The Reprimo-Like Gene Is an Epigenetic-Mediated Tumor Suppressor and a Candidate Biomarker for the Non-Invasive Detection of Gastric Cancer
Abstract
1. Introduction
2. Results
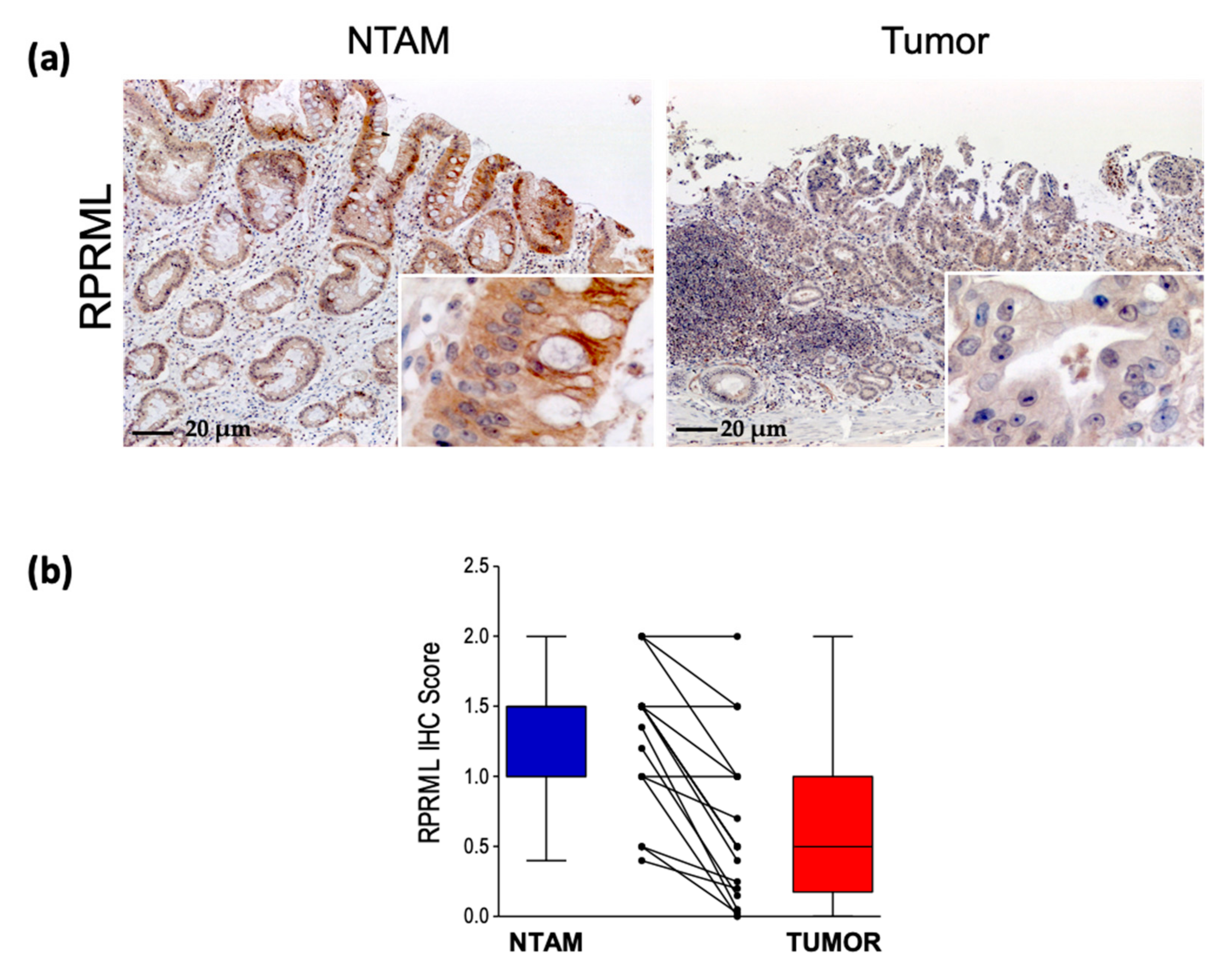
2.1. RPRML Expression in Clinical Samples
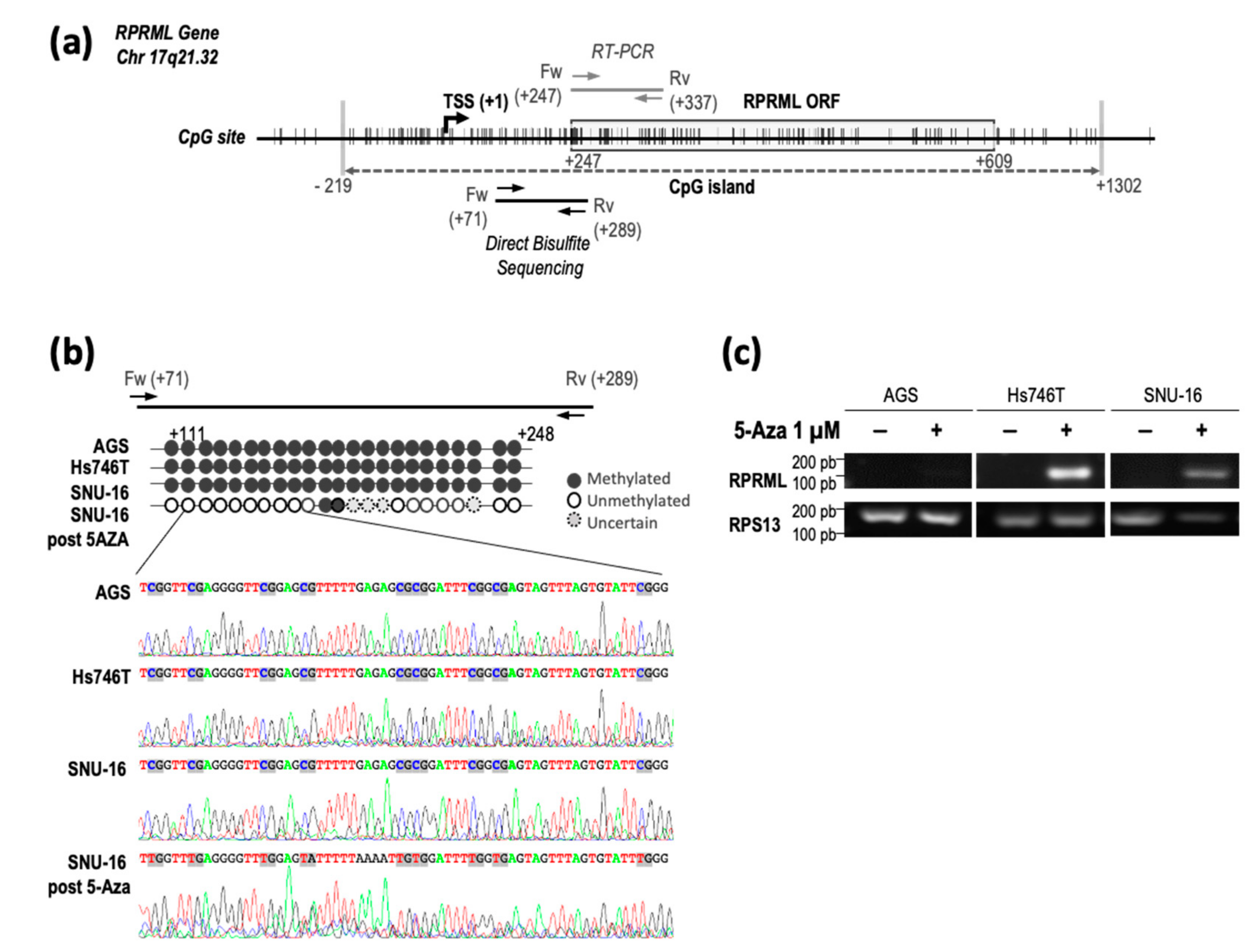
2.2. Regulatory Mechanisms of RPRML Expression in GC
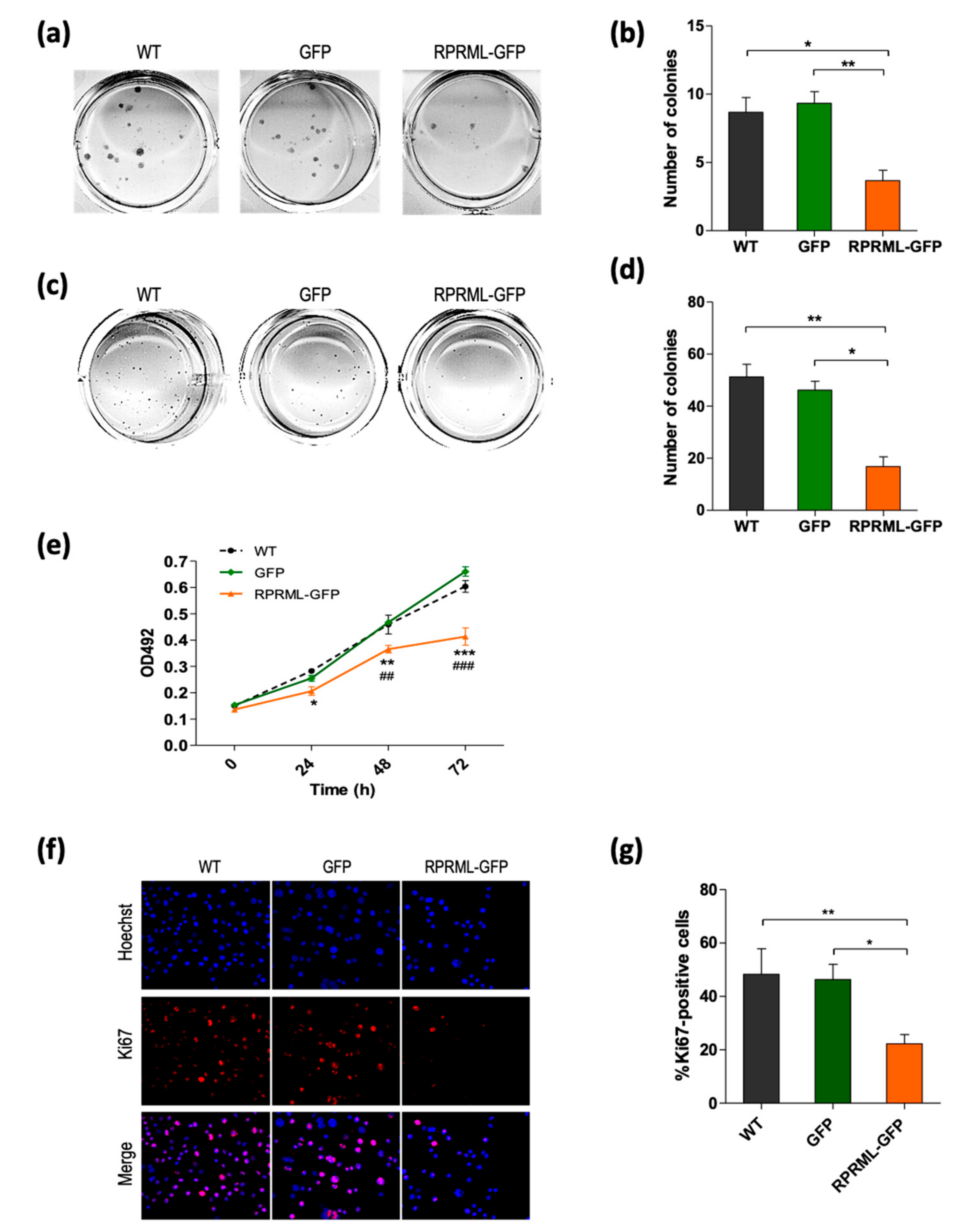
2.3. In Vitro Characterization of RPRML Functionality
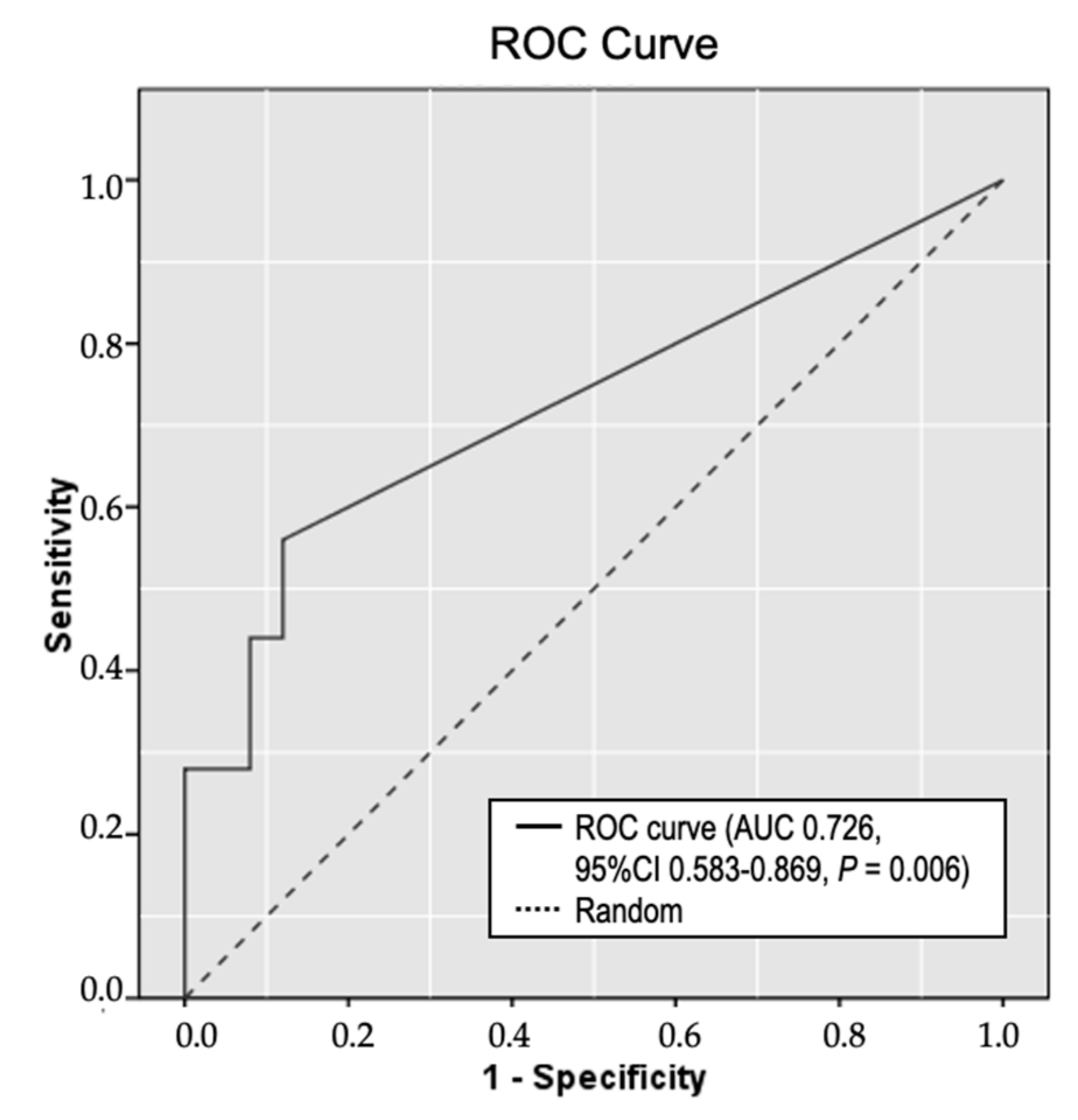
2.4. Circulating Methylated RPRML DNA in Plasma Samples for the Non-Invasive Detection of GC
3. Discussion
4. Materials and Methods
4.1. Clinical Samples and Pathological and Follow-Up Data
4.2. Immunohistochemical Analysis
4.3. Cell Culture and Transfections
4.4. Western Blot Analysis
4.5. RNA Isolation and RT-PCR
4.6. DNA Isolation and Bisulfite Modification
4.7. Genetic Screening
4.8. Direct Bisulfite Sequencing
4.9. 5-Azacytidine Assay
4.10. Colony Formation and Soft Agar Assays
4.11. MTS Assay
4.12. Ki67 Immunofluorescence
4.13. MethyLight Assay
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, Y.; Zhao, Q.; Zhao, X.; Li, Z. Identification of potential key genes in gastric cancer using bioinformatics analysis. Biomed. Rep. 2020, 12, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.S.; Lai, W.Y.X.; Lee, L.T.C.; Wong, W.L.C.; Pei, X.M.; Tsang, H.F.; Leung, J.J.; Cho, W.C.S.; Chu, M.K.M.; Wong, E.Y.L.; et al. Current and future molecular diagnostics of gastric cancer. Expert Rev. Mol. Diagn. 2019, 19, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, N.; Ushijima, T.; Tan, P. How to stomach an epigenetic insult: The gastric cancer epigenome. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.K.; Ramnarayanan, K.; Zhu, F.; Srivastava, S.; Xu, C.; Tan, A.L.K.; Lee, M.; Tay, S.; Das, K.; Xing, M.; et al. Genomic and Epigenomic Profiling of High-Risk Intestinal Metaplasia Reveals Molecular Determinants of Progression to Gastric Cancer. Cancer Cell 2018, 33, 137–150.e135. [Google Scholar] [CrossRef]
- Ho, S.W.T.; Tan, P. Dissection of gastric cancer heterogeneity for precision oncology. Cancer Sci. 2019, 110, 3405–3414. [Google Scholar] [CrossRef]
- Necula, L.; Matei, L.; Dragu, D.; Neagu, A.I.; Mambet, C.; Nedeianu, S.; Bleotu, C.; Diaconu, C.C.; Chivu-Economescu, M. Recent advances in gastric cancer early diagnosis. World J. Gastroenterol. 2019, 25, 2029–2044. [Google Scholar] [CrossRef]
- Puneet; Kazmi, H.R.; Kumari, S.; Tiwari, S.; Khanna, A.; Narayan, G. Epigenetic Mechanisms and Events in Gastric Cancer-Emerging Novel Biomarkers. Pathol. Oncol. Res. 2018, 24, 757–770. [Google Scholar] [CrossRef]
- Chen, D.; Xu, T.; Wang, S.; Chang, H.; Yu, T.; Zhu, Y.; Chen, J. Liquid Biopsy Applications in the Clinic. Mol. Diagn. Ther. 2020, 24, 125–132. [Google Scholar] [CrossRef]
- Sapari, N.S.; Loh, M.; Vaithilingam, A.; Soong, R. Clinical potential of DNA methylation in gastric cancer: A meta-analysis. PLoS ONE 2012, 7, e36275. [Google Scholar] [CrossRef]
- Lai, J.; Wang, H.; Luo, Q.; Huang, S.; Lin, S.; Zheng, Y.; Chen, Q. The relationship between DNA methylation and Reprimo gene expression in gastric cancer cells. Oncotarget 2017, 8, 108610–108623. [Google Scholar] [CrossRef] [PubMed]
- Satherley, L.K.; Ye, L.; Jiang, W.G.; Hargest, R. Tumour suppressor genes in gastric carcinogenesis: An up-todate review of genetic and epigenetic changes in the development of gastric adenocarcinoma. Transl. Gastrointest. Cancer 2015, 4. [Google Scholar] [CrossRef]
- Figueroa, R.J.; Carrasco-Avino, G.; Wichmann, I.A.; Lange, M.; Owen, G.I.; Siekmann, A.F.; Corvalan, A.H.; Opazo, J.C.; Amigo, J.D. Reprimo tissue-specific expression pattern is conserved between zebrafish and human. PLoS ONE 2017, 12, e0178274. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, I.A.; Zavala, K.; Hoffmann, F.G.; Vandewege, M.W.; Corvalan, A.H.; Amigo, J.D.; Owen, G.I.; Opazo, J.C. Evolutionary history of the reprimo tumor suppressor gene family in vertebrates with a description of a new reprimo gene lineage. Gene 2016, 591, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Opazo, P.; Valenzuela-Valderrama, M.; Wichmann, I.; Rodriguez, A.; Contreras-Reyes, D.; Fernandez, E.A.; Carrasco-Avino, G.; Corvalan, A.H.; Quest, A.F.G. Inverse expression of survivin and reprimo correlates with poor patient prognosis in gastric cancer. Oncotarget 2018, 9, 12853–12867. [Google Scholar] [CrossRef]
- Ohki, R.; Nemoto, J.; Murasawa, H.; Oda, E.; Inazawa, J.; Tanaka, N.; Taniguchi, T. Reprimo, a new candidate mediator of the p53-mediated cell cycle arrest at the G2 phase. J. Biol. Chem. 2000, 275, 22627–22630. [Google Scholar] [CrossRef]
- Saavedra, K.; Valbuena, J.; Olivares, W.; Marchant, M.J.; Rodriguez, A.; Torres-Estay, V.; Carrasco-Avino, G.; Guzman, L.; Aguayo, F.; Roa, J.C.; et al. Loss of Expression of Reprimo, a p53-induced Cell Cycle Arrest Gene, Correlates with Invasive Stage of Tumor Progression and p73 Expression in Gastric Cancer. PLoS ONE 2015, 10, e0125834. [Google Scholar] [CrossRef]
- Bernal, C.; Aguayo, F.; Villarroel, C.; Vargas, M.; Diaz, I.; Ossandon, F.J.; Santibanez, E.; Palma, M.; Aravena, E.; Barrientos, C.; et al. Reprimo as a potential biomarker for early detection in gastric cancer. Clin. Cancer Res. 2008, 14, 6264–6269. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Lai, J.; Luo, Q.; Ke, H.; Chen, Q. Methylation-Sensitive Melt Curve Analysis of the Reprimo Gene Methylation in Gastric Cancer. PLoS ONE 2016, 11, e0168635. [Google Scholar] [CrossRef]
- Amigo, J.D.; Opazo, J.C.; Garcia-Bloj, B.A.; Alarcon, M.A.; Owen, G.I.; Corvalan, A.H. The Reprimo Gene Family: A Novel Gene Lineage in Gastric Cancer with Tumor Suppressive Properties. Int. J. Mol. Sci. 2018, 19, 1862. [Google Scholar] [CrossRef]
- Consortium, T.C.G.A. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar]
- Cordova-Delgado, M.; Pinto, M.P.; Retamal, I.N.; Muñoz-Medel, M.; Bravo, M.L.; Fernández, M.F.; Cisternas, B.; Mondaca, S.; Sanchez, C.; Galindo, H.; et al. High Proportion of Potential Candidates for Immunotherapy in a Chilean Cohort of Gastric Cancer Patients: Results of the FORCE1 Study. Cancers 2019, 11, 1275. [Google Scholar] [CrossRef] [PubMed]
- Norero, E.; Alarcon, M.A.; Hakkaart, C.; de Mayo, T.; Mellado, C.; Garrido, M.; Aguayo, G.; Lagos, M.; Torres, J.; Calvo, A.; et al. Identification of c.1531C>T Pathogenic Variant in the CDH1 Gene as a Novel Germline Mutation of Hereditary Diffuse Gastric Cancer. Int. J. Mol. Sci. 2019, 20, 4980. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.E.P. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Nian, J.; Sun, X.; Ming, S.Y.; Yan, C.; Ma, Y.; Feng, Y.; Yang, L.; Yu, M.; Zhang, G.; Wang, X. Diagnostic Accuracy of Methylated SEPT9 for Blood-Based Colorectal Cancer Detection: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2017, 8, e216. [Google Scholar] [CrossRef]
- Yamashita, K.; Hosoda, K.; Nishizawa, N.; Katoh, H.; Watanabe, M. Epigenetic biomarkers of promoter DNA methylation in the new era of cancer treatment. Cancer Sci. 2018, 109, 3695–3706. [Google Scholar] [CrossRef]
- Wen, J.; Zheng, T.; Hu, K.; Zhu, C.; Guo, L.; Ye, G. Promoter methylation of tumor-related genes as a potential biomarker using blood samples for gastric cancer detection. Oncotarget 2017, 8, 77783–77793. [Google Scholar] [CrossRef]
- Anderson, B.W.; Suh, Y.-S.; Choi, B.; Lee, H.-J.; Yab, T.C.; Taylor, W.R.; Dukek, B.A.; Berger, C.K.; Cao, X.; Foote, P.H.; et al. Detection of Gastric Cancer with Novel Methylated DNA Markers: Discovery, Tissue Validation, and Pilot Testing in Plasma. Clin. Cancer Res. 2018, 24, 5724–5734. [Google Scholar] [CrossRef]
- Gue Shin, D.; Chol Kim, K. A Methylation Profile of Circulating Cell Free DNA for the Early Detection of Gastric Cancer and the Effects after Surgical Resection. J. Clin. Exp. Oncol. 2016, 5. [Google Scholar] [CrossRef]
- Saliminejad, K.; Soleymani Fard, S.; Khorram Khorshid, H.R.; Yaghmaie, M.; Mahmoodzadeh, H.; Mousavi, S.A.; Ghaffari, S.H. Methylation Analysis of P16, RASSF1A, RPRM, and RUNX3 in Circulating Cell-Free DNA for Detection of Gastric Cancer: A Validation Study. Avicenna J. Med. Biotechnol. 2020, 12, 99–106. [Google Scholar] [PubMed]
- Berger, A.H.; Knudson, A.G.; Pandolfi, P.P. A continuum model for tumour suppression. Nature 2011, 476, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R., 3rd; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-H.; Wu, C.-F.; Rajasekaran, N.; Shin, Y.K. Loss of Tumor Suppressor Gene Function in Human Cancer: An Overview. Cell. Physiol. Biochem. 2018, 51, 2647–2693. [Google Scholar] [CrossRef]
- Garcia-Bloj, B.; Moses, C.; Sgro, A.; Plani-Lam, J.; Arooj, M.; Duffy, C.; Thiruvengadam, S.; Sorolla, A.; Rashwan, R.; Mancera, R.L.; et al. Waking up dormant tumor suppressor genes with zinc fingers, TALEs and the CRISPR/dCas9 system. Oncotarget 2016, 7, 60535. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Feng, Z.; Tang, R.; Ren, F.; Wei, K.; Chen, G. Prognostic value of Caspase-3 expression in cancers of digestive tract: A meta-analysis and systematic review. Int. J. Clin. Exp. Med. 2015, 8, 10225–10234. [Google Scholar]
- Qu, Y.; Dang, S.; Hou, P. Gene methylation in gastric cancer. Clin. Chim. Acta 2013, 424, 53–65. [Google Scholar] [CrossRef]
- Rugge, M.; Pennelli, G.; Pilozzi, E.; Fassan, M.; Ingravallo, G.; Russo, V.M.; di Mario, F. Gastritis: The histology report. Dig. Liver Dis. 2011, 43 (Suppl. S4), S373–S384. [Google Scholar] [CrossRef]
- Wu, S.G.; Chang, Y.L.; Lin, J.W.; Wu, C.T.; Chen, H.Y.; Tsai, M.F.; Lee, Y.C.; Yu, C.J.; Shih, J.Y. Including total EGFR staining in scoring improves EGFR mutations detection by mutation-specific antibodies and EGFR TKIs response prediction. PLoS ONE 2011, 6, e23303. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, L.; Chang, N.; Hou, L.; Zhou, X.; Yang, L.; Li, L. Neutrophils undergo switch of apoptosis to NETosis during murine fatty liver injury via S1P receptor 2 signaling. Cell Death Dis. 2020, 11, 379. [Google Scholar] [CrossRef]
- Borowicz, S.; van Scoyk, M.; Avasarala, S.; Karuppusamy Rathinam, M.K.; Tauler, J.; Bikkavilli, R.K.; Winn, R.A. The soft agar colony formation assay. J. Vis. Exp. 2014, e51998. [Google Scholar] [CrossRef] [PubMed]
- Eads, C.A.; Danenberg, K.D.; Kawakami, K.; Saltz, L.B.; Blake, C.; Shibata, D.; Danenberg, P.V.; Laird, P.W. MethyLight: A high-throughput assay to measure DNA methylation. Nucleic Acids Res. 2000, 28, E32. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Hayashi, H.; Suzuki, H.; Yamamoto, E.; Sugisaki, H.; Higashimoto, H. Comparative analysis of methods to determine DNA methylation levels of a tumor-related microRNA gene. Anal. Biochem. 2015, 484, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Eads, C.A.; Danenberg, K.D.; Kawakami, K.; Saltz, L.B.; Danenberg, P.V.; Laird, P.W. CpG island hypermethylation in human colorectal tumors is not associated with DNA methyltransferase overexpression. Cancer Res. 1999, 59, 2302–2306. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]

| Subgroup | Hazard Ratio | p-Value | |
|---|---|---|---|
| RPRML IHC Score | Stage I–II | 3.26 (0.30–35.84) | 0.334 |
| Stage III–IV | 0.07 (0.01–0.46) | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarcón, M.A.; Olivares, W.; Córdova-Delgado, M.; Muñoz-Medel, M.; de Mayo, T.; Carrasco-Aviño, G.; Wichmann, I.; Landeros, N.; Amigo, J.; Norero, E.; et al. The Reprimo-Like Gene Is an Epigenetic-Mediated Tumor Suppressor and a Candidate Biomarker for the Non-Invasive Detection of Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 9472. https://doi.org/10.3390/ijms21249472
Alarcón MA, Olivares W, Córdova-Delgado M, Muñoz-Medel M, de Mayo T, Carrasco-Aviño G, Wichmann I, Landeros N, Amigo J, Norero E, et al. The Reprimo-Like Gene Is an Epigenetic-Mediated Tumor Suppressor and a Candidate Biomarker for the Non-Invasive Detection of Gastric Cancer. International Journal of Molecular Sciences. 2020; 21(24):9472. https://doi.org/10.3390/ijms21249472
Chicago/Turabian StyleAlarcón, María Alejandra, Wilda Olivares, Miguel Córdova-Delgado, Matías Muñoz-Medel, Tomas de Mayo, Gonzalo Carrasco-Aviño, Ignacio Wichmann, Natalia Landeros, Julio Amigo, Enrique Norero, and et al. 2020. "The Reprimo-Like Gene Is an Epigenetic-Mediated Tumor Suppressor and a Candidate Biomarker for the Non-Invasive Detection of Gastric Cancer" International Journal of Molecular Sciences 21, no. 24: 9472. https://doi.org/10.3390/ijms21249472
APA StyleAlarcón, M. A., Olivares, W., Córdova-Delgado, M., Muñoz-Medel, M., de Mayo, T., Carrasco-Aviño, G., Wichmann, I., Landeros, N., Amigo, J., Norero, E., Villarroel-Espíndola, F., Riquelme, A., Garrido, M., Owen, G. I., & Corvalán, A. H. (2020). The Reprimo-Like Gene Is an Epigenetic-Mediated Tumor Suppressor and a Candidate Biomarker for the Non-Invasive Detection of Gastric Cancer. International Journal of Molecular Sciences, 21(24), 9472. https://doi.org/10.3390/ijms21249472






