Interferon Beta Activity Is Modulated via Binding of Specific S100 Proteins
Abstract
1. Introduction
2. Results and Discussion
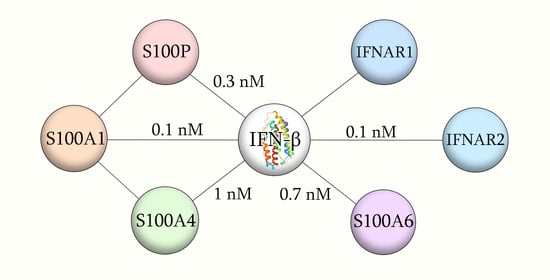
2.1. Conformation-Dependent Interactions between IFN-β and Specific S100 Proteins
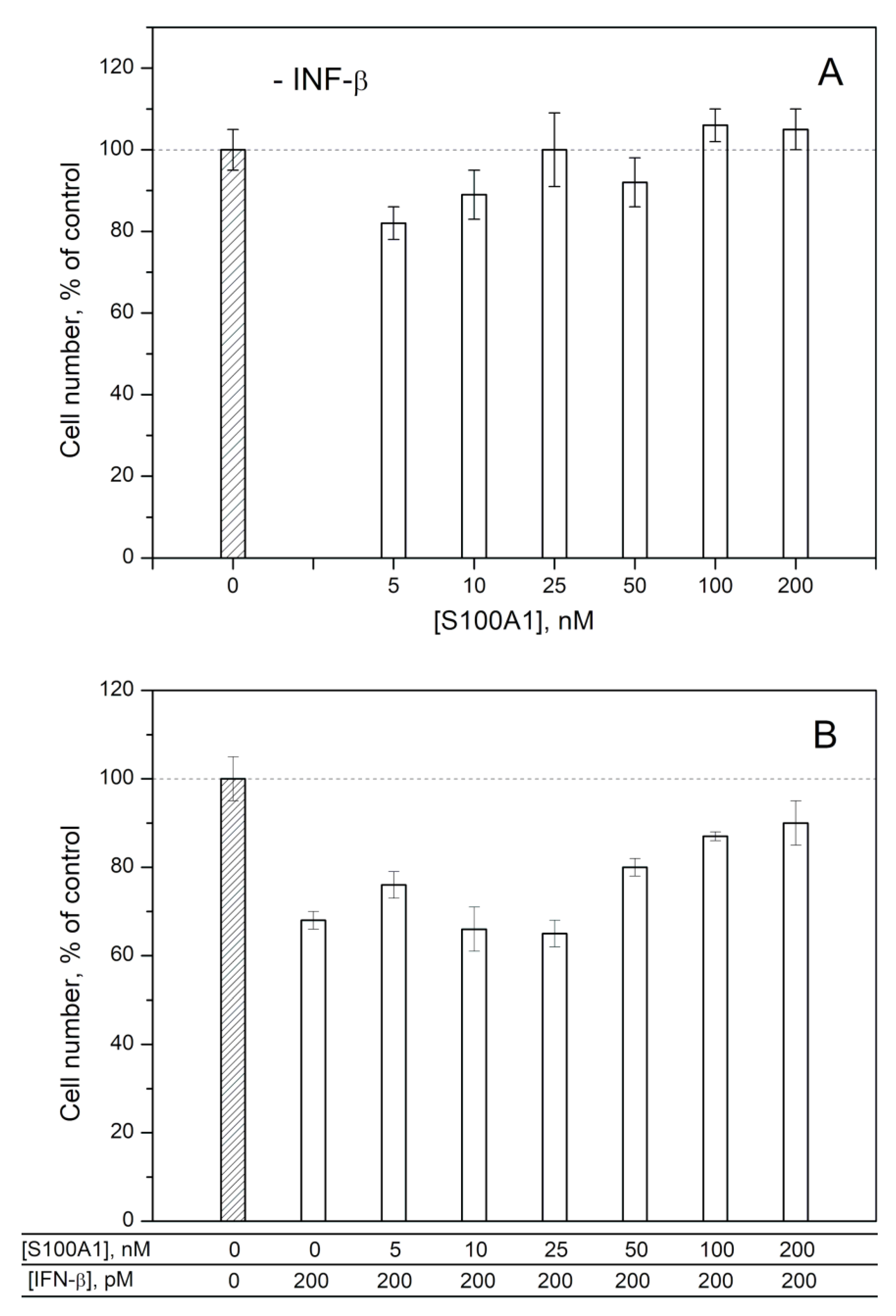
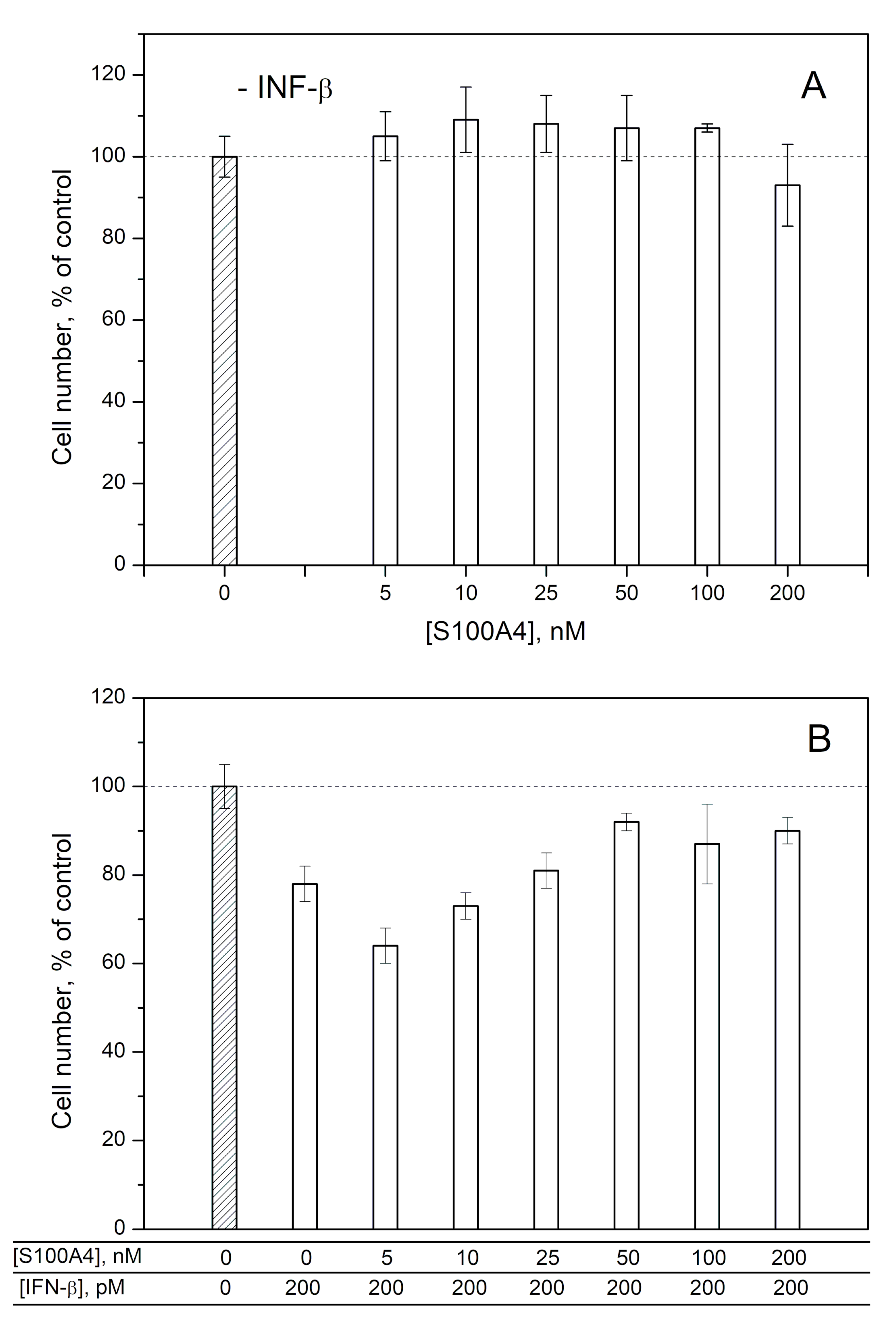
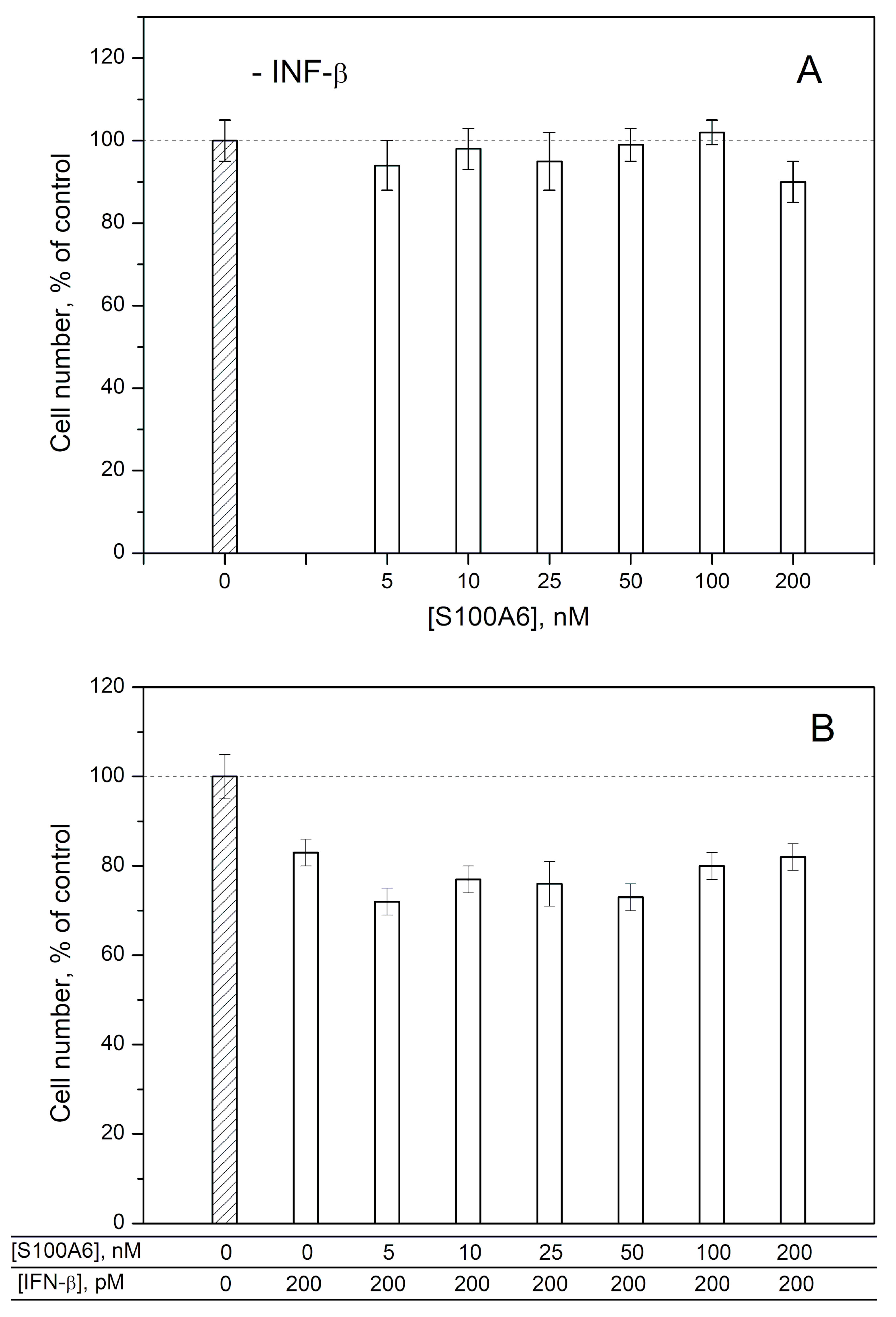
2.2. Modulation of IFN-β Cytotoxicity towards MCF-7 Cells by S100A1/A4/A6
2.3. Human Diseases Associated to Dysregulation of IFN-β and S100A1/A4/A6
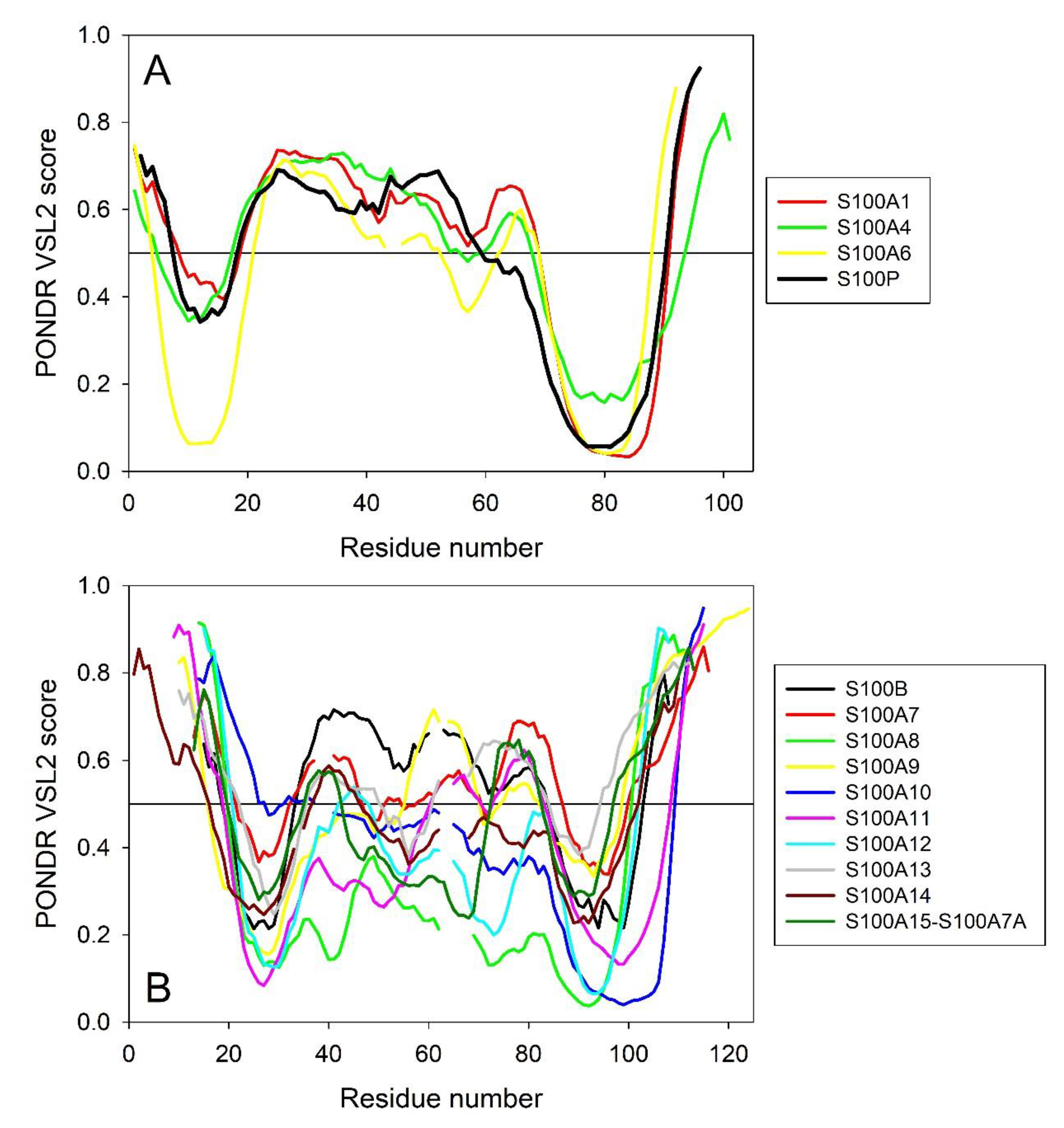
2.4. Intrinsic Disorder Predisposition of Human S100 Proteins
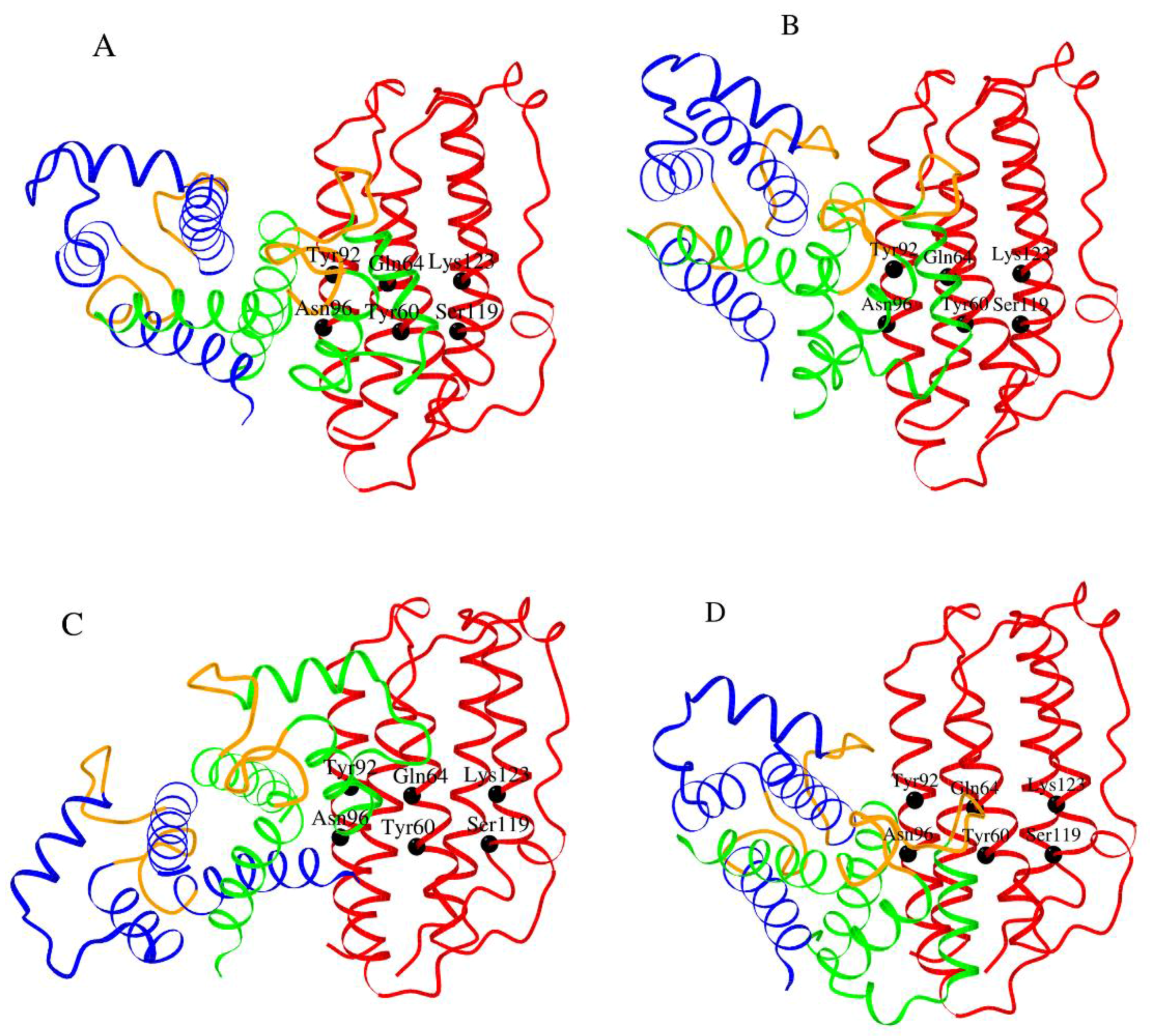
2.5. Modeling of S100—IFN-β Complexes
3. Materials and Methods
3.1. Materials
3.2. Construction of Plasmids
3.3. Expression and Purification of S100 Proteins
3.4. Surface Plasmon Resonance Studies
L1 + A ↔ L1A; L2 + A ↔ L2A
kd1 kd2
3.5. Chemical Crosslinking
3.6. Cell Viability Studies
3.7. Search of Diseases Associated to IFN-β and S100 Proteins
3.8. Evaluation of Intrinsic Disorder Predispositions of S100 Proteins
3.9. Multiple Sequence Alignments of S100 Proteins
3.10. Modeling of S100—IFN-β Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DTT | 1,4-dithiothreitol |
| EDAC | N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide |
| EDTA | ethylenediaminetetraacetic acid |
| EGTA | ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid |
| FBS | fetal bovine serum |
| HEPES | 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid |
| IFN-α | interferon alpha |
| IFN-β | interferon beta |
| IPTG | isopropyl-β-d-thiogalactoside |
| mAbs | monoclonal antibodies |
| MS | multiple sclerosis |
| OTP | Open Targets Platform |
| PBS | phosphate-buffered saline |
| PDB | Protein Data Bank |
| PMSF | phenylmethylsulfonyl fluoride; 2-ME, 2-mercaptoethanol |
| RA | rheumatoid arthritis |
| RAGE | receptor for advanced glycation end products |
| RU | resonance unit |
| SDS-PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SPR | surface plasmon resonance |
| sulfo-NHS | N-hydroxysulfosuccinimide |
| Tricine | N-[tris(hydroxymethyl)methyl]glycine |
| Tris | tris(hydroxymethyl)aminomethane |
| Usp2 | mouse ubiquitin specific peptidase 2 |
References
- Ali, S.; Mann-Nuttel, R.; Schulze, A.; Richter, L.; Alferink, J.; Scheu, S. Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver’s Seat. Front. Immunol. 2019, 10, 778. [Google Scholar] [CrossRef]
- Perkins, D.J.; Vogel, S.N. Space and time: New considerations about the relationship between Toll-like receptors (TLRs) and type I interferons (IFNs). Cytokine 2015, 74, 171–174. [Google Scholar] [CrossRef]
- Abdolvahab, M.H.; Mofrad, M.R.K.; Schellekens, H. Interferon Beta: From Molecular Level to Therapeutic Effects. Int. Rev. Cell Mol. Biol. 2016, 326, 343–372. [Google Scholar]
- Khsheibun, R.; Paperna, T.; Volkowich, A.; Lejbkowicz, I.; Avidan, N.; Miller, A. Gene Expression Profiling of the Response to Interferon Beta in Epstein-Barr-Transformed and Primary B Cells of Patients with Multiple Sclerosis. PLoS ONE 2014, 9, e102331. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef] [PubMed]
- de Weerd, N.A.; Vivian, J.P.; Nguyen, T.K.; Mangan, N.E.; Gould, J.A.; Braniff, S.J.; Zaker-Tabrizi, L.; Fung, K.Y.; Forster, S.C.; Beddoe, T.; et al. Structural basis of a unique interferon-beta signaling axis mediated via the receptor IFNAR1. Nat. Immunol. 2013, 14, 901. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Gong, M.J.; Zhao, F.R.; Shao, J.J.; Xie, Y.L.; Zhang, Y.G.; Chang, H.Y. Type I Interferons: Distinct Biological Activities and Current Applications for Viral Infection. Cell. Physiol. Biochem. 2018, 51, 2377–2396. [Google Scholar] [CrossRef]
- Medrano, R.F.V.; Hunger, A.; Mendonca, S.A.; Barbuto, J.A.M.; Strauss, B.E. Immunomodulatory and antitumor effects of type I interferons and their application in cancer therapy. Oncotarget 2017, 8, 71249–71284. [Google Scholar] [CrossRef]
- Boxx, G.M.; Cheng, G.H. The Roles of Type I Interferon in Bacterial Infection. Cell Host Microbe 2016, 19, 760–769. [Google Scholar] [CrossRef]
- Bolivar, S.; Anfossi, R.; Humeres, C.; Vivar, R.; Boza, P.; Munoz, C.; Pardo-Jimenez, V.; Olivares-Silva, F.; Diaz-Araya, G. IFN-beta Plays Both Pro- and Anti-inflammatory Roles in the Rat Cardiac Fibroblast Through Differential STAT Protein Activation. Front. Pharmacol. 2018, 9, 1368. [Google Scholar] [CrossRef]
- Jakimovski, D.; Kolb, C.; Ramanathan, M.; Zivadinov, R.; Weinstock-Guttman, B. Interferon beta for Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a032003. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhao, M.; Chang, C.; Wu, H.; Lu, Q. Type I Interferons in the Pathogenesis and Treatment of Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2020, 59, 248–272. [Google Scholar] [CrossRef]
- Huard, C.; Gulla, S.V.; Bennett, D.V.; Coyle, A.J.; Vleugels, R.A.; Greenberg, S.A. Correlation of cutaneous disease activity with type 1 interferon gene signature and interferon in dermatomyositis. Br. J. Dermatol. 2017, 176, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Catalina, M.D.; Bachali, P.; Geraci, N.S.; Grammer, A.C.; Lipsky, P.E. Gene expression analysis delineates the potential roles of multiple interferons in systemic lupus erythematosus. Commun. Biol. 2019, 2, 140. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Kanda, T.; Nakamoto, S.; Haga, Y.; Nakamura, M.; Yasui, S.; Jiang, X.; Wu, S.; Arai, M.; Yokosuka, O. Natural interferon-beta treatment for patients with chronic hepatitis C in Japan. World J. Hepatol. 2015, 7, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, M.; Uchida, T.; Hiraga, N.; Kan, H.; Makokha, G.N.; Abe-Chayama, H.; Miki, D.; Imamura, M.; Ochi, H.; Hayes, C.N.; et al. Development of a Novel Site-Specific Pegylated Interferon Beta for Antiviral Therapy of Chronic Hepatitis B Virus. Antimicrob. Agents Chemother. 2017, 61, e00183-17. [Google Scholar] [CrossRef] [PubMed]
- Baroutjian, A.; Sanchez, C.; Boneva, D.; McKenney, M.; Elkbuli, A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am. J. Emerg. Med. 2020, 38, 2405–2415. [Google Scholar] [CrossRef]
- Konde, M.K.; Baker, D.P.; Traore, F.A.; Sow, M.S.; Camara, A.; Barry, A.A.; Mara, D.; Barry, A.; Cone, M.; Kaba, I.; et al. Interferon β-1a for the treatment of Ebola virus disease: A historically controlled, single-arm proof-of-concept trial. PLoS ONE 2017, 12, e0169255. [Google Scholar] [CrossRef]
- Watson, A.; Spalluto, C.M.; McCrae, C.; Cellura, D.; Burke, H.; Cunoosamy, D.; Freeman, A.; Hicks, A.; Huhn, M.; Ostridge, K.; et al. Dynamics of IFN-beta Responses during Respiratory Viral Infection. Insights for Therapeutic Strategies. Am. J. Respir. Crit. Care Med. 2020, 201, 83–94. [Google Scholar] [CrossRef]
- Kaplan, A.; Lee, M.W.; Wolf, A.J.; Limon, J.J.; Becker, C.A.; Ding, M.; Murali, R.; Lee, E.Y.; Liu, G.Y.; Wong, G.C.L.; et al. Direct Antimicrobial Activity of IFN-beta. J. Immunol. 2017, 198, 4036–4045. [Google Scholar] [CrossRef]
- Sabir, N.; Hussain, T.; Shah, S.Z.A.; Zhao, D.; Zhou, X. IFN-beta: A Contentious Player in Host-Pathogen Interaction in Tuberculosis. Int. J. Mol. Sci. 2017, 18, 2725. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.J.; Rajaiah, R.; Tennant, S.M.; Ramachandran, G.; Higginson, E.E.; Dyson, T.N.; Vogel, S.N. Salmonella Typhimurium Co-Opts the Host Type I IFN System To Restrict Macrophage Innate Immune Transcriptional Responses Selectively. J. Immunol. 2015, 195, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Borden, E.C. Interferons alpha and beta in cancer: Therapeutic opportunities from new insights. Nat. Rev. Drug Discov. 2019, 18, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Abdolvahab, M.H.; Darvishi, B.; Zarei, M.; Majidzadeh, A.K.; Farahmand, L. Interferons: Role in cancer therapy. Immunotherapy 2020, 12, 833–855. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Fu, M.L.; Weichselbaum, R.R.; Gajewski, T.F.; Guo, Y.; Fu, Y.X. Targeting the tumor microenvironment with interferon-beta bridges innate and adaptive immune responses. Cancer Cell 2014, 25, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, C.; Bertagna, M.; Cohen, M. Cutaneous Side-effects of Immunomodulators in MS. Int. Ms J. 2011, 17, 88–94. [Google Scholar]
- Durelli, L.; Ferrero, B.; Oggero, A.; Verdun, E.; Ghezzi, A.; Montanari, E.; Zaffaroni, M. Liver and thyroid function and autoimmunity during interferon-beta 1b treatment for MS. Neurology 2001, 57, 1363–1370. [Google Scholar] [CrossRef]
- Reder, A.T.; Oger, J.F.; Kappos, L.; O’Connor, P.; Rametta, M. Short-term and long-term safety and tolerability of interferon beta-1b in multiple sclerosis. Mult. Scler. Relat. Disord. 2014, 3, 294–302. [Google Scholar] [CrossRef]
- Lamken, P.; Lata, S.; Gavutis, M.; Piehler, J. Ligand-induced assembling of the type I interferon receptor on supported lipid bilayers. J. Mol. Biol. 2004, 341, 303–318. [Google Scholar] [CrossRef]
- Kazakov, A.S.; Mayorov, S.A.; Deryusheva, E.I.; Avkhacheva, N.V.; Denessiouk, K.A.; Denesyuk, A.I.; Rastrygina, V.A.; Permyakov, E.A.; Permyakov, S.E. Highly specific interaction of monomeric S100P protein with interferon beta. Int. J. Biol. Macromol. 2020, 143, 633–639. [Google Scholar] [CrossRef]
- Prica, F.; Radon, T.; Cheng, Y.; Crnogorac-Jurcevic, T. The life and works of S100P-from conception to cancer. Am. J. Cancer Res. 2016, 6, 562–576. [Google Scholar] [PubMed]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 Proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, L.L.; Garrie, K.; Turner, M.D. Role of S100 proteins in health and disease. Biochim. Biophys. Acta. Mol. Cell Res. 2020, 1867, 118677. [Google Scholar] [CrossRef] [PubMed]
- Allgower, C.; Kretz, A.L.; von Karstedt, S.; Wittau, M.; Henne-Bruns, D.; Lemke, J. Friend or Foe: S100 Proteins in Cancer. Cancers 2020, 12, 2037. [Google Scholar] [CrossRef]
- Kypriotou, M.; Huber, M.; Hohl, D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the ’fused genes’ family. Exp. Dermatol. 2012, 21, 643–649. [Google Scholar] [CrossRef]
- Consortium, T.C.S.a.A. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 2005, 437, 69–87. [Google Scholar]
- Chen, H.; Xu, C.; Jin, Q.; Liu, Z. S100 protein family in human cancer. Am. J. Cancer Res. 2014, 4, 89–115. [Google Scholar]
- Bresnick, A.R.; Weber, D.J.; Zimmer, D.B. S100 proteins in cancer. Nat. Rev. Cancer. 2015, 15, 96–109. [Google Scholar] [CrossRef]
- Donato, R.; Geczy, C.L.; Weber, D.J. S100 proteins. In Encyclopedia of Metalloproteins; Kretsinger, R.H., Uversky, V.N., Permyakov, E.A., Eds.; Springer Science+Business Media: New York, NY, USA, 2013; pp. 1863–1874. [Google Scholar]
- Bresnick, A.R. S100 proteins as therapeutic targets. Biophys. Rev. 2018, 10, 1617–1629. [Google Scholar] [CrossRef]
- Permyakov, S.E.; Denesyuk, A.I.; Denessiouk, K.A.; Permyakova, M.E.; Kazakov, A.S.; Ismailov, R.G.; Rastrygina, V.A.; Sokolov, A.S.; Permyakov, E.A. Monomeric state of S100P protein: Experimental and molecular dynamics study. Cell Calcium 2019, 80, 152–159. [Google Scholar] [CrossRef]
- Leclerc, E.; Fritz, G.; Weibel, M.; Heizmann, C.W.; Galichet, A. S100B and S100A6 differentially modulate cell survival by interacting with distinct RAGE (receptor for advanced glycation end products) immunoglobulin domains. J. Biol. Chem. 2007, 282, 31317–31331. [Google Scholar] [CrossRef] [PubMed]
- Dukhanina, E.A.; Romanova, E.A.; Dukhanin, A.S.; Kabanova, O.D.; Lukyanova, T.I.; Shatalov, Y.V.; Yashin, D.V.; Gnuchev, N.V.; Sashchenko, L.P. Interactions and possible functional characteristics of Tag7-S100A4 protein complex. Bull. Exp. Biol. Med. 2008, 145, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, S.; Fernig, D.G.; Martin-Fernandez, M.; Rudland, P.S.; Barraclough, R. Mutually antagonistic actions of S100A4 and S100A1 on normal and metastatic phenotypes. Oncogene 2005, 24, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Fritz, G.; Heizmann, C.W. 3D Structures of the Calcium and Zinc Binding S100 Proteins. In Handbook of Metalloproteins; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef]
- Rohde, D.; Schön, C.; Boerries, M.; Didrihsone, I.; Ritterhoff, J.; Kubatzky, K.F.; Völkers, M.; Herzog, N.; Mähler, M.; Tsoporis, J.N.; et al. S100A1 is released from ischemic cardiomyocytes and signals myocardial damage via Toll-like receptor 4. EMBO Mol. Med. 2014, 6, 778–794. [Google Scholar] [CrossRef]
- Lv, Y.; Niu, Z.; Guo, X.; Yuan, F.; Liu, Y. Serum S100 calcium binding protein A4 (S100A4, metatasin) as a diagnostic and prognostic biomarker in epithelial ovarian cancer. Br. J. Biomed. Sci. 2018, 75, 88–91. [Google Scholar] [CrossRef]
- Onsurathum, S.; Haonon, O.; Pinlaor, P.; Pairojkul, C.; Khuntikeo, N.; Thanan, R.; Roytrakul, S.; Pinlaor, S. Proteomics detection of S100A6 in tumor tissue interstitial fluid and evaluation of its potential as a biomarker of cholangiocarcinoma. Tumour. Biol. 2018, 40. [Google Scholar] [CrossRef]
- Wu, Z.; Boonmars, T.; Nagano, I.; Boonjaraspinyo, S.; Srinontong, P.; Ratasuwan, P.; Narong, K.; Nielsen, P.S.; Maekawa, Y. Significance of S100P as a biomarker in diagnosis, prognosis and therapy of opisthorchiasis-associated cholangiocarcinoma. Int. J. Cancer 2016, 138, 396–408. [Google Scholar] [CrossRef]
- Khan, O.A.; Dhib-Jalbut, S.S. Serum interferon beta-1a (Avonex) levels following intramuscular injection in relapsing-remitting MS patients. Neurology 1998, 51, 738–742. [Google Scholar] [CrossRef]
- Lindner, D.J.; Kolla, V.; Kalvakolanu, D.V.; Borden, E.C. Tamoxifen enhances interferon-regulated gene expression in breast cancer cells. Mol. Cell. Biochem. 1997, 167, 169–177. [Google Scholar] [CrossRef]
- Lindner, D.J.; Hofmann, E.R.; Karra, S.; Kalvakolanu, D.V. The interferon-β and tamoxifen combination induces apoptosis using thioredoxin reductase. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2000, 1496, 196–206. [Google Scholar] [CrossRef][Green Version]
- Ambjørn, M.; Ejlerskov, P.; Liu, Y.; Lees, M.; Jäättelä, M.; Issazadeh-Navikas, S. IFNB1/interferon-β-induced autophagy in MCF-7 breast cancer cells counteracts its proapoptotic function. Autophagy 2013, 9, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Moraga, I.; Levin, D.; Krutzik, P.O.; Podoplelova, Y.; Trejo, A.; Lee, C.; Yarden, G.; Vleck, S.E.; Glenn, J.S.; et al. Structural Linkage between Ligand Discrimination and Receptor Activation by Type I Interferons. Cell 2011, 146, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.A.; Chou, R.H.; Li, H.C.; Yang, L.W.; Yu, C. Structural insights into the interaction of human S100B and basic fibroblast growth factor (FGF2): Effects on FGFR1 receptor signaling. Biochim. Biophys. Acta Proteins Proteom. 2013, 1834, 2606–2619. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A.; Ecsedi, P.; Kovacs, G.M.; Poti, A.L.; Remenyi, A.; Kardos, J.; Gogl, G.; Nyitray, L. High-throughput competitive fluorescence polarization assay reveals functional redundancy in the S100 protein family. FEBS J. 2020, 287, 2834–2846. [Google Scholar] [CrossRef] [PubMed]
- Tovchigrechko, A.; Vakser, I.A. GRAMM-X public web server for protein-protein docking. Nucleic Acids Res. 2006, 34, W310–W314. [Google Scholar] [CrossRef]
- Holm, L. DALI and the persistence of protein shape. Protein Sci. 2020, 29, 128–140. [Google Scholar] [CrossRef]
- Sobolev, V.; Sorokine, A.; Prilusky, J.; Abola, E.E.; Edelman, M. Automated analysis of interatomic contacts in proteins. Bioinformatics 1999, 15, 327–332. [Google Scholar] [CrossRef]
- Kazakov, A.S.; Sokolov, A.S.; Vologzhannikova, A.A.; Permyakova, M.E.; Khorn, P.A.; Ismailov, R.G.; Denessiouk, K.A.; Denesyuk, A.I.; Rastrygina, V.A.; Baksheeva, V.E.; et al. Interleukin-11 binds specific EF-hand proteins via their conserved structural motifs. J. Biomol. Struct. Dyn. 2017, 35, 78–91. [Google Scholar] [CrossRef]
- Baker, R.T.; Catanzariti, A.M.; Karunasekara, Y.; Soboleva, T.A.; Sharwood, R.; Whitney, S.; Board, P.G. Using deubiquitylating enzymes as research tools. Ubiquitin Protein Degrad. Part A 2005, 398, 540–554. [Google Scholar]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, A.M.; Soboleva, T.A.; Jans, D.A.; Board, P.G.; Baker, R.T. An efficient system for high-level expression and easy purification of authentic recombinant proteins. Protein Sci. 2004, 13, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.S.; Kazakov, A.S.; Solovyev, V.V.; Ismailov, R.G.; Uversky, V.N.; Lapteva, Y.S.; Mikhailov, R.V.; Pavlova, E.V.; Terletskaya, I.O.; Ermolina, L.V.; et al. Expression, Purification, and Characterization of Interleukin-11 Orthologues. Molecules 2016, 21, 1632. [Google Scholar] [CrossRef] [PubMed]
- Pinero, J.; Bravo, A.; Queralt-Rosinach, N.; Gutierrez-Sacristan, A.; Deu-Pons, J.; Centeno, E.; Garcia-Garcia, J.; Sanz, F.; Furlong, L.I. DisGeNET: A comprehensive platform integrating information on human disease-associated genes and variants. Nucleic Acids Res. 2017, 45, D833–D839. [Google Scholar] [CrossRef]
- Carvalho-Silva, D.; Pierleoni, A.; Pignatelli, M.; Ong, C.; Fumis, L.; Karamanis, N.; Carmona, M.; Faulconbridge, A.; Hercules, A.; McAuley, E.; et al. Open Targets Platform: New developments and updates two years on. Nucleic Acids Res. 2019, 47, D1056–D1065. [Google Scholar] [CrossRef]
- Peng, K.; Vucetic, S.; Radivojac, P.; Brown, C.J.; Dunker, A.K.; Obradovic, Z. Optimizing long intrinsic disorder predictors with protein evolutionary information. J. Bioinform. Comput. Biol. 2005, 3, 35–60. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Kraulis, P.J. Molscript-a Program to Produce Both Detailed and Schematic Plots of Protein Structures. J. Appl. Crystallogr. 1991, 24, 946–950. [Google Scholar] [CrossRef]
- Zimmer, D.B.; Eubanks, J.O.; Ramakrishnan, D.; Criscitiello, M.F. Evolution of the S100 family of calcium sensor proteins. Cell Calcium 2013, 53, 170–179. [Google Scholar] [CrossRef]
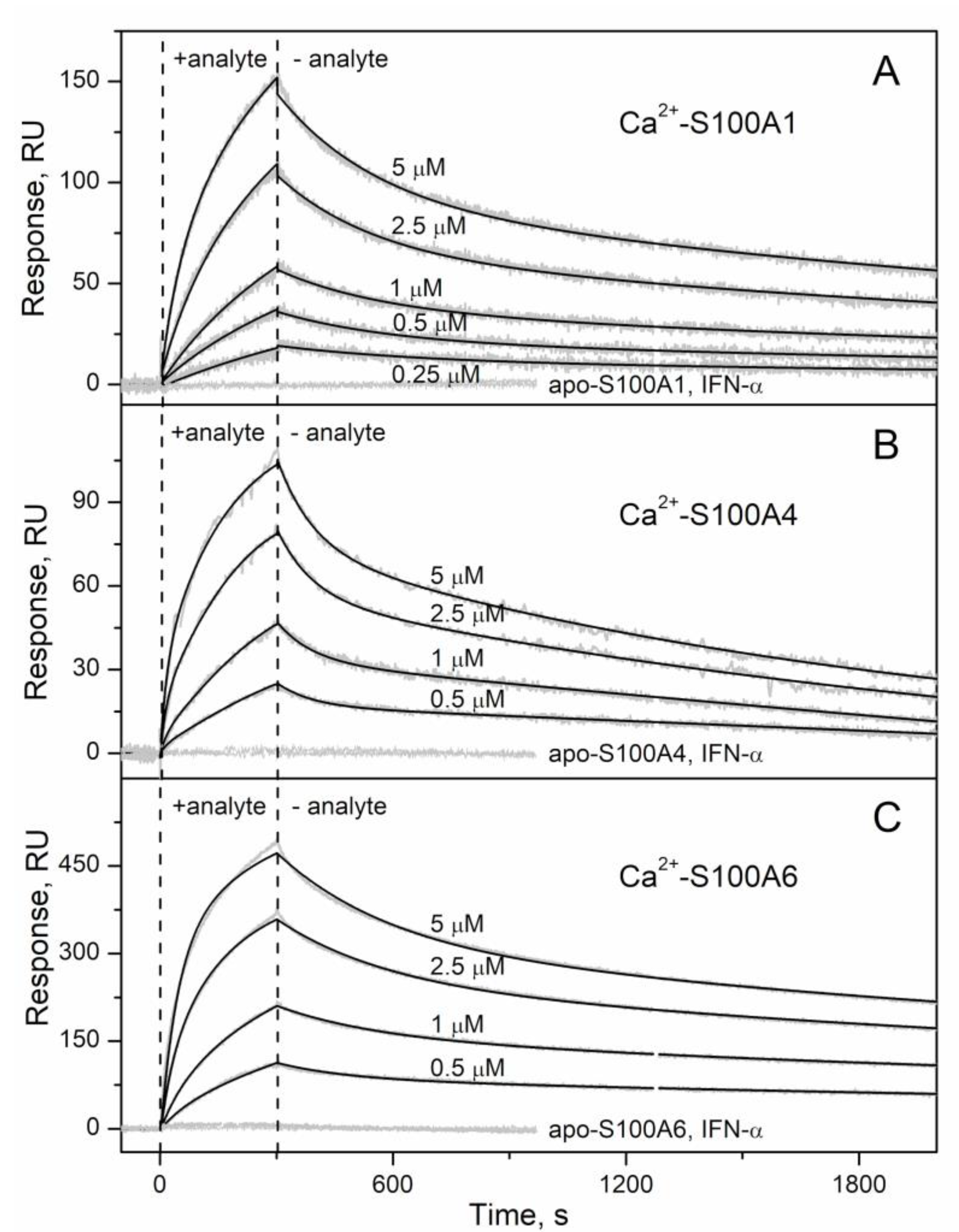
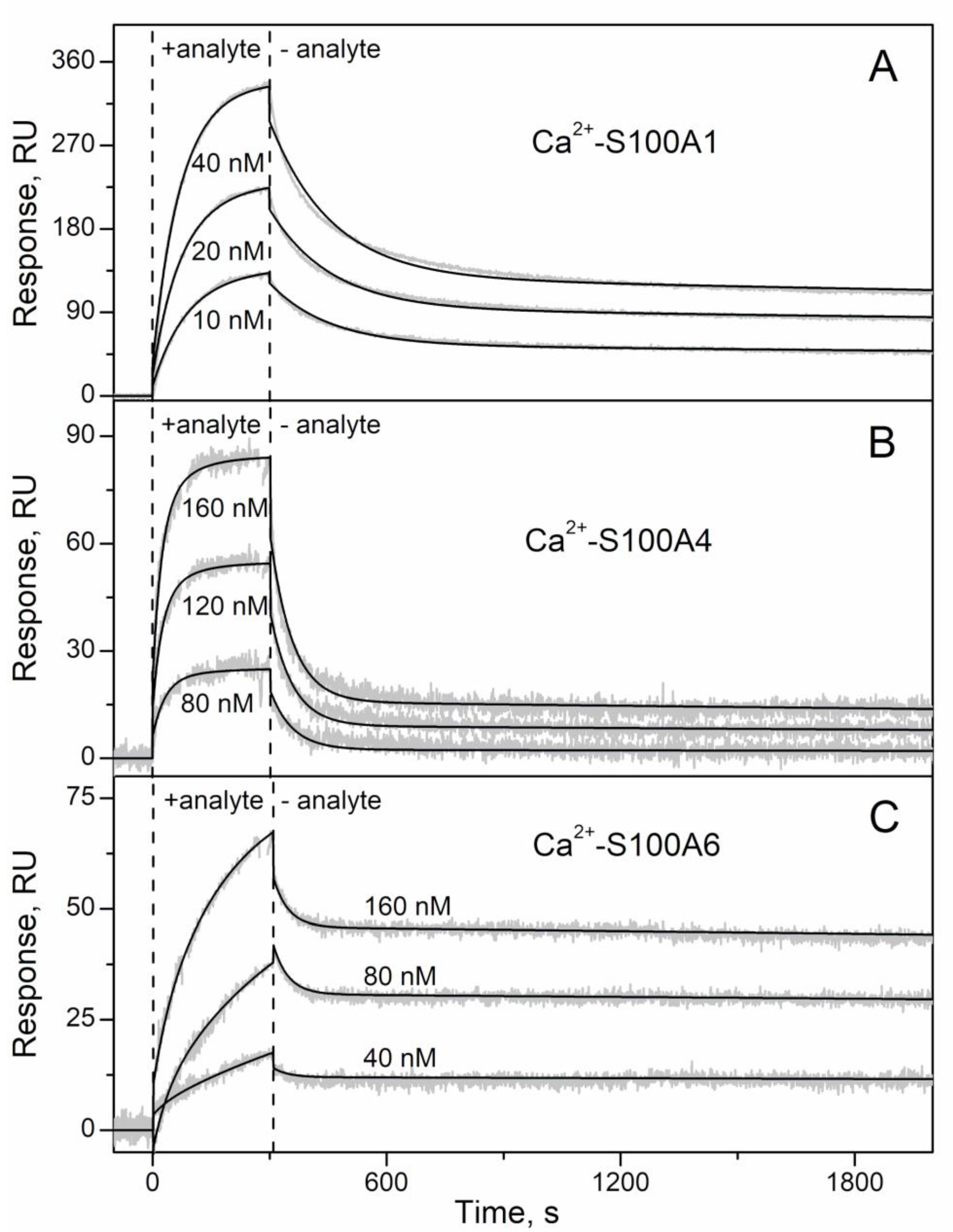


| Analyte | Ligand | kd1, s−1 | Kd1, nM | Rmax1 | kd2, s−1 | Kd2, nM | Rmax2 |
|---|---|---|---|---|---|---|---|
| S100A1 | IFN-β | (2.71 ± 0.13) × 10−4 | 41 ± 12 | 190 | (3.65 ± 0.54) × 10−3 | 1450 ± 544 | 68 |
| S100A4 | (5.97 ± 0.22) × 10−4 | 227 ± 19 | 98 | (1.08 ± 0.53) × 10−2 | 443 ± 249 | 38 | |
| S100A6 | (2.10 ± 0.57) × 10−4 | 82 ± 24 | 313 | (4.07 ± 0.97) × 10−3 | 267 ± 57 | 81 | |
| IFN-β | S100A1 | (6.46 ± 0.10) × 10−3 | 47 ± 10 | 361 | (6.80 ± 0.53) × 10−5 | 0.11 ± 0.06 | 108 |
| S100A4 | (1.75 ± 0.24) × 10−2 | 105 ± 11 | 112 | (8.03 ± 0.26) × 10−5 | 1.0 ± 0.1 | 19 | |
| S100A6 | (2.10 ± 0.57) × 10−5 | 0.70 ± 0.03 | 43 | (2.60 ± 0.27) × 10−2 | 281 ± 49 | 20 |
| Diseases Associated with S100A1 and IFN-β | Diseases Associated with S100A4 and IFN-β | Diseases Associated with S100A6 and IFN-β | Diseases Associated with S100P and IFN-β |
|---|---|---|---|
| neoplasm, cancer, carcinoma (lung, adenocarcinoma), infectious disease, vascular disease, pulmonary arterial hypertension, nervous system disease, heart disease, cardiomyopathy, hypertension | neoplasm (liver, lung, breast, skin, brain, ovarian), glioma, astrocytoma, cancer (lung, central nervous system, breast), carcinoma (non-small cell lung, lung, ovarian, breast, adenocarcinoma), glioblastoma multiforme, infectious disease, immune system disease, vascular disease, nervous system disease, neuropathy, lung disease, liver disease | neoplasm (lung), cancer (lung), carcinoma (lung), nervous system disease, lung disease | neoplasm (liver, brain), cancer (lung), carcinoma, adenocarcinoma, glioblastoma multiforme, respiratory system disease, liver disease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakov, A.S.; Sofin, A.D.; Avkhacheva, N.V.; Denesyuk, A.I.; Deryusheva, E.I.; Rastrygina, V.A.; Sokolov, A.S.; Permyakova, M.E.; Litus, E.A.; Uversky, V.N.; et al. Interferon Beta Activity Is Modulated via Binding of Specific S100 Proteins. Int. J. Mol. Sci. 2020, 21, 9473. https://doi.org/10.3390/ijms21249473
Kazakov AS, Sofin AD, Avkhacheva NV, Denesyuk AI, Deryusheva EI, Rastrygina VA, Sokolov AS, Permyakova ME, Litus EA, Uversky VN, et al. Interferon Beta Activity Is Modulated via Binding of Specific S100 Proteins. International Journal of Molecular Sciences. 2020; 21(24):9473. https://doi.org/10.3390/ijms21249473
Chicago/Turabian StyleKazakov, Alexey S., Alexander D. Sofin, Nadezhda V. Avkhacheva, Alexander I. Denesyuk, Evgenia I. Deryusheva, Victoria A. Rastrygina, Andrey S. Sokolov, Maria E. Permyakova, Ekaterina A. Litus, Vladimir N. Uversky, and et al. 2020. "Interferon Beta Activity Is Modulated via Binding of Specific S100 Proteins" International Journal of Molecular Sciences 21, no. 24: 9473. https://doi.org/10.3390/ijms21249473
APA StyleKazakov, A. S., Sofin, A. D., Avkhacheva, N. V., Denesyuk, A. I., Deryusheva, E. I., Rastrygina, V. A., Sokolov, A. S., Permyakova, M. E., Litus, E. A., Uversky, V. N., Permyakov, E. A., & Permyakov, S. E. (2020). Interferon Beta Activity Is Modulated via Binding of Specific S100 Proteins. International Journal of Molecular Sciences, 21(24), 9473. https://doi.org/10.3390/ijms21249473