Physicochemical and Biological Characterisation of Diclofenac Oligomeric Poly(3-hydroxyoctanoate) Hybrids as β-TCP Ceramics Modifiers for Bone Tissue Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Investigated Materials
2.2.1. Production of Poly(3-hydroxyoctanoate) (P(3HO)) Polymer
2.2.2. Preparation of Highly Porous Tricalcium Phosphate (TCP) Ceramics
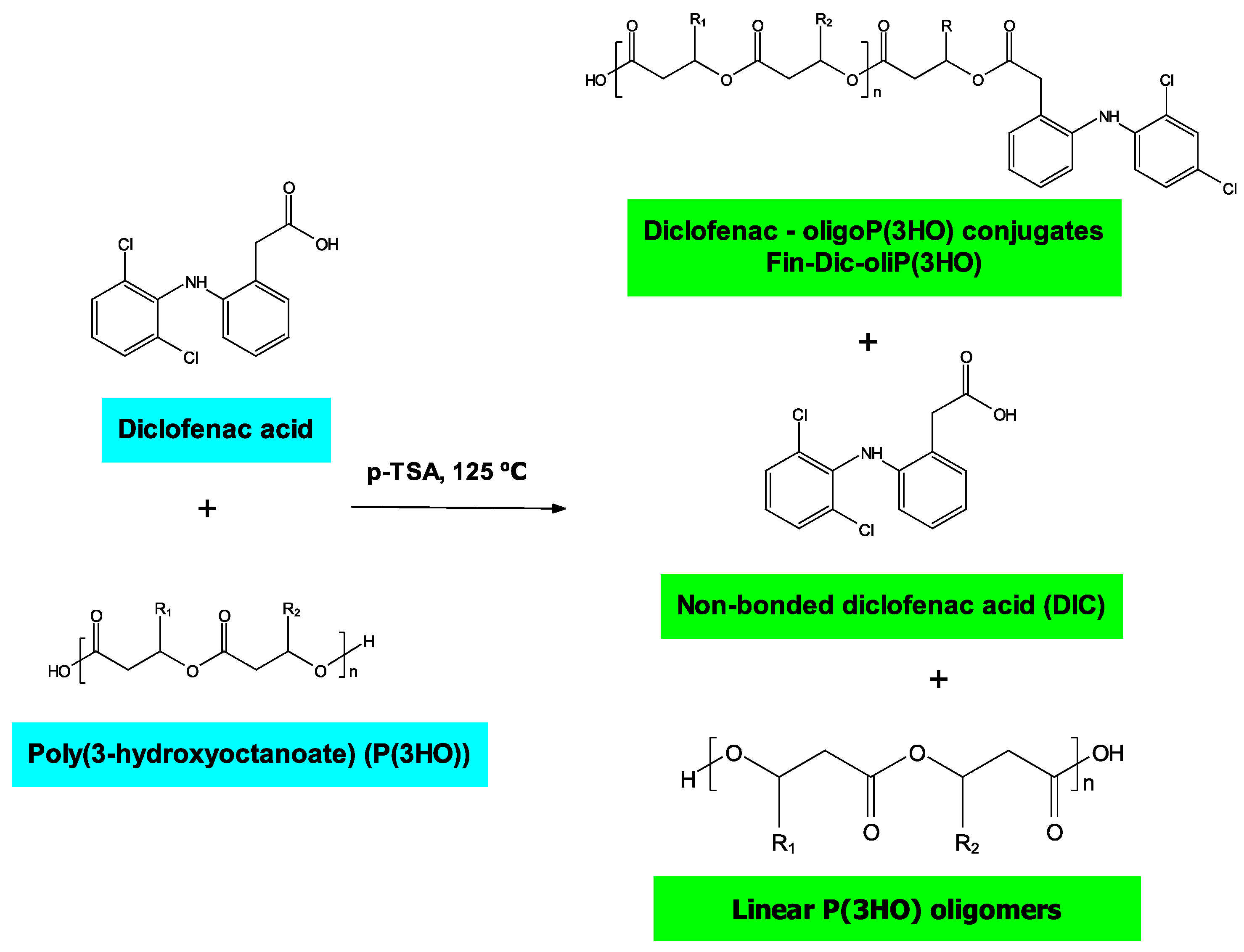
2.2.3. Preparation of Oligo-DIC Conjugates (Fin-Dic-oliP(3HO))
2.2.4. Preparation of Ceramic-Polymer Composites
2.3. Physicochemical Analysis of Oligo-DIC Conjugates and Their Blends
2.3.1. Nuclear Magnetic Resonance (1H NMR) Analysis
2.3.2. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Analysis
2.3.3. Gel Permeation Chromatography (GPC) Analysis
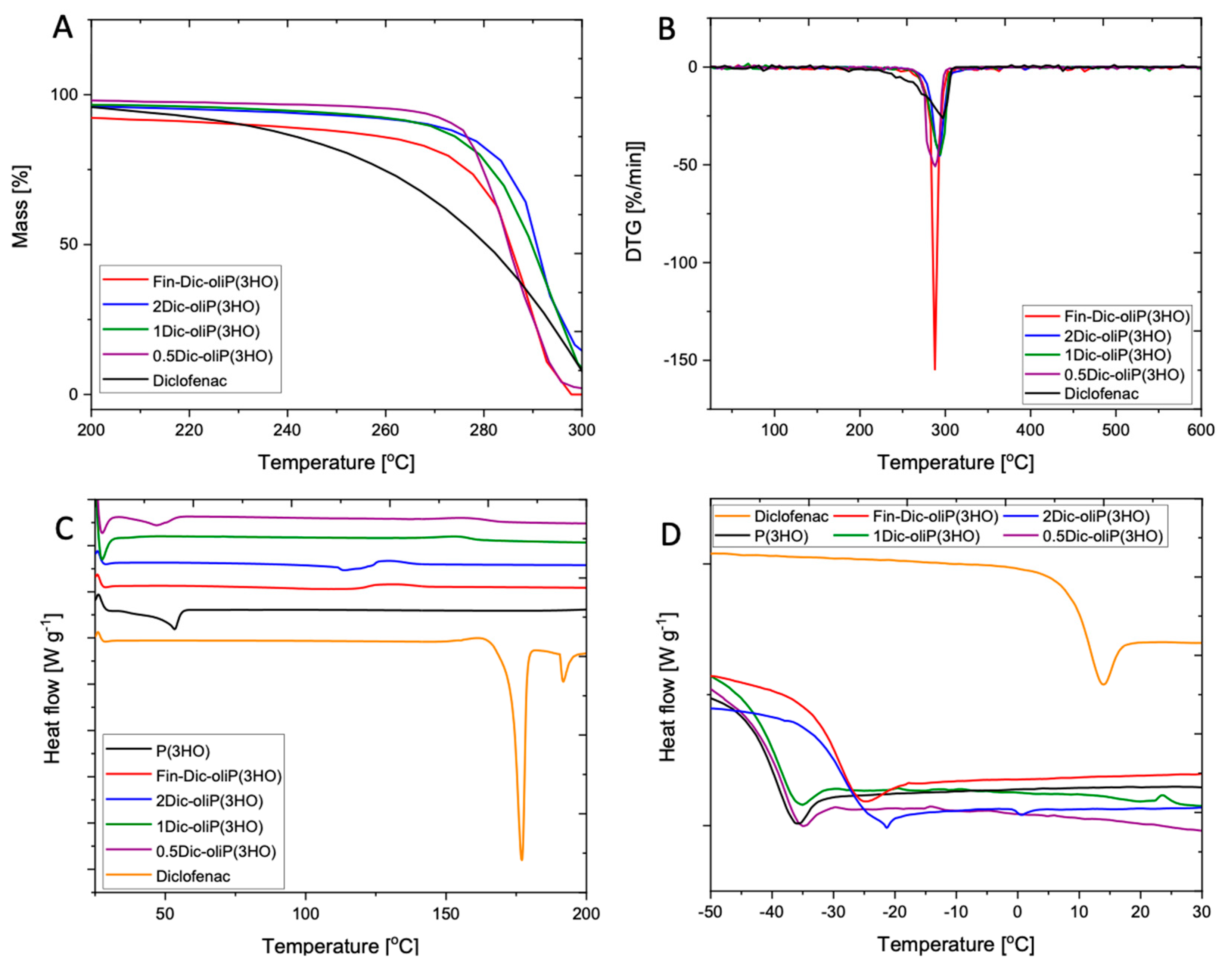
2.3.4. Thermal Analysis
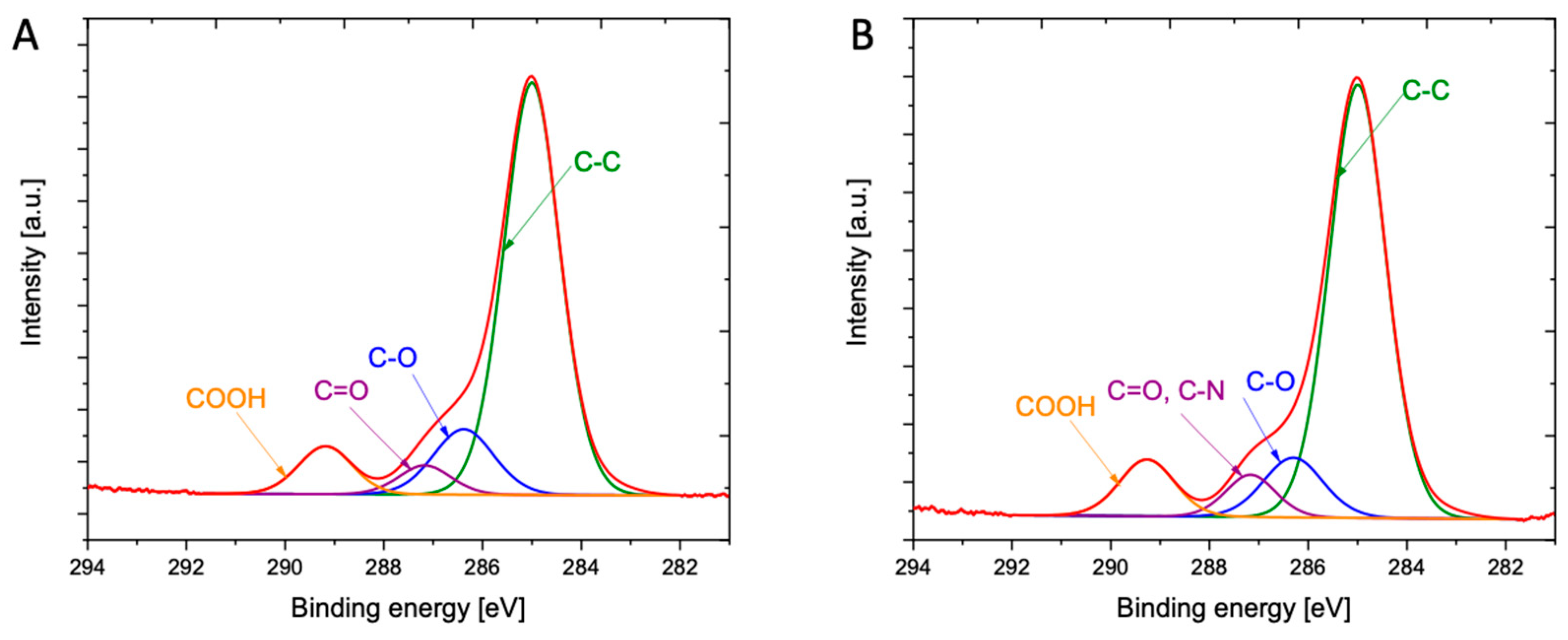
2.3.5. X-ray Photoelectron Spectroscopy (XPS)
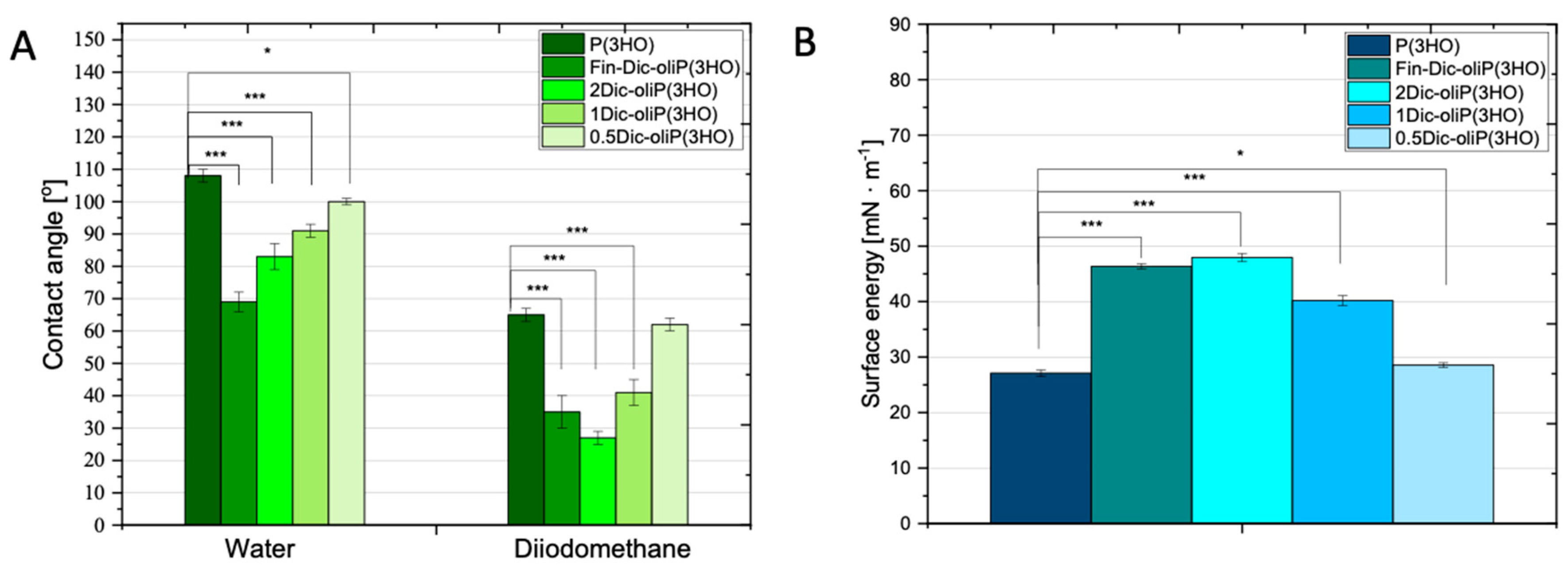
2.3.6. Measurements of the Contact Angle and Determination of Surface Free Energy
2.3.7. Atomic Force Microscopy (AFM) Imaging
2.3.8. SEM Imaging
2.3.9. Confocal Imaging
2.4. Biological Characterisation of Prepared Ceramic-Polymer-Scaffold Composites
2.4.1. The Mouse Calvaria-Derived Pre-Osteoblastic (MC3T3-E1) Cell Culture
2.4.2. The Cytotoxicity Test of Scaffold Extracts (Indirect Cytotoxicity)
2.4.3. In Vitro Biocompatibility Studies
2.4.4. Alamar Blue Assay
2.4.5. Cell Morphology Observations
2.5. Statistical Analysis
3. Results
3.1. Spectroscopic Insights into Structure of Obtained Drug-oligoP(3HO) Conjugates and Their Blends
3.2. Physicochemical Characteristics of Fin-Dic-oligoP(3HO) Conjugates and Their Blends
3.3. Wettability and Surface Free Energy Determination
3.4. Surface Roughness Determination
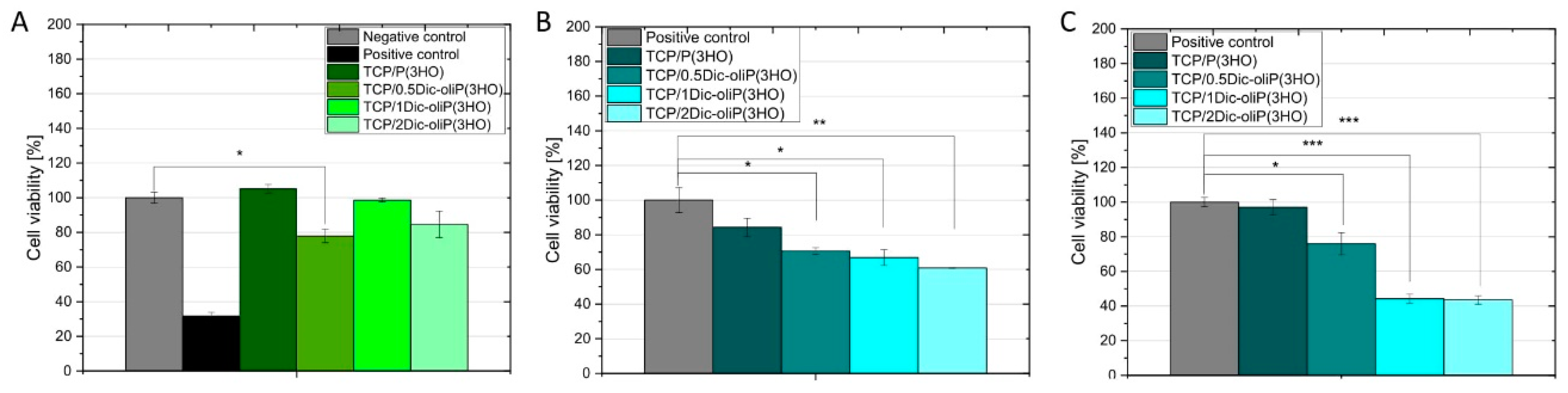
3.5. Cytotoxicity of Dic-oliP(3HO) Conjugates-Based Blends
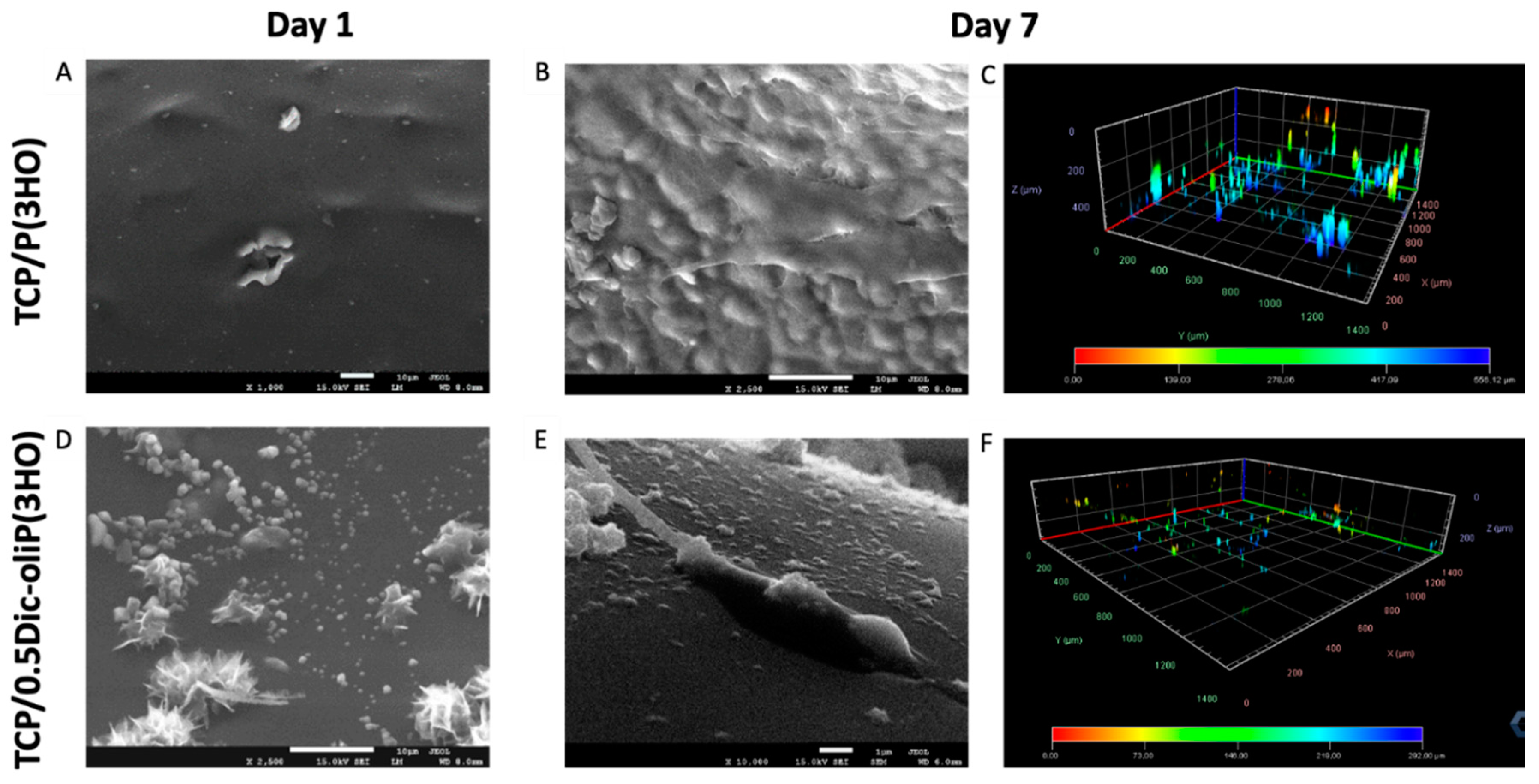
3.6. In Vitro Biocompatibility and Cell Morphology on Hybrid Composites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 0.5Dic-oliP(3HO) | Blend containing half doses of active substance per 1 g of polymer |
| 1Dic-oliP(3HO) | Blend containing one dose of active substance per 1 g of polymer |
| 1H NMR | Proton nuclear magnetic resonance |
| 2Dic-oliP(3HO) | Blend containing two doses of active substance per 1 g of polymer |
| ACP | Amorphous tricalcium phosphate |
| ATR-FTIR | Attenuated total reflection Fourier transform infrared |
| C2C12 | mouse myoblast cell line |
| Cdk2 | Cell division protein kinase 2 |
| DI | Dispersity index |
| DIC | Diclofenac acid |
| DSC | Differential scanning calorimetry |
| Fin-Dic-oliP(3HO)) | Oligo-diclofenac conjugates |
| GPC | Gel permeation chromatography |
| HAp | Hydroxyapatite |
| hOBs | Human osteoblasts |
| MC3T3-E1 | The mouse calvaria-derived pre-osteoblastic cell line |
| mcl-PHAs | Medium chain length polyhydroxyalkanoates |
| MEF3T3 | Mouse embryonic fibroblast cell line |
| MG-63 | osteosarcoma cell line |
| NIH 3T3 | Mouse fibroblast cell line |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| P(3HB-co-4HB) | Copolymer of 3-hydroxybutyric acid and 4-hydroxybutyric acid |
| P(3HB)-co-(3HV) | Copolymer of 3-hydroxybutyric acid and 3-hydroxyvaleric acid |
| P(3HO) | Poly(3-hydroxyoctanoate) |
| p27Kip1 | Cyclin-dependent kinase inhibitor 1B |
| PCL | Poly(caprolactone) |
| PEG | Poly(ethylene glycol) |
| PHAs | Polyhydroxyalkanoates |
| PLGA | Copolymer of dl-lactic acid and glycolic acid |
| PLLA | Poly(l-lactic acid) |
| PS | Polystyrene |
| PVAB | Copolymer of polyvinyl alcohol and boric acid |
| SAPAE | Copolymer of salicylic acid and succinic anhydride |
| SEM | Scanning electron microscopy |
| Sq | Root mean square roughness |
| TCP | Tricalcium phosphate |
| Tg | Glass transition temperature |
| TGA | Thermogravimetric analysis |
| Tm | Melting temperature |
| Tonset | Onset of thermal decomposition process |
| XPS | X-ray photoelectron spectroscopy |
| α-TCP | Alpha-tricalcium phosphate |
| β-TCP | Beta-tricalcium phosphate |
References
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative biomaterials for bone regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef] [PubMed]
- Madhumathi, K.; Rubaiya, Y.; Doble, M.; Venkateswari, R.; Kumar, T.S.S. Antibacterial, anti-inflammatory, and bone-regenerative dual-drug-loaded calcium phosphate nanocarriers—In vitro and in vivo studies. Drug Deliv. Transl. Res. 2018, 8, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Sarigol-Calamak, E.; Hascicek, C. Tissue Scaffolds As a Local Drug Delivery System for Bone Regeneration. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Advances in Experimental Medicine and Biology; Chun, H., Park, C., Kwon, I., Khang, G., Eds.; Springer: Singapore, 2018; Volume 1078. [Google Scholar] [CrossRef]
- Beck, A.; Krischak, G.; Sorg, T.; Augat, P.; Farker, K.; Merkel, U.; Kinzl, L.; Claes, L. Influence of diclofenac (group of nonsteroidal anti-inflammatory drugs) on fracture healing. Arch. Orthop. Trauma Surg. 2003, 123, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Sidney, L.E.; Heathman, T.R.J.; Britchford, E.R.; Abed, A.; Rahman, C.V.; Buttery, L.D.K. Investigation of localized delivery of diclofenac sodium from poly(D,L-lactic acid-co-glycolic acid)/poly(ethylene glycol) scaffolds using an in vitro osteoblast inflammation model. Tissue Eng. Part A 2015, 21, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, B.; Kosson, D.; Domaracka, K. Positives and negatives of nonsteroidal anti-inflammatory drugs in bone healing: The effects of these drugs on bone repair. Drug Des. Devel. Ther. 2018, 12, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.K.; Li, C.J.; Liao, H.J.; Wang, C.K.; Wang, G.J.; Ho, M.L. Anti-inflammatory drugs suppress proliferation and induce apoptosis through altering expressions of cell cycle regulators and pro-apoptotic factors in cultured human osteoblasts. Toxicology 2009, 258, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, L.; García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C. Effect of ibuprofen on proliferation, differentiation, antigenic expression, and phagocytic capacity of osteoblasts. J. Bone Miner. Metab. 2012, 30, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Kwong, F.N.K.; Harris, M.B. Recent developments in the biology of fracture repair. J. Am. Acad. Orthop. Surg. 2008, 16, 619–625. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 1–11. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S. Hybrid Ceramic/Polymer Composites for Bone Tissue Regeneration In Hybrid Polymer Composite Materials Processing; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 125–155. ISBN 9780081007891. [Google Scholar] [CrossRef]
- Sulistio, A.; Reyes-Ortega, F.; D’Souza, A.M.; Ng, S.M.Y.; Valade, D.; Quinn, J.F.; Donohue, A.C.; Mansfeld, F.; Blencowe, A.; Qiao, G.; et al. Precise control of drug loading and release of an NSAID-polymer conjugate for long term osteoarthritis intra-articular drug delivery. J. Mater. Chem. B 2017, 5, 6221–6226. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Mitchell, A.; Yu, W.; Snyder, S.; Uhrich, K.; Connor, J.P.O. Salicylic Acid-Based Polymers for Guided Bone Regeneration Using Bone Morphogenetic Protein-2. Tissue Eng. Part A 2015, 21, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Kim, B.; Cottrell, J.; Snyder, S.; Witek, L.; Ricci, J.; Uhrich, K.E.; Connor, J.P.O. Development of a guided bone regeneration device using salicylic acid-poly (anhydride-ester) polymers and osteoconductive scaffolds. J. Biomed. Mater. Res. Part A 2013, 102, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, F.; Duane, G.; Casey, E.; Davis, R.; Belton, I.; Kenny, S.T.; Guzik, M.W.; Woods, T.; Babu, R.P.; O’Connor, K. Fed-batch strategies using butyrate for high cell density cultivation of Pseudomonas putida and its use as a biocatalyst. Appl. Microbiol. Biotechnol. 2014, 98, 9217–9228. [Google Scholar] [CrossRef] [PubMed]
- Cichoń, E.; Haraźna, K.; Skibiński, S.; Witko, T.; Zima, A.; Ślósarczyk, A.; Zimowska, M.; Witko, M.; Leszczyński, B.; Wróbel, A.; et al. Novel bioresorbable tricalcium phosphate/polyhydroxyoctanoate (TCP/PHO) composites as scaffolds for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 98, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Valappil, S.P.; Roy, I.; Boccaccini, A.R. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 2006, 7, 2249–2258. [Google Scholar] [CrossRef]
- Francis, L.; Meng, D.; Knowles, J.C.; Roy, I.; Boccaccini, A.R. Multi-functional P (3HB) microsphere/45S5 Bioglass-based composite scaffolds for bone tissue engineering. Acta Biomater. 2010, 6, 2773–2786. [Google Scholar] [CrossRef]
- Misra, S.K.; Watts, P.C.P.; Valappil, S.P.; Silva, S.R.P.; Boccaccini, A.R.; Roy, I. Poly (3-hydroxybutyrate)/Bioglass composite films containing carbon nanotubes. Nanotechnology 2007, 18, 1–7. [Google Scholar] [CrossRef]
- Sofińska, K.; Barbasz, J.; Witko, T.; Dryzek, J.; Haraźna, K.; Witko, M.; Kryściak-Czerwenka, J.; Guzik, M. Structural, topographical, and mechanical characteristics of purified polyhydroxyoctanoate polymer. J. Appl. Polym. Sci. 2018, 47192. [Google Scholar] [CrossRef]
- Haraźna, K.; Szyk-Warszyńska, L.; Witko, M.; Guzik, M. Preparation, characterisation and modification of bacterial polyhydroxyoctanoate for construction of wound patches. In 7th International Seminar including Special Session “Recent Advances in Polymer Nanocomposites and Hybrid Materials”; Pielichowski, K., Ed.; Tomasz Mariusz Majka Publisher: Tarnów, Poland, 2019; pp. 139–152. ISBN 978-83-937270-6-3. [Google Scholar]
- van der Walle, G.A.M.; de Koning, G.J.M.; Weusthuis, R.A.; Eggink, G. Properties, Modifications and Applications of Biopolyesters. In Biopolyesters. Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2001; pp. 263–291. ISBN 978-3-540-40021-9. [Google Scholar] [CrossRef]
- Szwej, E.; Devocelle, M.; Kenny, S.; Guzik, M.; O’Connor, S.; Nikodinovic-Runic, J.; Radivojevic, J.; Maslak, V.; Byrne, A.T.; Gallagher, W.M.; et al. The chain length of biologically produced (R)-3-hydroxyalkanoic acid affects biological activity and structure of anti-cancer peptides. J. Biotechnol. 2015, 204, 7–12. [Google Scholar] [CrossRef]
- Zawidlak-Węgrzyńska, B.; Kawalec, M.; Bosek, I.; Łuczyk-Juzwa, M.; Adamus, G.; Rusin, A.; Filipczak, P.; Głowala-Kosińska, M.; Wolańska, K.; Krawczyk, Z.; et al. Synthesis and antiproliferative properties of ibuprofen—oligo (3-hydroxybutyrate) conjugates. Eur. J. Med. Chem. 2010, 45, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Wu, Q. The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef] [PubMed]
- Rajaratanam, D.D.; Ariffin, H.; Hassan, M.A.; Abd Rahman, N.M.A.N.; Nishida, H. In vitro cytotoxicity of superheated steam hydrolyzed oligo((R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate) and characteristics of its blend with poly(L-lactic acid) for biomaterial applications. PLoS ONE 2018, 13, e0199742. [Google Scholar] [CrossRef] [PubMed]
- Nigmatullin, R.; Thomas, P.; Lukasiewicz, B.; Puthussery, H.; Roy, I.; Biotechnology, A. Polyhydroxyalkanoates, a family of natural polymers, and their applications in drug delivery. J. Chem. Technol. Biotechnol. 2015, 90, 1209–1221. [Google Scholar] [CrossRef]
- Witko, T.; Guzik, M.; Sofińska, K.; Stepien, K.; Podobinska, K. Novel Biocompatible Polymers for Biomedical Applications. Biophys. J. 2018, 114, 363a. [Google Scholar] [CrossRef]
- Witko, T.; Solarz, D.; Feliksiak, K.; Rajfur, Z.; Guzik, M. Cellular architecture and migration behavior of fibroblast cells on polyhydroxyoctanoate (PHO): A natural polymer of bacterial origin. Biopolymers 2019, 110, e23324. [Google Scholar] [CrossRef]
- Lukasiewicz, B.; Basnett, P.; Nigmatullin, R.; Matharu, R.; Knowles, J.C.; Roy, I. Binary polyhydroxyalkanoate systems for soft tissue engineering. Acta Biomater. 2018, 71, 225–234. [Google Scholar] [CrossRef]
- Basnett, P.; Ching, K.Y.; Stolz, M.; Knowles, J.C.; Boccaccini, A.R.; Smith, C.; Locke, I.C.; Keshavarz, T.; Roy, I. Novel Poly(3-hydroxyoctanoate)/Poly(3-hydroxybutyrate) blends for medical applications. React. Funct. Polym. 2013, 73, 1340–1348. [Google Scholar] [CrossRef]
- Pramanik, N.; Dutta, K.; Basu, R.K.; Kundu, P.P. Aromatic π-Conjugated Curcumin on Surface Modified Polyaniline/Polyhydroxyalkanoate Based 3D Porous Scaffolds for Tissue Engineering Applications. ACS Biomater. Sci. Eng. 2016, 2, 2365–2377. [Google Scholar] [CrossRef]
- Ang, S.L.; Shaharuddin, B.; Chuah, J.A.; Sudesh, K. Electrospun poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)/silk fibroin film is a promising scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 145, 173–188. [Google Scholar] [CrossRef]
- Kwiecień, I.; Radecka, I.; Kowalczuk, M.; Adamus, G. Transesterification of PHA to Oligomers Covalently Bonded with (Bio) Active Compounds Containing Either Carboxyl or Hydroxyl Functionalities. PLoS ONE 2015, 10, e0120149. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, I.; Radecka, I.; Kwiecień, M.; Adamus, G. Synthesis and Structural Characterization of Bioactive PHA and γ-PGA Oligomers for Potential Applications as a Delivery System. Materials 2016, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Francis, B.L.; Meng, D.; Locke, I.C.; Mordan, N.; Salih, V.; Knowles, J.C.; Boccaccini, A.R.; Roy, I. The Influence of Tetracycline Loading on the Surface Morphology and Biocompatibility of Films Made from P (3HB) Microspheres. Adv. Biomater. 2010, 7, 260–268. [Google Scholar] [CrossRef]
- Francis, L.; Meng, D.; Knowles, J.; Keshavarz, T.; Boccaccini, A.R.; Roy, I. Controlled Delivery of Gentamicin Using Poly (3-hydroxybutyrate) Microspheres. Int. J. Mol. Sci. 2011, 12, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Guzik, M.; Narancic, T.; Ilic-Tomic, T.; Vojnovic, S.; Kenny, S.T.; Casey, W.T.; Duane, G.F.; Casey, E.; Woods, T.; Babu, R.P.; et al. Identification and characterization of an acyl-CoA dehydrogenase from Pseudomonas putida KT2440 that shows preference towards medium to long chain length fatty acids. Microbiology 2014, 160, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Small, R.E. Diclofenac sodium. Clin. Pharm. 1989, 8, 545–558. [Google Scholar]
- Skibiński, S.; Cichoń, E.; Haraźna, K.; Marcello, E.; Roy, I.; Witko, M.; Ślósarczyk, A.; Czechowska, J.; Guzik, M.; Zima, A. Functionalized tricalcium phosphate and poly(3-hydroxyoctanoate) derived composite scaffolds as platforms for the controlled release of diclofenac. Ceram. Int. 2020. [Google Scholar] [CrossRef]
- Haraźna, K.; Walas, K.; Urbańska, P.; Witko, T.; Snoch, W.; Siemek, A.; Jachimska, B.; Krzan, M.; Napruszewska, B.D.; Witko, M.; et al. Polyhydroxyalkanoate-derived hydrogen-bond donors for the synthesis of new deep eutectic solvents. Green Chem. 2019, 21, 3116–3126. [Google Scholar] [CrossRef]
- Klapetek, P.; David, N.; Anderson, C. Gwyddion User Guide. Available online: http://gwyddion.net/download/user-guide/gwyddion-user-guide-en.pdf (accessed on 27 November 2020).
- David, N.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Witko, T.; Solarz, D.; Feliksiak, K.; Haraźna, K.; Rajfur, Z.; Guzik, M. Insights into in vitro wound closure on two biopolyesters—Polylactide and polyhydroxyoctanoate. Materials 2020, 17, 2793. [Google Scholar] [CrossRef]
- Rai, R.; Yunos, D.M.; Boccaccini, A.R.; Knowles, J.C.; Barker, I.A.; Howdle, S.M.; Tredwell, G.D.; Keshavarz, O.T.; Roy, I. Poly-3-hydroxyoctanoate P(3HO), a Medium Chain Length Polyhydroxyalkanoate Homopolymer from Pseudomonas mendocina. Biomacromolecules 2011, 12, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, E.; Ramukutty, S. Growth, morphology, spectral and thermal studies of gel grown diclofenac acid crystals Growth, morphology, spectral and thermal studies of gel grown diclofenac acid crystals. J. Cryst. Growth 2017, 389, 78–82. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, S.M.; Heidelberg, T. Single-step lipase-catalyzed functionalization of medium-chain-length polyhydroxyalkanoates. J. Chem. Technol. Biotechnol. 2013, 88, 1328–1335. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, P.; Lv, H.; Qin, Y.; Deng, L. Gentamicin modified chitosan film with improved antibacterial property and cell biocompatibility. Int. J. Biol. Macromol. 2017, 98, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Wadhwa, P.; Won, J.; Gi, Y.; Jeon, J. Lipase mediated functionalization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with ascorbic acid into an antioxidant active biomaterial. Int. J. Biol. Macromol. 2019, 123, 117–123. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull. Am. Phys. Soc. 1956, 1, 123–132. [Google Scholar]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Yul, J.; Dreiss, A.D.; Zhou, Z.; Hansen, J.C.; Siedlecki, C.A.; Hengstebeck, R.W.; Cheng, J.; Winograd, N.; Donahue, H.J. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials 2007, 28, 1787–1797. [Google Scholar] [CrossRef]
- Ailincai, D.; Gavril, G.; Marin, L. Polyvinyl alcohol boric acid—A promising tool for the development of sustained release drug delivery systems. Mater. Sci. Eng. C 2020, 107, 110316. [Google Scholar] [CrossRef]
- Sarkar, N.; Aakeröy, C.B. Evaluating hydrogen-bond propensity, hydrogen-bond coordination and hydrogen-bond energy as tools for predicting the outcome of attempted co-crystallisations. Supramol. Chem. 2020, 32, 81–90. [Google Scholar] [CrossRef]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Díaz, E.; Puerto, I.; Ribeiro, S.; Lanceros-Mendez, S.; Barandiarán, J.M. The influence of copolymer composition on PLGA/nHA scaffolds’ cytotoxicity and in vitro degradation. Nanomaterials 2017, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Goreva, A.V.; Shishatskaya, E.I.; Volova, T.G.; Sinskey, A.J. Characterization of Polymeric Microparticles Based on Resorbable Polyesters of Oxyalkanoic Acids as a Platform for Deposition and Delivery of Drugs. Med. Polym. 2012, 54, 224–236. [Google Scholar] [CrossRef]
- Rai, R.; Boccaccini, A.R.; Knowles, J.C.; Mordon, N.; Salih, V.; Locke, I.C.; Keshavarz, T.; Roy, I. The Homopolymer Poly (3-hydroxyoctanoate) as a Matrix Material for Soft Tissue Engineering. J. Appl. Polym. Sci. 2011, 122, 3606–3617. [Google Scholar] [CrossRef]
- Fini, A.; Garuti, M.; Fazio, G.; Holgado, M.A. Diclofenac Salts. I. Fractal and Thermal Analysis of Sodium and Potassium Diclofenac Salts. J. Pharm. Sci. 2001, 90, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, C.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug—Polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Palacio, J.; Orozco, V.H.; López, B.L. Effect of the Molecular Weight on the Physicochemical Properties of Poly(lactic acid) Nanoparticles and on the Amount of Ovalbumin Adsorption. J. Braz. Chem. Soc. 2011, 22, 2304–2311. [Google Scholar] [CrossRef]
- Yanez, M.; Blanchette, J.; Jabbarzadeh, E. Modulation of Inflammatory Response to Implantes Biomaterials Using Natural Compounds. Curr. Pharm. Des. 2017, 23, 6347–6357. [Google Scholar] [CrossRef]
- Braunecker, J.; Baba, M.; Milroy, G.E.; Cameron, R.E. The effects of molecular weight and porosity on the degradation and drug release from polyglycolide. Int. J. Pharm. 2004, 282, 19–34. [Google Scholar] [CrossRef]
- Naveen, S.V.; Tan, I.K.P.; Goh, Y.S.; Balaji Raghavendran, H.R.; Murali, M.R.; Kamarul, T. Unmodified medium chain length polyhydroxyalkanoate (uMCL-PHA) as a thin film for tissue engineering application—Characterization and in vitro biocompatibility. Mater. Lett. 2015, 141, 55–58. [Google Scholar] [CrossRef]
- Xu, J.L.; Lesniak, A.; Gowen, A.A. Predictive Modeling of the in Vitro Responses of Preosteoblastic MC3T3-E1 Cells on Polymeric Surfaces Using Fourier Transform Infrared Spectroscopy. ACS Appl. Mater. Interfaces 2020, 12, 24466–24478. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Tsukimura, N.; Iwasa, F.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.A.; Ogawa, T. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials 2009, 30, 5319–5329. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, J.M.; Wei, F.; Guo, J.; Guillem-Marti, J.; Lin, Z.; Ginebra, M.P.; Xiao, Y. The effect of biomimetic calcium deficient hydroxyapatite and sintered β-tricalcium phosphate on osteoimmune reaction and osteogenesis. Acta Biomater. 2019, 96, 605–618. [Google Scholar] [CrossRef] [PubMed]
- De Luna-Bertos, E.; Ramos-Torrecillas, J.; Garcia-Martinez, O.; Guildford, A.; Santin, M.; Ruiz, C. Therapeutic doses of nonsteroidal anti-inflammatory drugs inhibit osteosarcoma MG-63 osteoblast-like cells maturation, viability, and biomineralization potential. Sci. World J. 2013, 2013, 809891. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Illescas-Montes, R.; Manzano-moreno, F.J.; Ruiz, C.; De Luna-Bertos, E. Effects of Therapeutic Doses of Celecoxib on Several Physiological Parameters of Cultured Human Osteoblasts. Int. J. Med. Sci. 2019, 16, 1466–1472. [Google Scholar] [CrossRef]
- Krischak, G.D.; Augat, P.; Blakytny, R.; Claes, L.; Kinzl, L.; Beck, A. The non-steroidal anti-inflammatory drug diclofenac reduces appearance of osteoblasts in bone defect healing in rats. Arch. Orthop. Trauma Surg. 2007, 127, 453–458. [Google Scholar] [CrossRef]
- Ahmed, S.; Chauhan, V.M.; Ghaemmaghami, A.M.; Aylott, J.W. New generation of bioreactors that advance extracellular matrix modelling and tissue engineering. Biotechnol. Lett. 2019, 41, 1–25. [Google Scholar] [CrossRef]

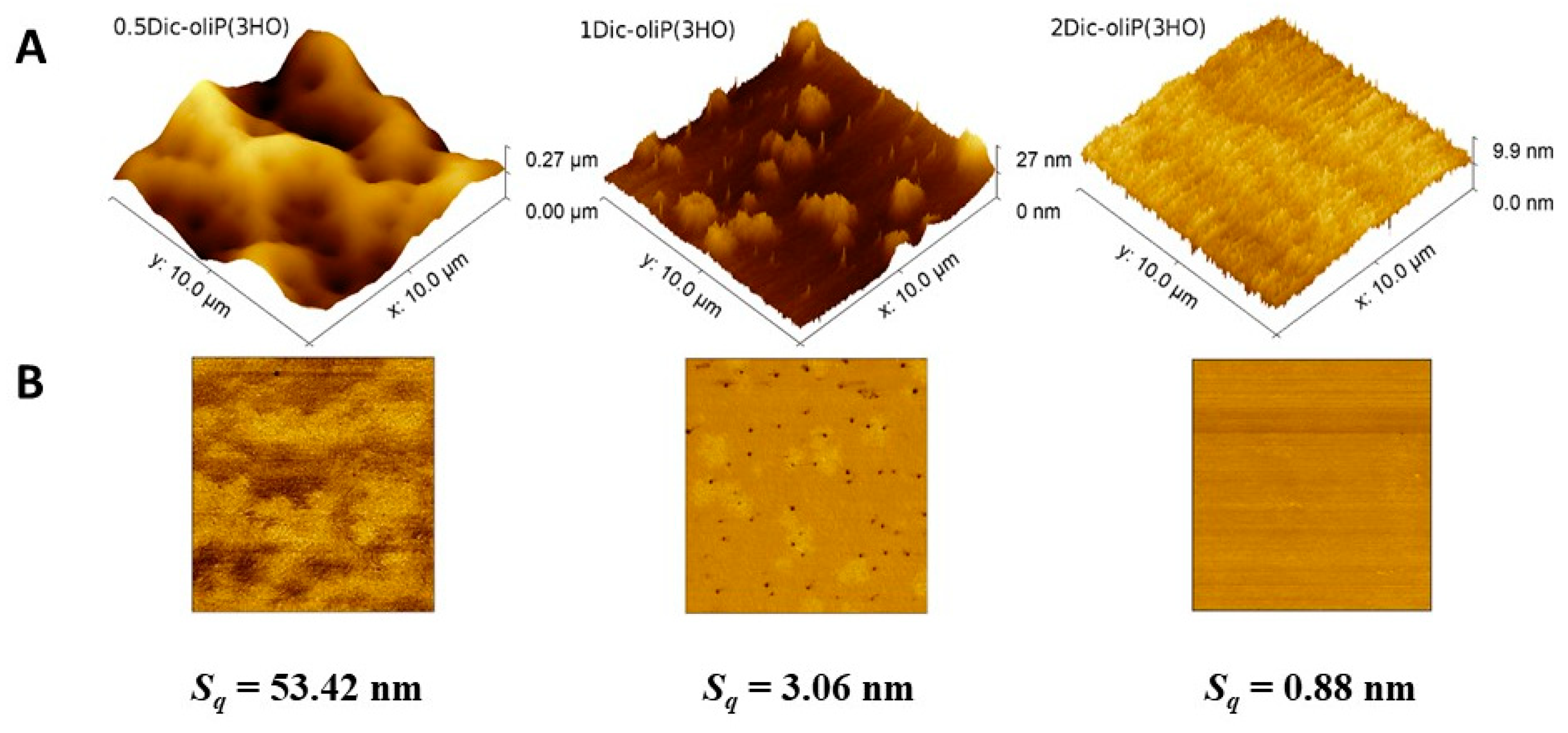
| Name | Fin-Dic-oliP(3HO) | P(3HO) |
|---|---|---|
| 2Dic-oliP(3HO) | 0.97 | 0.03 |
| 1Dic-oliP(3HO) | 0.48 | 0.52 |
| 0.5Dic-oliP(3HO) | 0.24 | 0.76 |
| C (%) | O (%) | O/C Ratio | N (%) | Cl (%) | |
|---|---|---|---|---|---|
| P(3HO) | 77.25 | 22.75 | 0.29 | n.d. | n.d. |
| Fin-Dic-oliP(3HO) | 79.27 | 20.11 | 0.25 | 0.46 | 0.17 |
| Mn [kDa] | Mw [kDa] | Dispersity Index | Tonset (°C) | TDTG (°C) | Residue (1%) (°C) | Tg (°C) | Tm (°C) | |
|---|---|---|---|---|---|---|---|---|
| P(3HO) [21] | 73 | 137 | 1.88 | 279 | 296 | 306 | −41 | 53 |
| Fin-Dic-oliP(3HO) | 4.93 ± 0.0 | 8.21 ± 0.12 | 1.66 ± 0.02 | 285 | 293 | 298 | −30 | - |
| 2Dic-oliP(3HO) | 4.93 ± 0.0 | 8.21 ± 0.12 | 1.66 ± 0.02 | 285 | 293 | 367 | −29 | - |
| 1Dic-oliP(3HO) | 78.88 ± 4.55 | 133.74 ± 4.81 | 1.70 ± 0.04 | 280 | 294 | 305 | −40 | - |
| 0.5Dic-oliP(3HO) | 79.59 ± 0.12 | 139.26 ± 0.00 | 1.75 ± 0.00 | 281 | 288 | 308 | −40 | 50 |
| DIC | N/A | N/A | N/A | 266 | 297 | 307 | 10 | 177 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haraźna, K.; Cichoń, E.; Skibiński, S.; Witko, T.; Solarz, D.; Kwiecień, I.; Marcello, E.; Zimowska, M.; Socha, R.; Szefer, E.; et al. Physicochemical and Biological Characterisation of Diclofenac Oligomeric Poly(3-hydroxyoctanoate) Hybrids as β-TCP Ceramics Modifiers for Bone Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 9452. https://doi.org/10.3390/ijms21249452
Haraźna K, Cichoń E, Skibiński S, Witko T, Solarz D, Kwiecień I, Marcello E, Zimowska M, Socha R, Szefer E, et al. Physicochemical and Biological Characterisation of Diclofenac Oligomeric Poly(3-hydroxyoctanoate) Hybrids as β-TCP Ceramics Modifiers for Bone Tissue Regeneration. International Journal of Molecular Sciences. 2020; 21(24):9452. https://doi.org/10.3390/ijms21249452
Chicago/Turabian StyleHaraźna, Katarzyna, Ewelina Cichoń, Szymon Skibiński, Tomasz Witko, Daria Solarz, Iwona Kwiecień, Elena Marcello, Małgorzata Zimowska, Robert Socha, Ewa Szefer, and et al. 2020. "Physicochemical and Biological Characterisation of Diclofenac Oligomeric Poly(3-hydroxyoctanoate) Hybrids as β-TCP Ceramics Modifiers for Bone Tissue Regeneration" International Journal of Molecular Sciences 21, no. 24: 9452. https://doi.org/10.3390/ijms21249452
APA StyleHaraźna, K., Cichoń, E., Skibiński, S., Witko, T., Solarz, D., Kwiecień, I., Marcello, E., Zimowska, M., Socha, R., Szefer, E., Zima, A., Roy, I., Raftopoulos, K. N., Pielichowski, K., Witko, M., & Guzik, M. (2020). Physicochemical and Biological Characterisation of Diclofenac Oligomeric Poly(3-hydroxyoctanoate) Hybrids as β-TCP Ceramics Modifiers for Bone Tissue Regeneration. International Journal of Molecular Sciences, 21(24), 9452. https://doi.org/10.3390/ijms21249452










