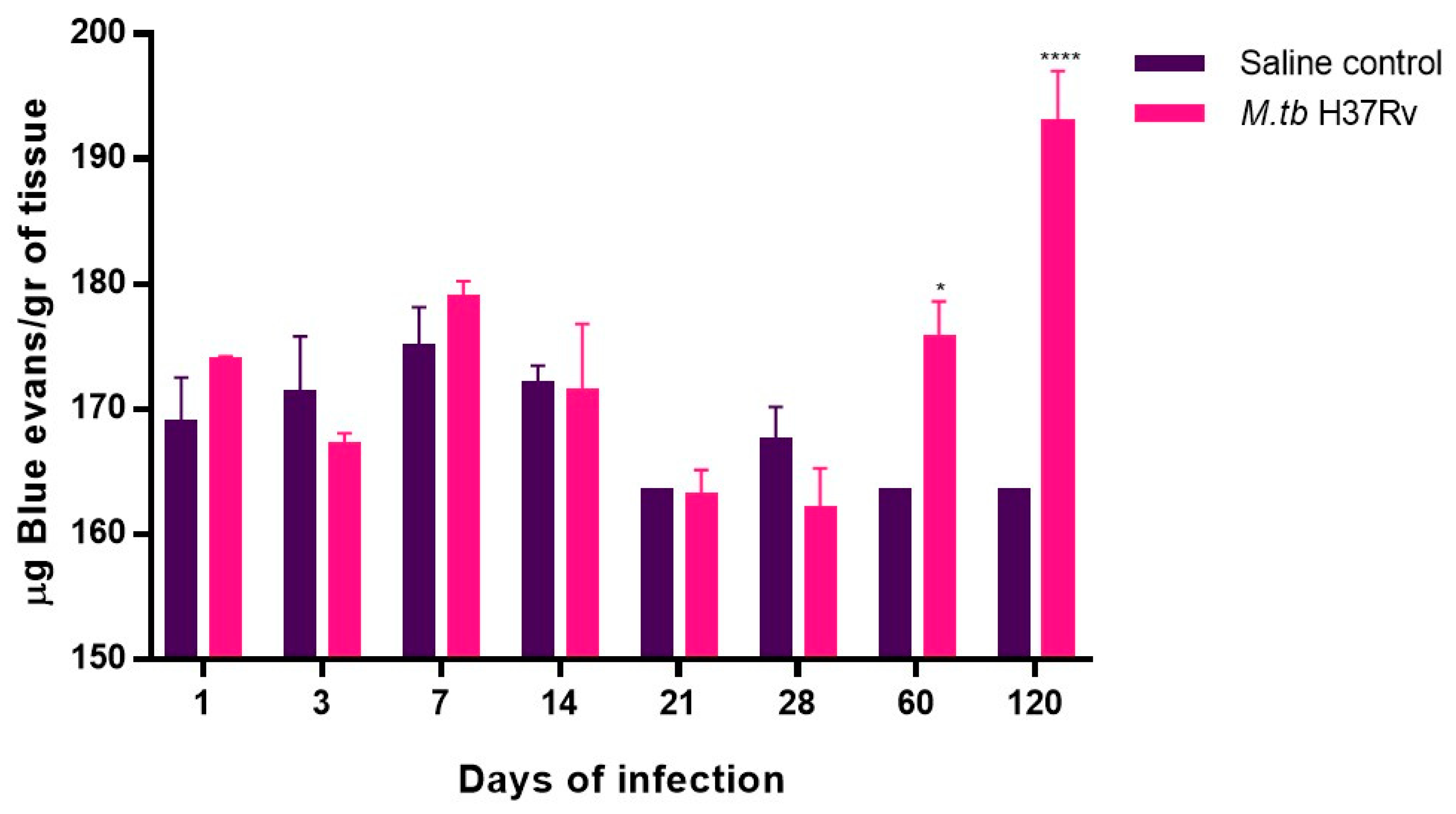
2.4. Pulmonary TB Is Related to Neuronal Injury during Early Infection and Neuronal Death in the Hippocampus and BBB Dysfunction during Progressive Late Disease
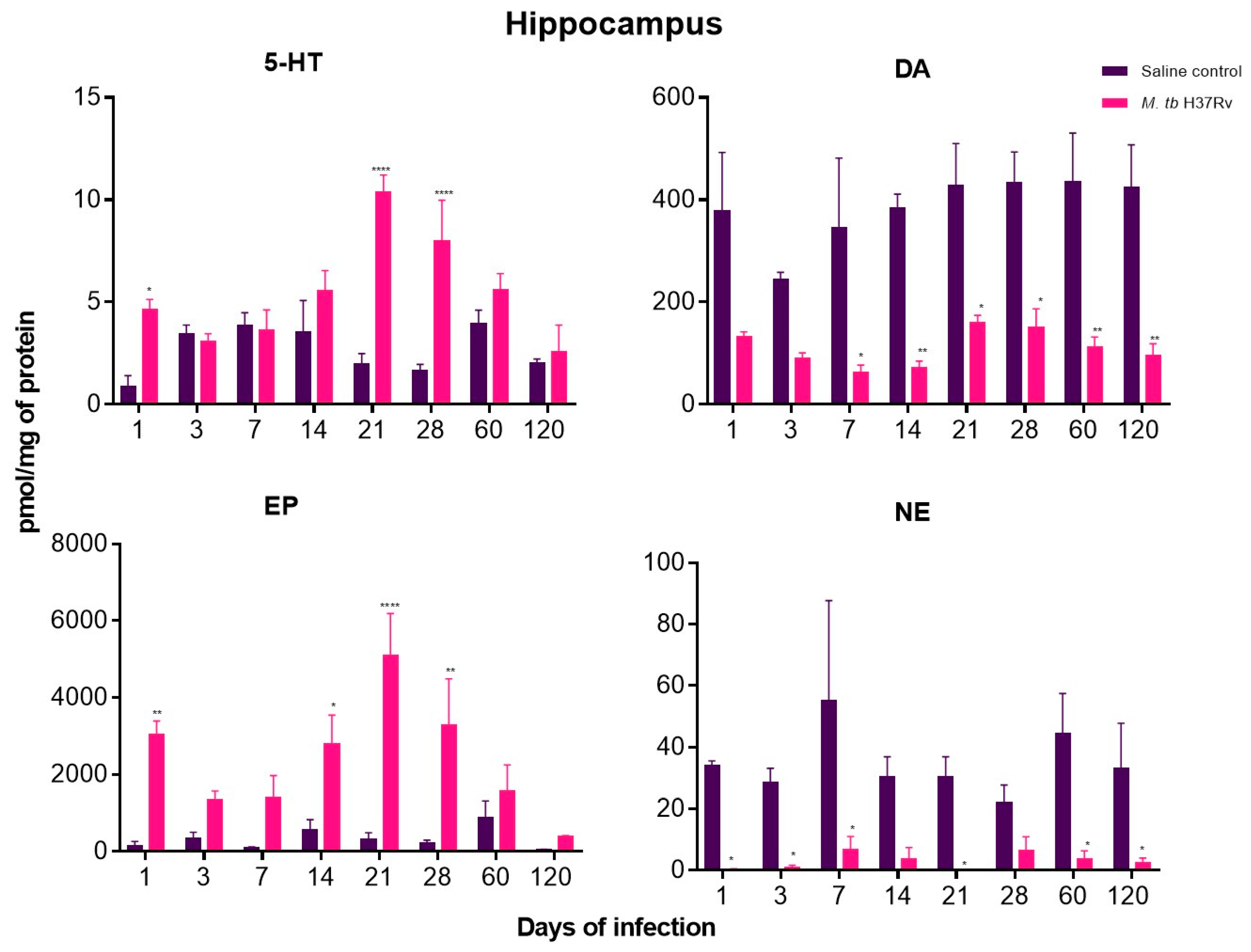
2.5. There Are Significant Changes in the Concentrations of Neurotransmitters during Pulmonary TB
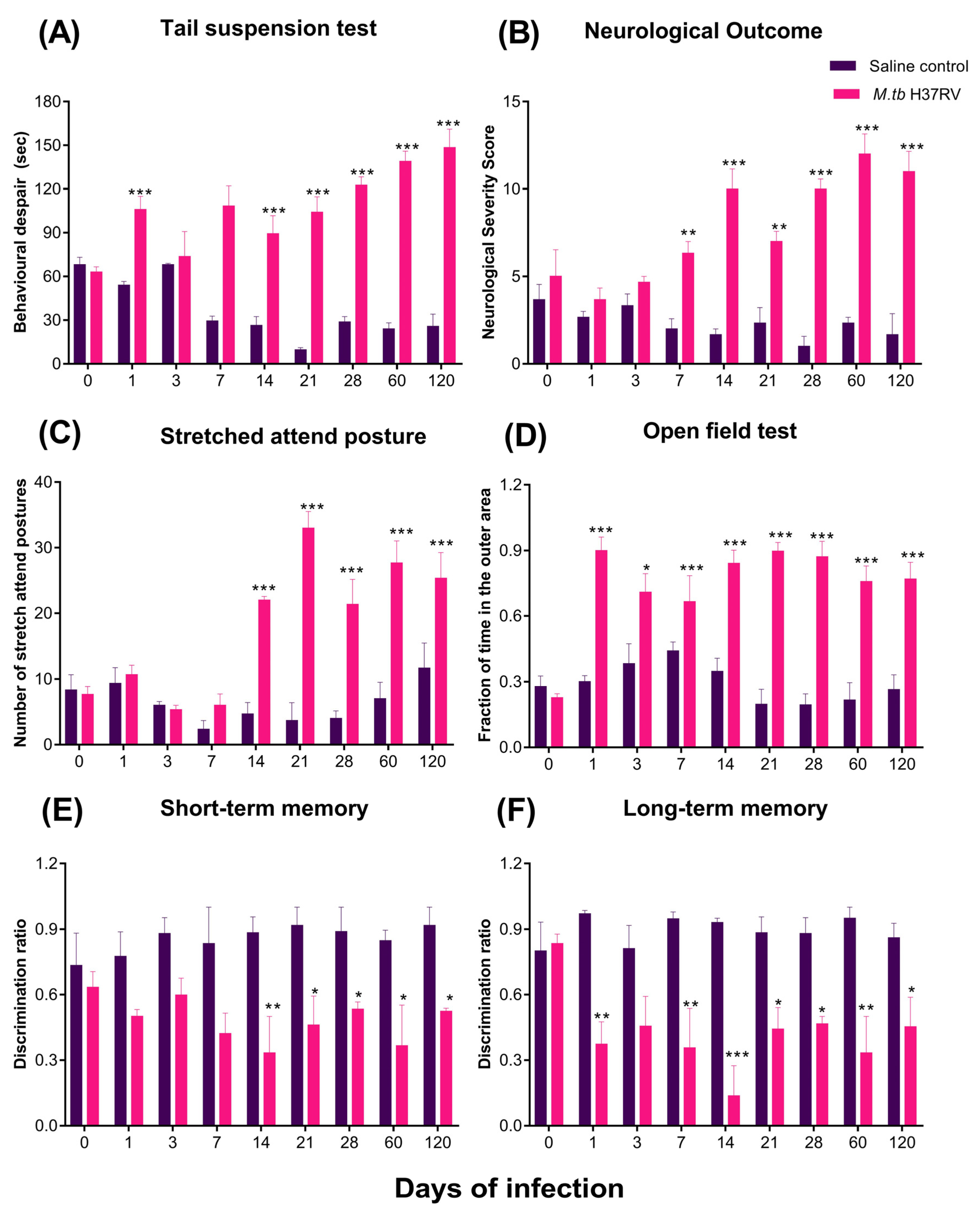
2.6. Pulmonary TB Is Associated with Diverse Behavioural Abnormalities
2.7. The Immune Response in the Hippocampus Is Correlated with the Presence of Behavioural Changes during Pulmonary TB
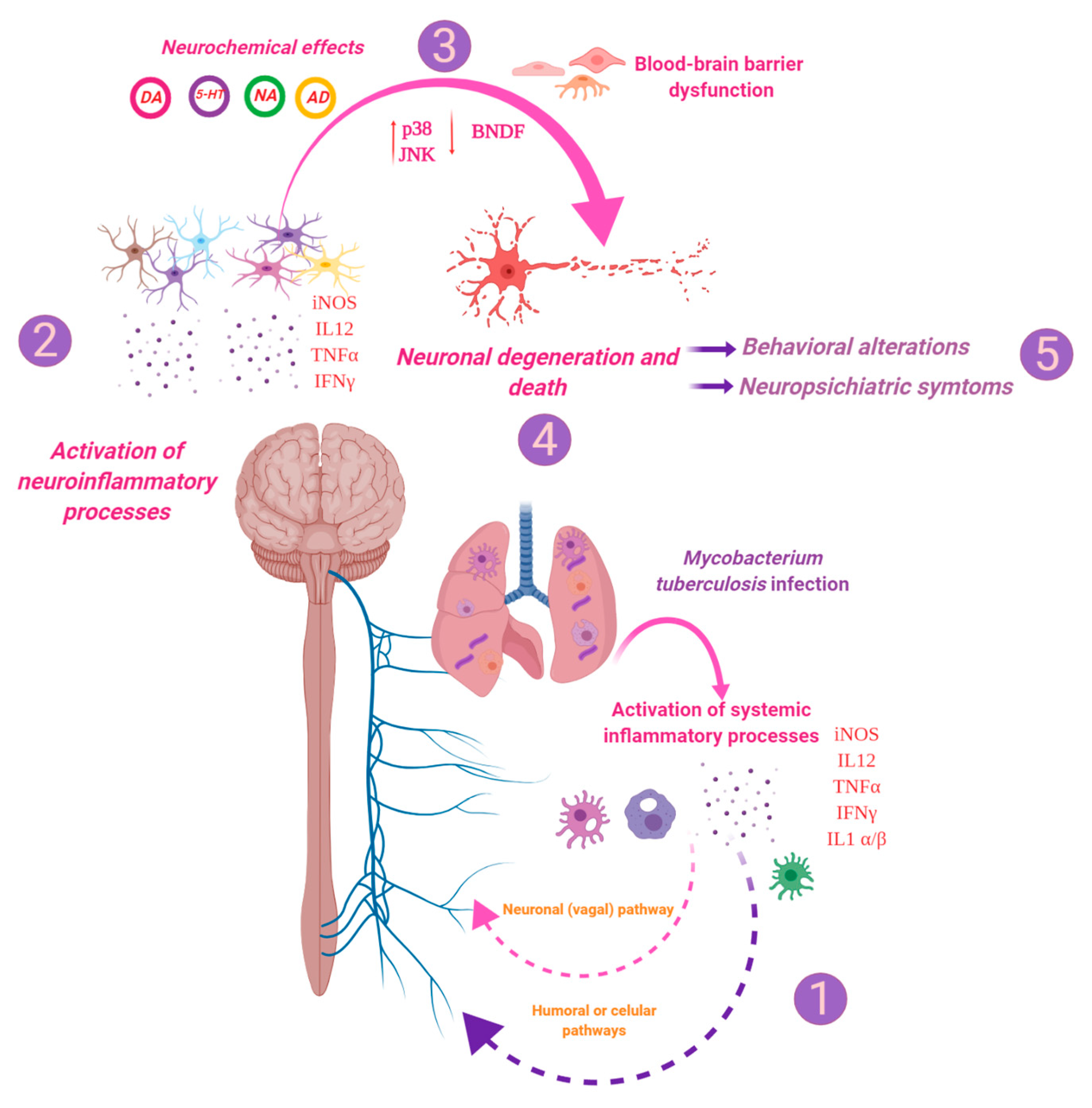
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animals
4.3. The Experimental Model of Pulmonary TB
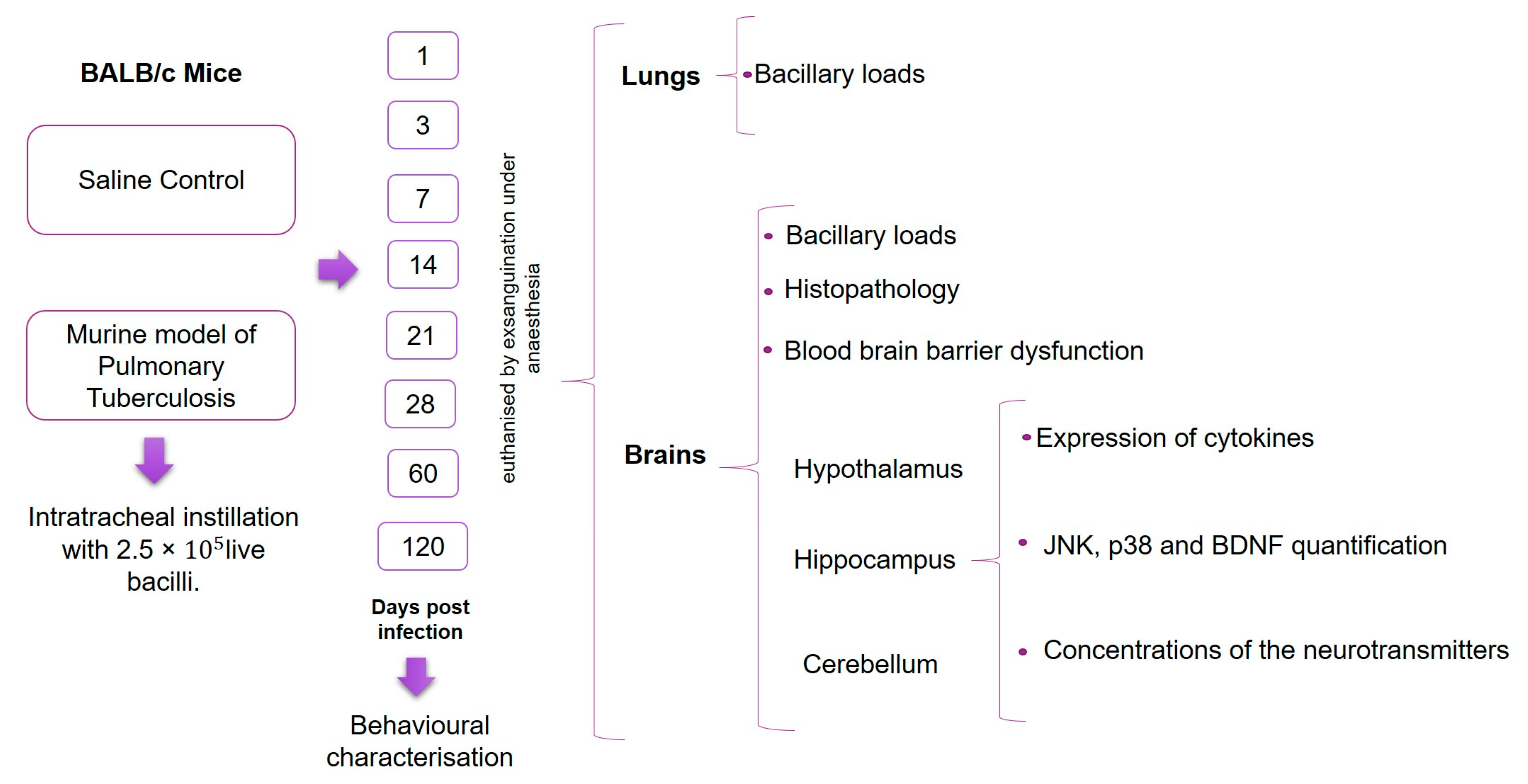
4.4. Experimental Design
4.5. Determination of Colony-Forming Units (CFU) in Infected Lungs and Brain
4.6. Expression of Cytokines by RT-PCR
4.7. Study of the Activation of MAPK Signalling by Western Blot Assays
4.8. Neuronal Damage in the Hippocampus Determined by FJ-B Staining
4.9. Preparation of Brain Tissue for Histological Analysis
4.10. Assessment of BBB Dysfunction Using Evans Blue Staining
4.11. Neurotransmitter Quantification by HPLC
4.12. Behaviour Tests
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BCG | Bacillus Calmette–Guerin |
| BSL-3 | Biosafety laboratory (level three) |
| BBB BSA CSF CNS CVOs JNK CFU COX-2 DG DA EP EDTA EB ERK FJ-B GAPDH GM-CSF HPLC HPA IDO iNOS IFNα IFNγ IL i.p. LMA LOD LOQ MAPK MCP-1 MPA MPB M. tb NSS BDNF NE NFκB NTS OADC PD PAMPS PMSF TB RT–PCR 5-HT SEM SAP TLR TGFβ TNFα Th1 Th2 uPA VEGF | Blood–brain barrier Bovine serum albumin Cerebrospinal fluid Central nervous system Circumventricular organs c-Jun NH2-terminal kinase Colony forming units Cyclooxygenase-2 Dentate gyrus Dopamine Epinephrine Ethylenediaminetetraacetic acid Evans blue Extracellular signal-regulated kinase Fluoro-Jade B Glyceraldehyde-3-phosphate dehydrogenase Granulocyte-macrophage colony-stimulating factor High-performance liquid chromatography Hypothalamic–pituitary–adrenal axis Indolamine 2,3-dioxygenase Induced nitric oxide synthase Interferon-alpha Interferon-gamma Interleukin Intraperitoneal Locomotor activity Limit of detection Limit of quantification Mitogen-activated protein kinases Monocyte chemoattractant protein-1 Mobile phase A Mobile phase B Mycobacterium tuberculosis Neurological severity score Neurotrophin brain-derived nerve growth factor Norepinephrine Nuclear factor kappa B Nucleus tractus solitarius Oleic acid, albumin, dextrose and catalase Pixel densities Pathogen-associated molecular patterns Phenylmethylsulfonyl fluoride Tuberculosis Reverse transcription-polymerase chain reaction Serotonin Standard error of the mean Stretched attend posture Toll-like receptor Transforming growth factor type-beta Tumour necrosis factor-alpha Type 1 cooperating lymphocytes Type 2 Cooperating lymphocytes Urokinase-type plasminogen activator Vascular endothelial growth factor |
References
- Pai, M.; Behr, M.A.; Dowdy, D.; Dheda, K.; Divangahi, M.; Boehme, C.C.; Ginsberg, A.; Swaminathan, S.; Spigelman, M.; Getahun, H.; et al. Tuberculosis. Nat. Rev. Dis. Primers 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020; Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Wells, K.B.; Golding, J.M.; Burnam, M.A. Psychiatric disorder in a sample of the general population with and without chronic medical conditions. Am. J. Psychiatry 1988, 145, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Xavier, P.B.; Peixoto, B. Emotional distress in angolan patients with several types of tuberculosis. Afr. Health Sci. 2015, 15, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Paulo, B.X.; Peixoto, B. Emotional distress patients with several types of tuberculosis. A pilot study with patients from the Sanatorium Hospital of Huambo. Int. J. Mycobacteriol. 2016, 5, S58. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Mehreen, S.; Khan, M.A.; Ashiq, N.; Ihtesham, M. Depression and its Associated Factors with Multidrug-Resistant Tuberculosis at Baseline. J. Depress Anxiety 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Aydin, I.O.; Uluşahin, A. Depression, anxiety comorbidity, and disability in tuberculosis and chronic obstructive pulmonary disease patients: Applicability of GHQ-12. Gen. Hosp. Psychiatry 2001, 23, 77–83. [Google Scholar] [CrossRef]
- Issa, B.A.; Yussuf, A.D.; Kuranga, S.I. Depression comorbidity among patients with tuberculosis in a university teaching hospital outpatient clinic in Nigeria. Ment. Health Fam. Med. 2009, 6, 133–138. [Google Scholar]
- Aamir, S. Aisha Co-morbid anxiety and depression among pulmonary tuberculosis patients. J. Coll. Physicians Surg. Pak. 2010, 20, 703–704. [Google Scholar] [CrossRef]
- Hernández-Pando, R.; Orozcoe, H.; Sampieri, A.; Pavón, L.; Velasquillo, C.; Larriva-Sahd, J.; Alcocer, J.M.; Madrid, M.V. Correlation between the kinetics of Th1, Th2 cells and pathology in a murine model of experimental pulmonary tuberculosis. Immunology 1996, 89, 26–33. [Google Scholar]
- Khairova, R.A.; MacHado-Vieira, R.; Du, J.; Manji, H.K. A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int. J. Neuropsychopharmacol. 2009, 12, 561–578. [Google Scholar] [CrossRef]
- Arreola, R.; Becerril-Villanueva, E.; Cruz-Fuentes, C.; Velasco-Velázquez, M.A.; Garces-Alvarez, M.E.; Hurtado-Alvarado, G.; Quintero-Fabian, S.; Pavon, L. Immunomodulatory effects mediated by serotonin. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Dantzer, R., Capuron, L., Eds.; Current Topics in Behavioral Neurosciences; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Mondelli, V.; Vernon, A.C. From early adversities to immune activation in psychiatric disorders: The role of the sympathetic nervous system. Clin. Exp. Immunol. 2019, 197, 319–328. [Google Scholar] [CrossRef] [PubMed]
- D’Attilio, L.; Santucci, N.; Bongiovanni, B.; Bay, M.L.; Bottasso, O. Tuberculosis, the disrupted immune-endocrine response and the potential thymic repercussion as a contributing factor to disease physiopathology. Front. Endocrinol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Platt, B.; Schulenberg, J.; Klee, N.; Nizami, M.; Clark, J.A. A depressive phenotype induced by Bacille Calmette Guérin in “susceptible” animals: Sensitivity to antidepressants. Psychopharmacology 2013, 226, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Villanueva, E.; Ponce-Regalado, M.D.; Pérez-Sánchez, G.; Salazar-Juárez, A.; Arreola, R.; Álvarez-Sánchez, M.E.; Juárez-Ortega, M.; Falfán-Valencia, R.; Hernández-Pando, R.; Morales-Montor, J.; et al. Chronic infection with Mycobacterium lepraemurium induces alterations in the hippocampus associated with memory loss. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- André, C.; Dinel, A.L.; Ferreira, G.; Layé, S.; Castanon, N. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: Focus on brain indoleamine 2,3-dioxygenase activation. Brain. Behav. Immun. 2014, 41, 10–21. [Google Scholar] [CrossRef]
- Capuron, L.; Miller, A.H. Immune system to brain signaling: Neuropsychopharmacological implications. Pharmacol. Ther. 2011, 130, 226–238. [Google Scholar] [CrossRef]
- Barrios-Payán, J.; Revuelta, A.; Mata-Espinosa, D.; Marquina-Castillo, B.; Villanueva, E.B.; Gutiérrez, M.E.H.; Pérez-Sánchez, G.; Pavón, L.; Hernandez-Pando, R. The contribution of the sympathetic nervous system to the immunopathology of experimental pulmonary tuberculosis. J. Neuroimmunol. 2016, 298, 98–105. [Google Scholar] [CrossRef]
- Hernandez Pando, R.; Aguilar, D.; Cohen, I.; Guerrero, M.; Ribon, W.; Acosta, P.; Orozco, H.; Marquina, B.; Salinas, C.; Rembao, D.; et al. Specific bacterial genotypes of Mycobacterium tuberculosis cause extensive dissemination and brain infection in an experimental model. Tuberculosis 2010, 90, 268–277. [Google Scholar] [CrossRef]
- Zhou, X.; Gan, T.; Fang, G.; Wang, S.; Mao, Y.; Ying, C. Zeaxanthin improved diabetes-induced anxiety and depression through inhibiting inflammation in hippocampus. Metab. Brain Dis. 2018, 33, 705–711. [Google Scholar] [CrossRef]
- Abg Abd Wahab, D.Y.; Gau, C.H.; Zakaria, R.; Muthu Karuppan, M.K.; A-Rahbi, B.S.; Abdullah, Z.; Alrafiah, A.; Abdullah, J.M.; Muthuraju, S. Review on Cross Talk between Neurotransmitters and Neuroinflammation in Striatum and Cerebellum in the Mediation of Motor Behaviour. Biomed Res. Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Koistinaho, M.; Koistinaho, J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia 2002, 40, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Han, J.; Hui, L. MAPK signaling in inflammation-associated cancer development. Protein Cell 2010, 1, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Waetzig, V.; Czeloth, K.; Hidding, U.; Mielke, K.; Kanzow, M.; Brecht, S.; Goetz, M.; Lucius, R.; Herdegen, T.; Hanisch, U.R. c-Jun N-terminal kinases (JNKs) mediate pro-inflammatory actions of microglia. Glia 2005, 50, 235–246. [Google Scholar] [CrossRef]
- Schieven, G. The Biology of p38 Kinase: A Central Role in Inflammation. Curr. Top. Med. Chem. 2005, 5, 921–928. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Santana-Martínez, R.A.; León-Contreras, J.C.; Barrera-Oviedo, D.; Pedraza-Chaverri, J.; Hernández-Pando, R.; Maldonado, P.D. Sustained Activation of JNK Induced by Quinolinic Acid Alters the BDNF/TrkB Axis in the Rat Striatum. Neuroscience 2018, 383, 22–32. [Google Scholar] [CrossRef]
- Boveri, M.; Kinsner, A.; Berezowski, V.; Lenfant, A.M.; Draing, C.; Cecchelli, R.; Dehouck, M.P.; Hartung, T.; Prieto, P.; Bal-Price, A. Highly purified lipoteichoic acid from gram-positive bacteria induces in vitro blood-brain barrier disruption through glia activation: Role of pro-inflammatory cytokines and nitric oxide. Neuroscience 2006, 137, 1193–1209. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Orozco, E.H.; Arriaga, K.; Sampieri, A.; Larriva-Sahd, J.; Madrid-Marina, V. Analysis of the local kinetics and localisation of interleukin-1α, tumour necrosis factor-α and transforming growth factor-β, during the course of experimental pulmonary tuberculosis. Immunology 1997, 90, 607–617. [Google Scholar] [CrossRef]
- Chandra, M.; Rana, P.; Chandra, K.; Arora, V.K. Tuberculosis—Depression syndemic: A public health challenge. Indian J. Tuberc. 2019, 66, 197–202. [Google Scholar] [CrossRef]
- Hernandez-Pando, R.; Orozco, H.; Honour, J.; Silva, P.; Leyva, R.; Rook, G.A.W. Adrenal changes in murine pulmonary tuberculosis; a clue to pathogenesis? FEMS Immunol. Med. Microbiol. 1995, 12, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Wollman, E.E. Les inter-relations entre le système nerveux et le système immunitaire [Relationships between the brain and the immune system]. J. Soc. Biol. 2003, 197, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.; Tizabi, Y. Neuroinflammation, Neurodegeneration and Depression. Neurotox. Res. 2013, 23, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of Cytokines across the Blood-Brain Barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef]
- Katsuura, G.; Arimura, A.; Koves, K.; Gottschall, P.E. Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1β-induced ACTH release. Am. J. Physiol.-Endocrinol. Metab. 1990, 258. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F.; Goehler, L.E. Cytokine-to-brain communication: A review & analysis of alternative mechanisms. Life Sci. 1995, 57, 1011–1026. [Google Scholar] [CrossRef]
- Jiang, N.M.; Cowan, M.; Moonah, S.N.; Petri, W.A., Jr. The Impact of Systemic Inflammation on Neurodevelopment. Trends Mol. Med. 2018, 24, 794–804. [Google Scholar] [CrossRef]
- Ugalde-Muñiz, P.; Fetter-Pruneda, I.; Navarro, L.; García, E.; Chavarría, A. Chronic Systemic Inflammation Exacerbates Neurotoxicity in a Parkinson’s Disease Model. Oxidative Med. Cell. Longev. 2020, 2020. [Google Scholar] [CrossRef]
- De Miranda, A.S.; Lacerda-Queiroz, N.; de Carvalho Vilela, M.; Rodrigues, D.H.; Rachid, M.A.; Quevedo, J.; Teixeira, A.L. Anxiety-like behavior and pro-inflammatory cytokine levels in the brain of C57BL/6 mice infected with Plasmodium berghei (strain ANKA). Neurosci. Lett. 2011, 491, 202–206. [Google Scholar] [CrossRef]
- de Queiroz, M.L.; Viel, T.A.; Papa, C.H.G.; Lescano, S.A.Z.; Chieffi, P.P. Alterações comportamentais em Rattus norvegicus coinfectados por Toxocara canis e Toxoplasma gondii. Rev. Inst. Med. Trop. Sao Paulo 2013, 55, 51–53. [Google Scholar] [CrossRef]
- Corrêa, F.M.; Chieffi, P.P.; Lescano, S.A.Z.; dos Santos, S.V. Alterações comportamentais e na memória de Mus musculus coinfectado por Toxocara canis e Toxoplasma gondii. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Dean, J.L.E.; Saklatvala, J. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 2003, 546, 37–44. [Google Scholar] [CrossRef]
- Plastira, I.; Bernhart, E.; Joshi, L.; Koyani, C.N.; Strohmaier, H.; Reicher, H.; Malle, E.; Sattler, W. MAPK signaling determines lysophosphatidic acid (LPA)-induced inflammation in microglia. J. Neuroinflamm. 2020, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Gozdz, A.; Zawadzka, M.; Ellert-Miklaszewska, A.; Lipko, M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat. Rec. 2009, 292, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Kothari, V.; Luo, Y.; Tornabene, T.; O’Neill, A.M.; Greene, M.W.; Geetha, T.; Babu, J.R. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 499–508. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, W.; Hashimoto, K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Queirós, J.; Alves, P.C.; Vicente, J.; Gortázar, C.; De La Fuente, J. Genome-wide associations identify novel candidate loci associated with genetic susceptibility to tuberculosis in wild boar. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Zheng, Y.; Fang, W.; Fan, S.; Liao, W.; Xiong, Y.; Liao, S.; Li, Y.; Xiao, S.; Liu, J. Neurotropin inhibits neuroinflammation via suppressing NF-κB and MAPKs signaling pathways in lipopolysaccharide-stimulated BV2 cells. J. Pharmacol. Sci. 2018, 136, 242–248. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhou, Q.; Xu, J.; Qian, Q.; Ni, P. Mild Endoplasmic Reticulum Stress Protects Against Astrocytic Activation and Blood-Brain Barrier Hyperpermeability. Front. Cell. Neurosci. 2018, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Van Dyken, P.; Lacoste, B. Impact of Metabolic Syndrome on Neuroinflammation and the Blood–Brain Barrier. Front. Neurosci. 2018, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Haroon, E.; Raison, C.L.; Felger, J.C. Cytokine targets in the brain: Impact on neurotransmitters and neurocircuits. Depress. Anxiety 2013, 30, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Borisov, A.S.; Majer, M.; Drake, D.F.; Pagnoni, G.; Woolwine, B.J.; Vogt, G.J.; Massung, B.; Miller, A.H. Activation of Central Nervous System Inflammatory Pathways by Interferon-Alpha: Relationship to Monoamines and Depression. Biol. Psychiatry 2009, 65, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Lawson, M.A.; André, C.; Briley, E.M.; Szegedi, S.S.; Lestage, J.; Castanon, N.; Herkenham, M.; Dantzer, R.; Kelley, K.W. Induction of IDO by Bacille Calmette-Guérin Is Responsible for Development of Murine Depressive-Like Behavior. J. Immunol. 2009, 182, 3202–3212. [Google Scholar] [CrossRef]
- Zhu, C.B.; Carneiro, A.M.; Dostmann, W.R.; Hewlett, W.A.; Blakely, R.D. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J. Biol. Chem. 2005, 280, 15649–15658. [Google Scholar] [CrossRef]
- Zhu, C.B.; Lindler, K.M.; Owens, A.W.; Daws, L.C.; Blakely, R.D.; Hewlett, W.A. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 2010, 35, 2510–2520. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2013; Volume 246. [Google Scholar]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade B: A high affinity fluorescent marker for the localisation of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]
- Yang, J.X.; Jiang, Y.Y.; Guo, Y.B. Measuring blood-brain-barrier permeability using evans blue in mice. BioProtocol 5 2015. [Google Scholar] [CrossRef]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Castagné, V.; Moser, P.; Roux, S.; Porsolt, R.D. Rodent Models of Depression: Forced Swim and Tail Suspension Behavioral Despair Tests in Rats and Mice. Curr. Protoc. Pharmacol. 2010, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Multani, P.K.; Saini, N.; Kaur, R.; Sharma, P. Biomarkers for Drugs of Abuse and Neuropsychiatric Disorders: Models and Mechanisms; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124046306. [Google Scholar]
- Kaesermann, H.P. Stretched attend posture, a non-social form of ambivalence, is sensitive to a conflict-reducing drug action. Psychopharmacology 1986, 89, 31–37. [Google Scholar] [CrossRef]
- Holly, K.S.; Orndorff, C.O.; Murray, T.A. MATSAP: An automated analysis of stretch-Attend posture in rodent behavioral experiments. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Stahel, P.F.; Shohami, E.; Younis, F.M.; Kariya, K.; Otto, V.I.; Lenzlinger, P.M.; Grosjean, M.B.; Eugster, H.P.; Trentz, O.; Kossmann, T.; et al. Experimental closed head injury: Analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J. Cereb. Blood Flow Metab. 2000, 20, 369–380. [Google Scholar] [CrossRef]
- Ennaceur, A.; Neave, N.; Aggleton, J.P. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp. Brain Res. 1997, 113, 509–519. [Google Scholar] [CrossRef]







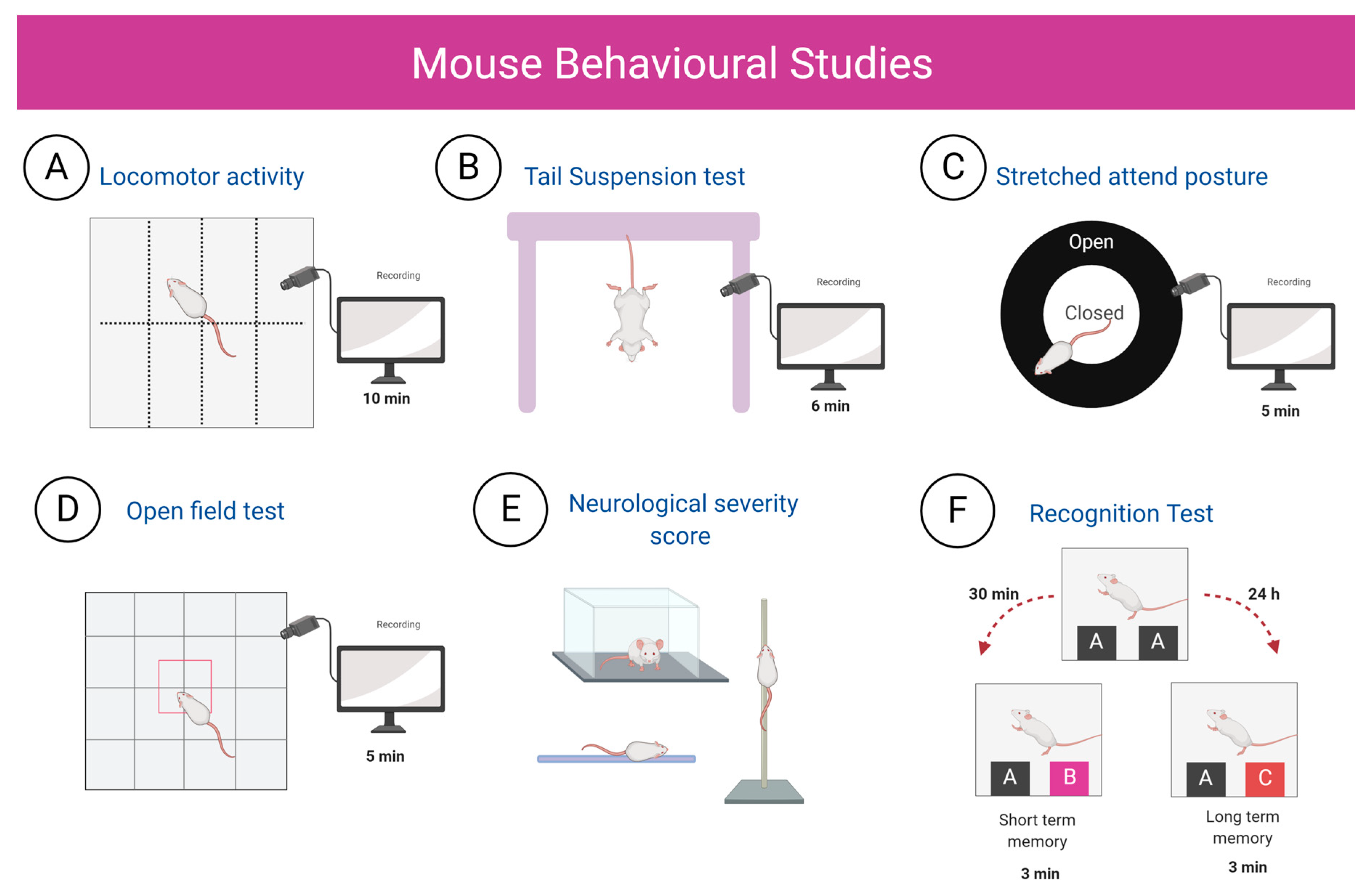
| LMA | TST | SAP | OFT | NSS | STM | LTM | |
|---|---|---|---|---|---|---|---|
| Hypothalamus | r | r | r | r | r | r | r |
| TNFa | 0.8833 ** | 0.7889 * | 0.7559 * | −0.07808 | 0.7317 * | 0.1095 | 0.3309 |
| IL12 | 0.8184 * | 0.5717 | 0.4409 | −0.1747 | 0.6993 | −0.3207 | −0.01169 |
| IFNg | 0.7815 * | 0.6809 | 0.7112 * | −0.09366 | 0.6088 | 0.08314 | 0.3786 |
| IL4 | 0.7899 * | 0.6198 | 0.7099 * | 0.01956 | 0.7502 * | −0.2002 | 0.2053 |
| TGFb | 0.6945 | 0.5908 | 0.399 | 0.01386 | 0.6948 | −0.2504 | 0.177 |
| iNOS | 0.02219 | 0.1924 | 0.4995 | 0.3337 | 0.2651 | −0.1323 | 0.2914 |
| IDO | 0.6636 | 0.4863 | 0.5386 | 0.6636 | 0.6561 | −0.3265 | 0.08468 |
| Hippocampus | r | r | r | r | r | r | r |
| TNFa | −0.1889 | 0.9552 ** | 0.9348 * | 0.9710 ** | 0.2837 | −0.7992 * | −0.8205 * |
| IL12 | −0.04769 | 0.8692 * | 0.7412 * | 0.9112 ** | 0.4015 | −0.7820 * | −0.7183 * |
| IFNg | 0.495 | 0.631 | 0.7785 * | 0.9417 ** | 0.4031 | 0.2029 | 0.2087 |
| IL4 | −0.1975 | −0.3987 | −0.5243 | −0.5828 | −0.1602 | −0.3492 | −0.2862 |
| TGFb | −0.3224 | 0.05267 | −0.4841 | 0.06559 | 0.2175 | −0.6465 | −0.905 ** |
| iNOS | 0.4823 | 0.6369 | 0.361 | −0.1349 | 0.4144 | 0.1765 | −0.805 ** |
| IDO | 0.06284 | 0.4678 | −0.1296 | −0.01114 | 0.4456 | −0.3844 | −0.5953 |
| Cerebellum | r | r | r | r | r | r | r |
| TNFa | 0.8385 ** | 0.7098 * | 0.42 | −0.2635 | 0.6887 | −0.2252 | 0.04278 |
| IL12 | 0.7584 * | 0.7564 * | 0.4769 | −0.2273 | 0.5967 | 0.04006 | 0.1963 |
| IFNg | 0.5856 | 0.3561 | 0.1751 | −0.1976 | 0.513 | −0.4544 | −0.1623 |
| IL4 | 0.7871 * | 0.7646 * | 0.4776 | −0.2379 | 0.624 | −0.00214 | 0.1755 |
| TGFb | 0.7687 * | 0.5827 | 0.3275 | −0.2482 | 0.647 | −0.3513 | −0.05443 |
| IL10 | −0.3965 | −0.03638 | −0.3613 | −0.2648 | −0.4922 | 0.4757 | 0.07207 |
| iNOS | 0.5856 | 0.3561 | 0.1751 | −0.1976 | 0.513 | −0.4544 | −0.1623 |
| IDO | 0.5856 | 0.3561 | 0.1751 | −0.1976 | 0.513 | −0.4544 | −0.1623 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Espinosa, J.V.; Santana-Martínez, R.A.; Maldonado, P.D.; Zetter, M.; Becerril-Villanueva, E.; Pérez-Sánchez, G.; Pavón, L.; Mata-Espinosa, D.; Barrios-Payán, J.; López-Torres, M.O.; et al. Experimental Pulmonary Tuberculosis in the Absence of Detectable Brain Infection Induces Neuroinflammation and Behavioural Abnormalities in Male BALB/c Mice. Int. J. Mol. Sci. 2020, 21, 9483. https://doi.org/10.3390/ijms21249483
Lara-Espinosa JV, Santana-Martínez RA, Maldonado PD, Zetter M, Becerril-Villanueva E, Pérez-Sánchez G, Pavón L, Mata-Espinosa D, Barrios-Payán J, López-Torres MO, et al. Experimental Pulmonary Tuberculosis in the Absence of Detectable Brain Infection Induces Neuroinflammation and Behavioural Abnormalities in Male BALB/c Mice. International Journal of Molecular Sciences. 2020; 21(24):9483. https://doi.org/10.3390/ijms21249483
Chicago/Turabian StyleLara-Espinosa, Jacqueline V., Ricardo A. Santana-Martínez, Perla D. Maldonado, Mario Zetter, Enrique Becerril-Villanueva, Gilberto Pérez-Sánchez, Lenin Pavón, Dulce Mata-Espinosa, Jorge Barrios-Payán, Manuel O. López-Torres, and et al. 2020. "Experimental Pulmonary Tuberculosis in the Absence of Detectable Brain Infection Induces Neuroinflammation and Behavioural Abnormalities in Male BALB/c Mice" International Journal of Molecular Sciences 21, no. 24: 9483. https://doi.org/10.3390/ijms21249483
APA StyleLara-Espinosa, J. V., Santana-Martínez, R. A., Maldonado, P. D., Zetter, M., Becerril-Villanueva, E., Pérez-Sánchez, G., Pavón, L., Mata-Espinosa, D., Barrios-Payán, J., López-Torres, M. O., Marquina-Castillo, B., & Hernández-Pando, R. (2020). Experimental Pulmonary Tuberculosis in the Absence of Detectable Brain Infection Induces Neuroinflammation and Behavioural Abnormalities in Male BALB/c Mice. International Journal of Molecular Sciences, 21(24), 9483. https://doi.org/10.3390/ijms21249483











