Searching for New Beneficial Bacterial Isolates of Wild Raspberries for Biocontrol of Phytopathogens-Antagonistic Properties and Functional Characterization
Abstract
1. Introduction
2. Results
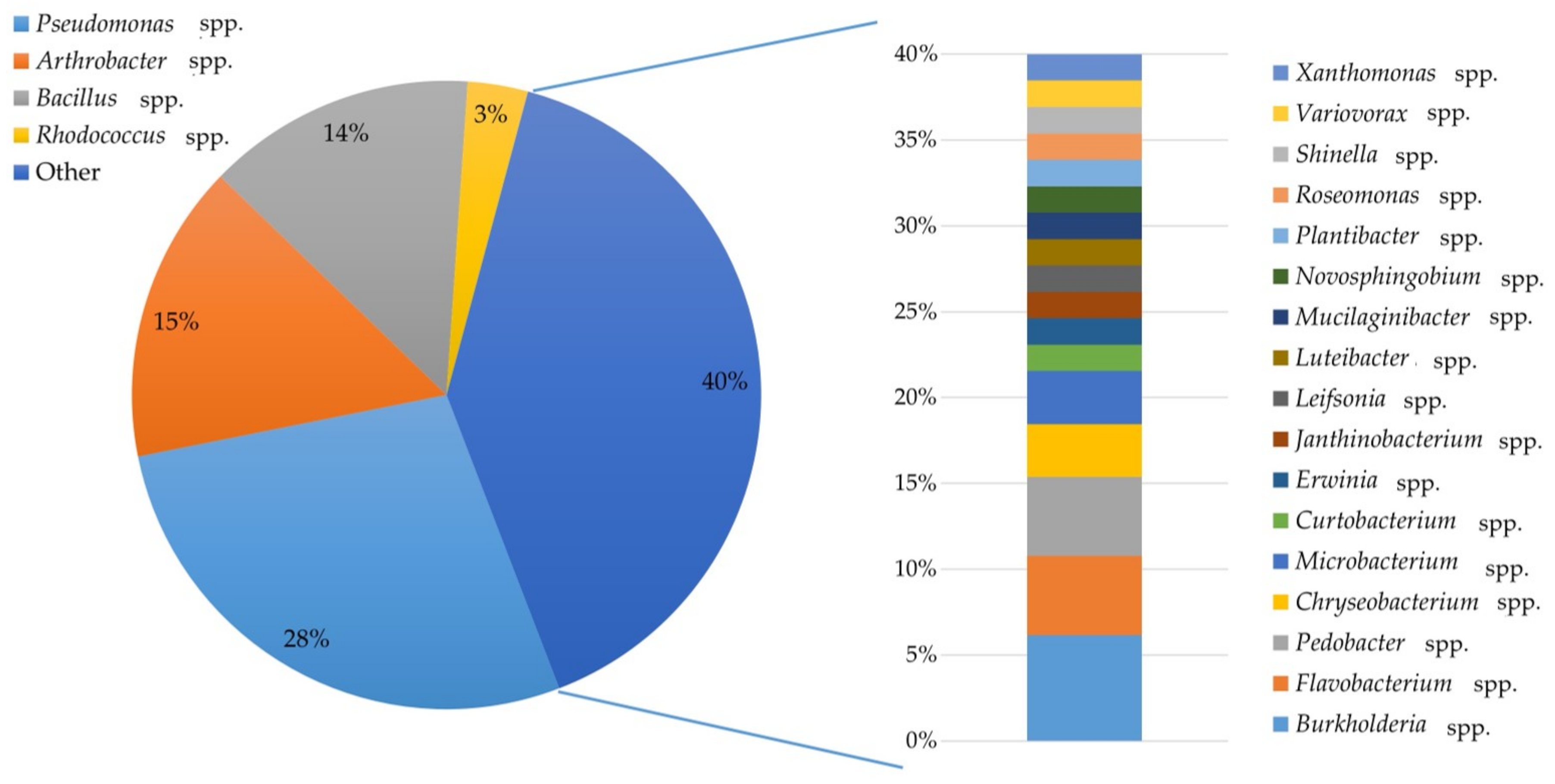
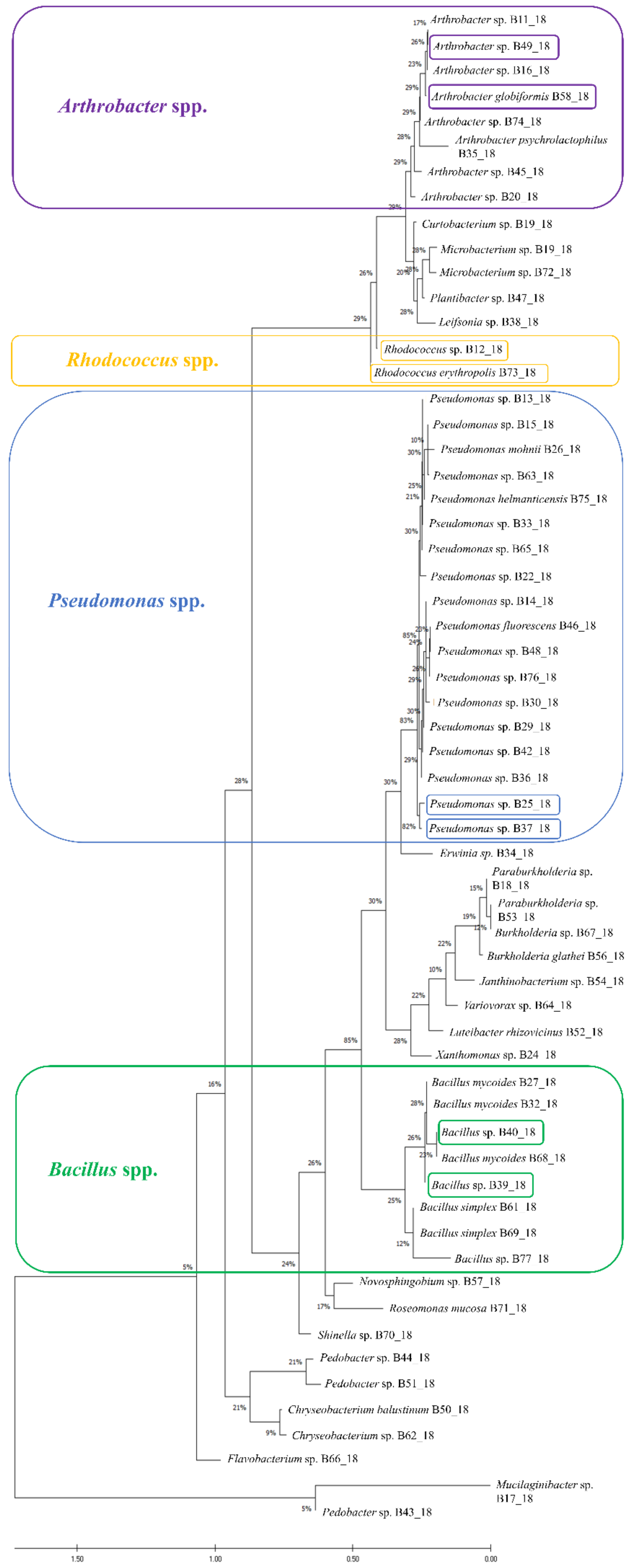
2.1. Identification of Bacterial Isolates
2.2. Antagonistic Properties
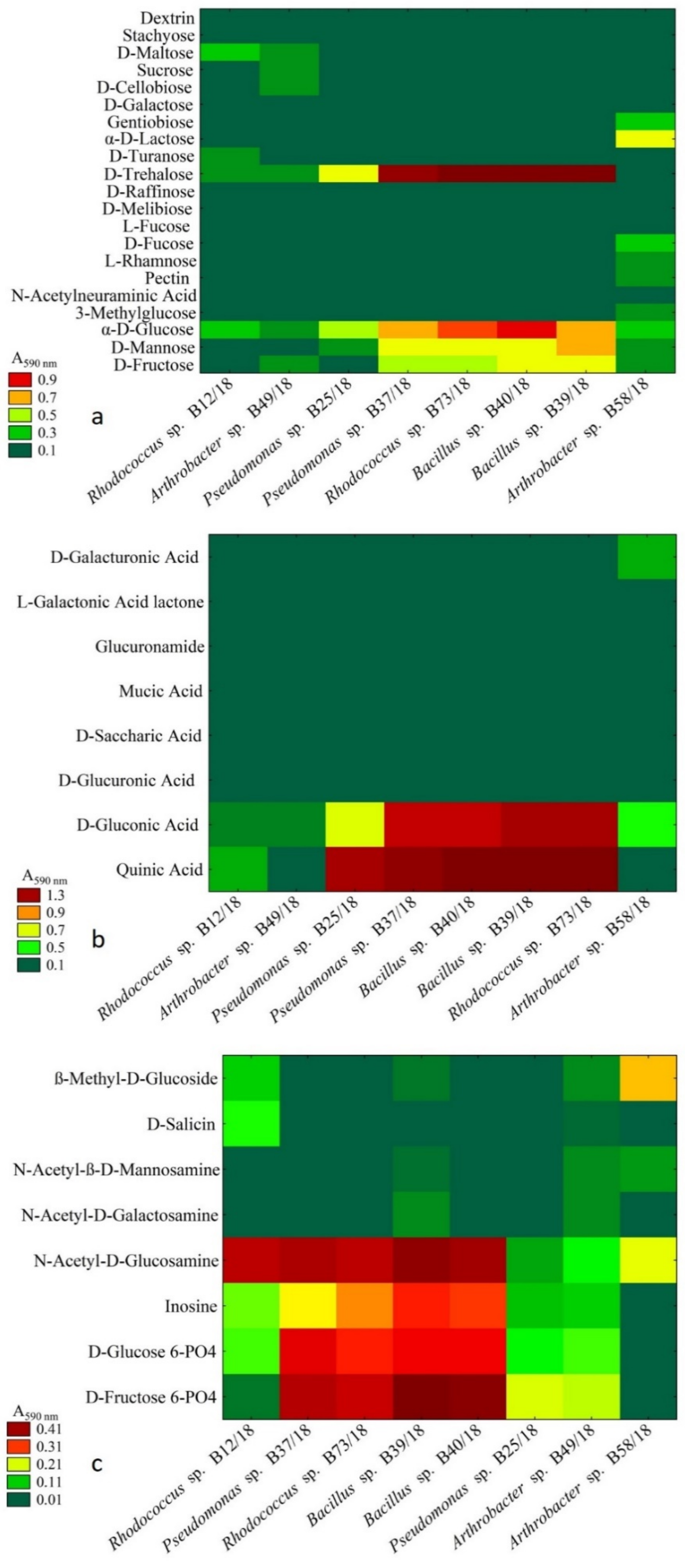
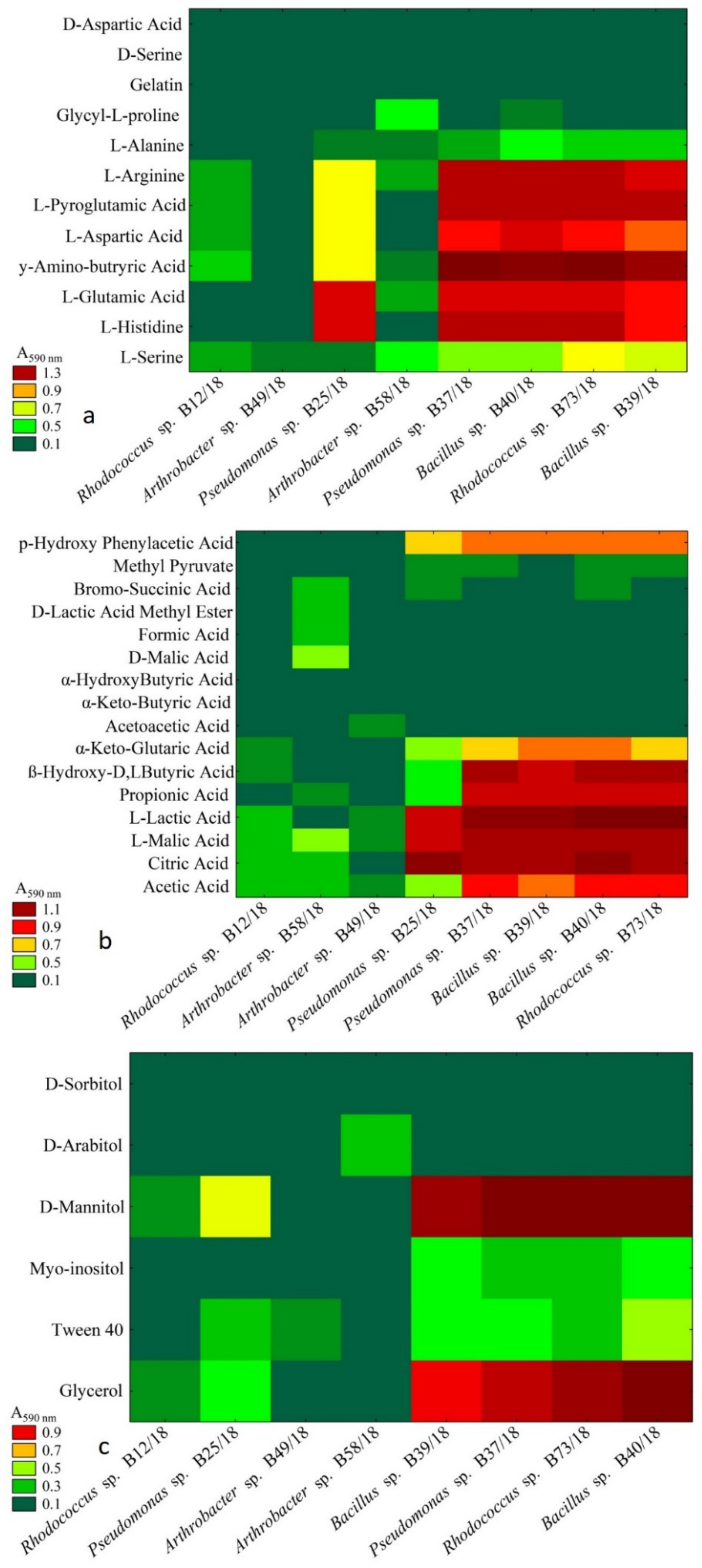
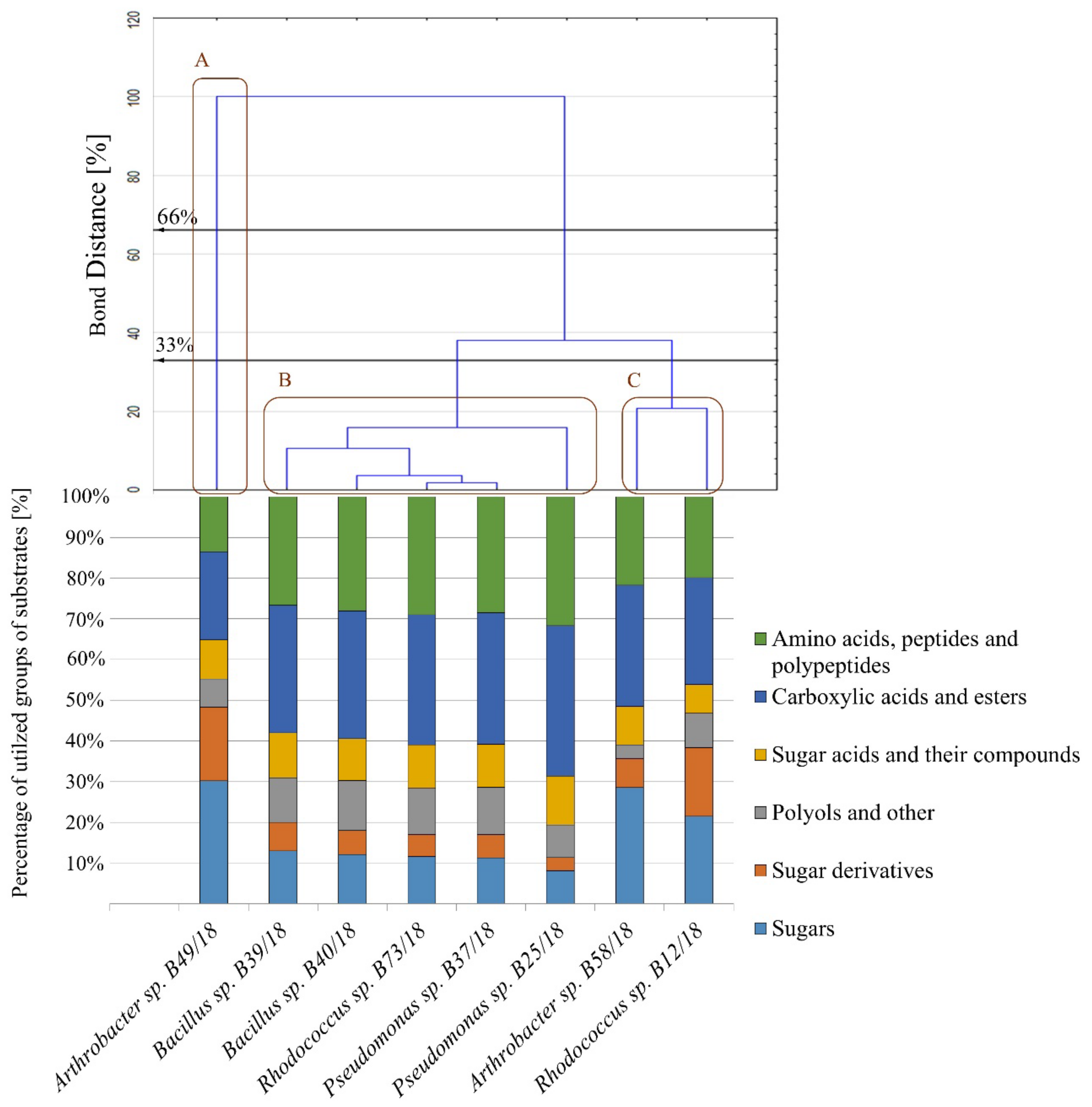
2.3. Analysis of Metabolic Abilities
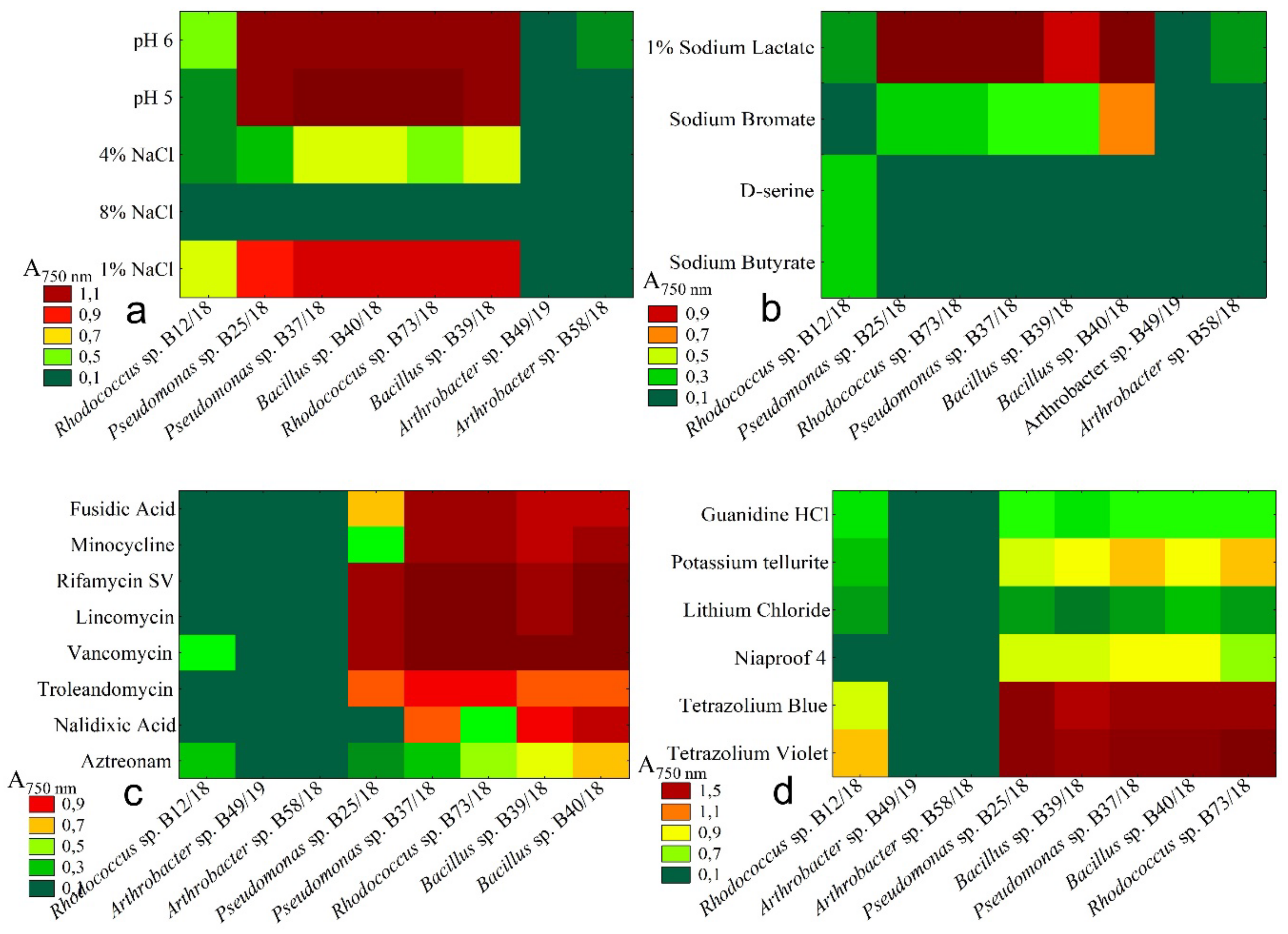
2.4. Resistance to Chemical Stresses
2.5. Enzymatic Activity of Selected Bacterial Isolates
2.6. Prebiotic Supplement Mixture against Fungal and Fungal-Like Pathogens
3. Discussion
3.1. The Properties of Bacteria Belonging to Arthrobacter Genus
3.2. The Properties of Bacteria Belonging to Rhodococcus Genus
3.3. The Properties of Bacteria Belonging to Bacillus Genus
3.4. The properties of Bacteria Belonging to Pseudomonas Genus
3.5. Summary of Bacterial Properties as Potential Candidates to Biopreparations for Agroecology
4. Materials and Methods
4.1. Bacterial Isolates Acquisition and Identification
4.2. Biocontrol Efficacy and Antagonistic Abilities of Bacterial Isolates
4.3. Metabolic Abilites of Isolated Beneficial Bacterial Isolates
4.4. Enzymatic Activity of Selected Bacterial Isolates
4.5. Metabolic Abilities of Selected Fungal and Fungal-Like Plant Pathogens
4.6. Prebiotic Supplement Blend as Possible Component of Future Biopreparation
5. Conclusions
- -
- Bacteria isolated from raspberry rhizosphere have antagonistic properties against common fungal and fungal-like plant pathogens such as Botrytis spp., Colletotrichum spp., Phytophthora spp., and Verticillium spp.
- -
- Describing the utilization abilities of different substrates by microorganisms such as bacteria is a study that might benefit from the application of a calculation method of the 590–750 nm ratio of absorbances. This may not only give the researchers a broader spectrum of results, but also emphasize differences between the tested isolates as far as functional response is concerned, namely the metabolic activity in juxtaposition with biomass production.
- -
- Bacteria to be used in biopreparation need to present some desired features. They should be antagonistic against pathogens, have a broad spectrum of utilized compounds to be used as a carbon source, survive in many different conditions such as different pH values. Due to the fact, that since resistance to antibiotics becomes more common in soil microorganisms it is worth considering that bacteria used in biopreparations are not resistant to antibiotic compounds. This practice reduces spreading this undesirable feature among other microorganisms. From tested bacteria, isolates B12/18 and B58/18 presented needed features and at the same time, they were not resistant to most tested antibiotics. Those two isolates might be worth to be used in future testing and formulations. It is worth considering using other isolates in some special cases and under restrictions due to their good antagonistic and metabolic properties.
- -
- A carbon substrate, such as D-trehalose was utilized by the tested bacteria in a balanced way, namely without causing a stressful metabolic situation that, might be a beneficial addition to a bacterial formulation providing enhanced growth and survivability.
- -
- Metabolic and enzymatic abilities analysis provides important information about particular environmental isolates which might be essential to achieving a complete understanding of their functioning in biopreparations or in future environmental niches. The more enzymatic abilities are demonstrated by the isolates, the easier it might be for those isolates to acclimatize to new environments or stress conditions.
- -
- Carefully selected chemical compounds are a valuable additive to biopreparations. Additives such as D-malic acid, D-saccharic acid, N-acetylo-D-galactosamine, and α-keto-glutaric acid may not only enhance or stimulate bacterial growth but also inhibit the growth of plant fungal and fungal-like pathogens.
- -
- Future research will focus on in planta testing bacterial formulations on raspberry plants combined with introducing fungal and fungal-like pathogens to the experimental treatments. Due to the fact that microbial inoculation may cause tremendous changes in the dynamics of soil microbial communities, subsequent research should include the soil microbiome and mycobiome status and its functional shifts after selected bacterial isolates application. Moreover, regarding the suggested blend of prebiotic supplements, it should be tested for its effect on the growth of beneficial microorganisms and pathogens. Moreover, the study should focus on the development of a biopreparation with carriers and technology conditions appropriate for the selected bacterial isolates, considering future needs arising from the Biodiversity Strategy for 2030.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 16S rDNA | 16S ribosomal DNA gene |
| ASM | Acibenzolar-S-methyl |
| MEGA | Molecular Evolutionary Genetics Analysis |
| OD590/OD750 OD490/OD750 | The ratio of absorbances value measured at 590 nm and 750 nm The ratio of absorbances value measured at 490 nm and 750 nm |
| PCA | Plate Count Agar |
| PCR | Polymerase Chain Reaction |
| PDA | Potato Dextrose Agar |
| T | Transmittance |
Appendix A
| Bacteria | Phytopathogenic Fungi and Fungal-Like Pathogens | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colletotrichum spp. | Verticillium spp. | Phytophthora spp. | Botrytis spp. | ||||||||||
| G172/18 | G371/18 | G166/18 | G293/18 | G296/18 | G297/18 | G368/18 | G373/18 | G369/18 | G275/16 | G277/18 | G276/18 | ||
| Flavobacterium sp. | B10/18 | − | − | − | − | − | − | − | − | − | - | − | − |
| Rhodococcus sp. | B12/18 | + | + | ++ | + | ++ | ++ | +++ | + | ++ | - | ++ | +++ |
| Pseudomonas sp. | B13/18 | − | − | − | − | − | − | − | − | − | − | − | − |
| Pseudomonas sp. | B14/18 | − | − | − | − | − | − | − | − | + | − | ++ | − |
| Burkholderia sp. | B18/18 | − | − | − | − | − | − | − | − | − | − | − | − |
| Curtobacterium sp. | B19/18 | − | − | − | − | − | − | − | − | − | − | − | − |
| Arthrobacter sp. | B20/18 | − | − | − | − | − | − | − | − | − | − | + | − |
| Pseudomonas sp. | B21/18 | − | − | − | − | − | − | − | + | − | − | − | + |
| Pseudomonas sp. | B22/18 | − | − | − | − | − | − | − | − | − | − | − | − |
| Microbacterium sp. | B23/18 | − | − | − | − | − | − | − | ++ | - | - | - | −- |
| Pseudomonas sp. | B25/18 | ++ | ++ | +++ | +++ | ++ | ++ | ++ | + | +++ | − | − | − |
| Pseudomonas sp. | B26/18 | − | − | − | − | − | − | − | − | − | − | − | − |
| Bacillus sp. | B27/18 | − | − | − | − | + | + | − | − | − | − | − | − |
| not identified | B28/18 | − | − | − | − | − | − | − | + | − | − | − | − |
| Pseudomonas sp. | B29/18 | − | − | − | ++ | + | − | − | − | − | − | − | − |
| Pseudomonas sp. | B30/18 | − | − | + | + | + | − | − | + | − | − | − | − |
| Bacillus sp. | B31/18 | − | − | − | − | − | − | − | − | − | − | − | − |
| Bacillus sp. | B32/18 | − | − | − | − | − | + | − | − | − | − | − | − |
| Pseudomonas sp. | B33/18 | + | − | − | + | + | − | − | ++ | − | − | − | − |
| Arthrobacter sp. | B35/18 | − | − | − | − | − | − | − | ++ | − | − | − | − |
| Pseudomonas sp. | B37/18 | − | − | ++ | +++ | +++ | ++ | +++ | + | ++ | − | ||
| Bacillus sp. | B39/18 | − | ++ | ++ | ++ | ++ | ++ | ++ | + | +++ | ++ | − | ++ |
| Bacillus sp. | B40/18 | − | − | − | ++ | + | ++ | + | + | + | − | − | − |
| Flavobacterium sp. | B41/18 | − | − | − | − | − | − | + | − | − | − | − | − |
| Pseudomonas sp. | B42/18 | − | − | − | − | − | − | − | ++ | + | − | − | − |
| Pedobacter sp. | B44/18 | − | − | − | − | − | + | ++ | ++ | + | − | − | − |
| Arthrobacter sp. | B45/18 | − | − | − | − | − | − | − | ++ | + | + | − | − |
| Pseudomonas sp. | B46/18 | - | - | - | - | + | + | - | + | - | - | − | − |
| Plantibacter sp. | B47/18 | − | − | − | − | − | − | − | − | − | − | − | − |
| Pseudomonas sp. | B48/18 | − | − | − | − | − | − | − | − | ++ | − | − | − |
| Arthrobacter sp. | B49/18 | - | - | ++ | ++ | ++ | ++ | +++ | + | ++ | - | +++ | - |
| Pedobacter sp. | B51/18 | − | − | − | − | − | − | − | − | − | − | − | − |
| Janthinobacterium sp. | B54/18 | − | − | − | − | − | − | − | − | − | − | − | − |
| Novosphingobium sp. | B57/18 | − | − | − | − | ++ | ++ | − | +++ | − | − | − | − |
| Arthrobacter sp. | B58/18 | ++ | ++ | ++ | ++ | + | +++ | + | + | − | − | ++ | − |
| Arthrobacter sp. | B59/18 | − | − | − | − | − | − | − | − | ++ | − | − | − |
| not identidied | B60/18 | − | − | − | − | + | + | ++ | − | − | − | − | − |
| Bacillus sp. | B61/18 | − | − | − | − | − | − | − | − | − | − | − | − |
| Flavobacterium sp. | B66/18 | − | − | − | ++ | + | ++ | − | +++ | − | − | − | − |
| Bacillus sp. | B68/18 | − | − | − | − | + | − | − | - | − | − | − | − |
| Bacillus sp. | B69/18 | − | − | − | ++ | - | − | − | +++ | − | − | − | − |
| Shinella sp. | B70/18 | − | − | − | − | − | − | − | +++ | − | − | − | − |
| Microbacterium sp. | B72/18 | − | − | − | − | − | − | ++ | + | + | − | − | − |
| Rhodococcus sp. | B73/18 | - | - | - | ++ | ++ | ++ | ++ | + | ++ | − | ++ | − |
| Arthrobacter sp. | B74/18 | + | − | ++ | + | − | − | − | ++ | − | − | − | − |
| Pseudomonas sp. | B75/18 | − | +++ | − | + | − | − | − | +++ | + | − | ++ | − |
| Bacillus sp. | B77/18 | − | − | − | − | + | + | ++ | + | − | − | + | − |
| Substrate | Beneficial Bacteria | Fungal and Fungal-Like Plant Pathogens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhodococcus sp. | Pseudomonas sp. | Arthrobacter sp. | Botrytis spp. | Colletotrichum spp. | Phytophthora spp. | Verticillium spp. | |||||||
| B12/18 | B37/18 | B58/18 | G275/18 | G276/18 | G277/18 | G166/18 | G172/18 | G371/18 | G408/18 | G293/18 | G296/18 | G297/18 | |
| Bromosuccinic Acid | 0.00 | 0.16 | 8.60 | 2.05 | 2.91 | 3.84 | 17.67 | 0.00 | 11.44 | 0.00 | 13.91 | 8.50 | 12.38 |
| D-Arabitol | 0.00 | 0.90 | 8.37 | 1.23 | 1.60 | 1.49 | 1.19 | 1.53 | 1.29 | 1.86 | 6.82 | 3.48 | 3.42 |
| D-Cellobiose | 0.00 | 0.00 | 3.17 | 1.55 | 1.44 | 1.20 | 1.81 | 1.65 | 1.16 | 1.24 | 2.53 | 2.05 | 2.42 |
| Dextrin | 9.32 | 0.00 | 1.56 | 0.00 | 1.85 | 4.14 | 2.38 | 2.98 | 1.74 | 1.93 | 2.27 | 2.08 | 2.31 |
| D-Fructose | 22.84 | 0.00 | 18.46 | 1.39 | 1.42 | 1.24 | 1.80 | 1.53 | 1.73 | 1.30 | 1.64 | 1.61 | 1.67 |
| D-Galactose | 0.00 | 0.00 | 0.00 | 1.38 | 1.42 | 1.37 | 1.35 | 1.59 | 1.31 | 1.39 | 1.61 | 1.59 | 1.65 |
| D-Galacturonic Acid | 0.00 | 0.00 | 16.97 | 1.65 | 1.95 | 1.43 | 2.49 | 2.03 | 1.78 | 1.64 | 0.00 | 0.00 | 0.00 |
| D-Glucuronic Acid | 0.00 | 0.00 | 30.14 | 1.54 | 2.16 | 49.57 | 1.73 | 3.40 | 2.35 | 7.11 | 2.18 | 1.99 | 2.04 |
| D-Lactic Acid Methyl Ester | 0.00 | 0.00 | 9.94 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| D-Malic Acid | 0.00 | 0.00 | 7.43 | 2.70 | 2.92 | 4.66 | 4.80 | 5.24 | 2.59 | 6.13 | 2.09 | 2.07 | 2.11 |
| D-Mannitol | 2.31 | 24.86 | 19.51 | 1.36 | 1.42 | 1.40 | 1.18 | 1.42 | 1.33 | 1.30 | 2.44 | 2.23 | 3.00 |
| D-Mannose | 3.30 | 3.76 | 7.48 | 1.62 | 1.54 | 1.34 | 3.28 | 1.90 | 1.55 | 1.31 | 1.73 | 1.66 | 1.80 |
| D-Melibiose | 0.00 | 0.00 | 0.00 | 1.70 | 1.43 | 1.43 | 1.34 | 1.55 | 1.21 | 1.41 | 1.46 | 1.38 | 1.51 |
| D-Raffinose | 6.03 | 0.00 | 2.70 | 1.40 | 1.45 | 1.34 | 1.24 | 1.39 | 1.33 | 1.23 | 1.52 | 1.51 | 1.52 |
| D-Saccharic Acid | 0.00 | 0.00 | 54.50 | 1.69 | 2.76 | 7.70 | 2.04 | 8.50 | 2.57 | 0.00 | 3.58 | 3.63 | 2.64 |
| D-Sorbitol | 19.00 | 0.00 | 0.00 | 1.38 | 1.36 | 1.20 | 1.46 | 1.93 | 1.41 | 1.22 | 6.71 | 7.47 | 6.36 |
| D-Trehalose | 3.76 | 0.00 | 4.15 | 1.76 | 1.43 | 1.54 | 1.76 | 1.30 | 1.35 | 1.71 | 1.52 | 1.56 | 1.54 |
| Gentiobiose | 68.20 | 0.00 | 9.61 | 1.56 | 1.50 | 1.46 | 2.40 | 1.65 | 1.41 | 1.23 | 1.68 | 1.57 | 1.70 |
| Glucuronamide | 0.00 | 0.00 | 17.05 | 0.00 | 0.00 | 3.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Glycerol | 2.50 | 6.47 | 0.00 | 1.39 | 1.63 | 1.27 | 1.66 | 1.57 | 1.63 | 1.33 | 1.70 | 1.97 | 1.96 |
| L-Alanine | 3.82 | 1.17 | 19.17 | 2.26 | 2.19 | 1.89 | 1.48 | 1.77 | 1.43 | 1.78 | 2.10 | 2.42 | 2.59 |
| L-Aspartic Acid | 3.44 | 2.93 | 24.17 | 3.09 | 5.22 | 4.05 | 2.85 | 3.28 | 2.69 | 0.00 | 3.35 | 3.70 | 3.56 |
| L-Fucose | 0.00 | 0.00 | 127.57 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.70 | 2.33 | 3.91 |
| L-Glutamic Acid | 0.00 | 2.46 | 9.13 | 2.27 | 2.42 | 2.00 | 2.94 | 5.13 | 1.63 | 3.34 | 3.23 | 2.46 | 3.97 |
| L-Lactic Acid | 3.42 | 3.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 13.15 | 0.00 | 255.50 | 5.43 | 14.76 |
| L-Malic Acid | 3.01 | 30.46 | 8.28 | 0.00 | 4.90 | 0.00 | 6.63 | 5.89 | 2.83 | 0.00 | 3.94 | 3.92 | 4.49 |
| L-Pyroglutamic Acid | 2.34 | 5.90 | 0.00 | 0.00 | 0.00 | 0.00 | 2.19 | 1.82 | 1.80 | 0.00 | 0.00 | 0.00 | 0.00 |
| L-Rhamnose | 0.00 | 0.00 | 82.45 | 1.27 | 1.47 | 1.33 | 1.26 | 1.18 | 1.25 | 1.20 | 1.53 | 1.51 | 1.58 |
| L-Serine | 3.30 | 0.52 | 6.45 | 1.92 | 24.50 | 3.03 | 2.28 | 2.96 | 1.90 | 6.94 | 2.58 | 2.54 | 2.82 |
| N-Acetyl-D-Galactosamine | 0.00 | 0.00 | 15.67 | 1.50 | 1.73 | 3.20 | 1.16 | 1.40 | 1.23 | 1.49 | 1.56 | 1.78 | 1.70 |
| N-Acetyl-D-Glucosamine | 2.23 | 0.00 | 3.99 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| N-Acetyl-D-Mannosamine | 0.00 | 0.00 | 6.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Quinic Acid | 2.26 | 0.00 | 11.40 | 1.57 | 1.94 | 1.74 | 1.41 | 1.70 | 1.30 | 2.57 | 3.61 | 2.65 | 2.87 |
| Stachyose | 21.00 | 0.00 | 5.45 | 1.37 | 1.33 | 1.43 | 1.33 | 1.62 | 1.26 | 1.34 | 1.46 | 1.46 | 1.50 |
| Sucrose | 9.46 | 0.00 | 3.71 | 1.45 | 1.40 | 1.46 | 1.68 | 1.53 | 1.73 | 1.32 | 1.54 | 1.55 | 1.56 |
| α-D-Glucose | 1.51 | 4.07 | 8.24 | 1.56 | 1.40 | 1.41 | 1.70 | 1.33 | 1.18 | 1.46 | 1.62 | 1.62 | 1.68 |
| α-D-Lactose | 139.50 | 0.00 | 13.03 | 1.42 | 1.39 | 1.33 | 1.46 | 1.83 | 1.41 | 1.26 | 0.00 | 0.00 | 0.00 |
| α-Keto-glutaric Acid | 5.97 | 0.00 | 0.00 | 78.29 | 5.07 | 0.00 | 3.80 | 5.58 | 8.88 | 21.06 | 8.26 | 5.57 | 6.92 |
| β-Methyl-D-Glucoside | 38.80 | 0.00 | 7.34 | 1.79 | 1.51 | 1.73 | 1.49 | 1.68 | 1.44 | 1.45 | 1.52 | 1.44 | 1.54 |
| γ-Amino-butyric Acid | 2.00 | 0.00 | 1.74 | 1.41 | 7.05 | 3.19 | 1.39 | 1.38 | 1.43 | 2.17 | 2.11 | 2.30 | 2.50 |
| Isolate Code LMEM | Identification | The Accession Number of Sequences in GenBank | Compartment | Microbiological Medium | Forest Location in Poland | Coordinates | Forest Distinct in Poland |
|---|---|---|---|---|---|---|---|
| B10/18 | Flavobacterium sp. | MW255682 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01061 E 022.01705 | Kraśnik |
| B11/18 | Arthrobacter sp. | MW255683 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01061 E 022.01705 | Kraśnik |
| B12/18 | Rhodococcus sp. | MW255650 | wild raspberry rhizosphere | Agar with soil extract | Chruślanki Józefowskie | N 51.01061 E 022.01705 | Kraśnik |
| B13/18 | Pseudomonas sp. | MW255684 | wild raspberry rhizosphere | Agar with soil extract | Chruślanki Józefowskie | N 51.01061 E 022.01705 | Kraśnik |
| B14/18 | Pseudomonas sp. | MW255685 | wild raspberry rhizosphere | Agar with soil extract | Chruślanki Józefowskie | N 51.01061 E 022.01705 | Kraśnik |
| B15/18 | Pseudomonas sp. | MW255686 | wild raspberry rhizosphere | Plate Count Agar | Pomorze | N 51.17137 E 021.95993 | Kraśnik |
| B16/18 | Arthrobacter sp. | MW255687 | wild raspberry rhizosphere | Agar with soil extract | Wierzchowiska | - | Janów Lubelski |
| B17/18 | Mucilaginibacter sp. | MW255688 | wild raspberry rhizosphere | Agar with soil extract | Wierzchowiska | - | Janów Lubelski |
| B18/18 | Burkholderia sp. | MW255689 | wild raspberry rhizosphere | Agar with soil extract | Świdnik | N 51.54462 E 022.28306 | Świdnik |
| B19/18 | Curtobacterium sp. | MW255690 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01083 E 022.01578 | Kraśnik |
| B20/18 | Arthrobacter sp. | MW255691 | wild raspberry rhizosphere | Plate Count Agar | Pomorze | N 51.17137 E 021.95993 | Kraśnik |
| B21/18 | Pseudomonas sp. | - | wild raspberry rhizosphere | Plate Count Agar | Pomorze | N 51.17137 E 021.95993 | Kraśnik |
| B22/18 | Pseudomonas sp. | MW255692 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01061 E 022.01705 | Kraśnik |
| B23/18 | Microbacterium sp. | MW255693 | wild raspberry rhizosphere | Agar with soil extract | Chruślanki Józefowskie | N 51.01051 E 022.01672 | Kraśnik |
| B24/18 | Xanthomonas sp. | MW255694 | wild raspberry rhizosphere | Plate Count Agar | Wierzchowiska | - | Janów Lubelski |
| B25/18 | Pseudomonas protegens | MW255695 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.00903 E 022.01139 | Kraśnik |
| B26/18 | Pseudomonas mohnii | MW255696 | wild raspberry rhizosphere | Plate Count Agar | Pomorze | N 51.17247 E 021.95057 | Kraśnik |
| B27/18 | Bacillus mycoides | MW255697 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.00903 E 022.01139 | Kraśnik |
| B29/18 | Pseudomonas sp. | MW255698 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01083 E 022.01578 | Kraśnik |
| B30/18 | Pseudomonas sp. | MW255699 | wild raspberry rhizosphere | Plate Count Agar | Wierzchowiska | - | Janów Lubelski |
| B31/18 | Bacillus sp. | MW255700 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.00936 E 022.01220 | Kraśnik |
| B32/18 | Bacillus mycoides | MW255701 | wild raspberry rhizosphere | Plate Count Agar | Bobowiska | N 51.41395 E 022.24402 | Puławy |
| B33/18 | Pseudomonas sp. | MW255702 | wild raspberry rhizosphere | Plate Count Agar | Bobowiska | N 51.41395 E 022.24402 | Puławy |
| B34/18 | Erwinia sp. | MW255703 | wild raspberry rhizosphere | Plate Count Agar | Krzywda | - | Łuków |
| B35/18 | Arthrobacter psychrolactophilus | MW255704 | wild raspberry rhizosphere | Plate Count Agar | Krzywda | - | Łuków |
| B36/18 | Pseudomonas sp. | MW255705 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01061 E 022.01705 | Kraśnik |
| B37/18 | Pseudomonas sp. | MW255651 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01061 E 022.01705 | Kraśnik |
| B38/18 | Leifsonia sp. | MW255706 | wild raspberry rhizosphere | Plate Count Agar | Śmiary | - | Siedlce |
| B39/18 | Bacillus thuringiensis | MW255707 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01051 E 022.01672 | Kraśnik |
| B40/18 | Bacillus sp. | MW255708 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01061 E 022.01705 | Kraśnik |
| B41/18 | Flavobacterium sp. | - | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.00926 E 022.01120 | Kraśnik |
| B42/18 | Pseudomonas sp. | MW255709 | wild raspberry rhizosphere | Plate Count Agar | Bobowiska | N 51.41395 E 022.24402 | Puławy |
| B43/18 | Pedobacter sp. | - | wild raspberry rhizosphere | Plate Count Agar | Bobowiska | N 51.41395 E 022.24402 | Puławy |
| B44/18 | Pedobacter sp. | MW255710 | wild raspberry rhizosphere | Plate Count Agar | Bobowiska | N 51.41395 E 022.24402 | Puławy |
| B45/18 | Arthrobacter sp. | MW255711 | wild raspberry rhizosphere | Plate Count Agar | Pomorze | N 51.17100 E 021.95998 | Kraśnik |
| B46/18 | Pseudomonas fluorescens | MW255712 | wild raspberry rhizosphere | Plate Count Agar | Pomorze | N 51.17100 E 021.95998 | Kraśnik |
| B47/18 | Plantibacter sp. | MW255713 | wild raspberry rhizosphere | Plate Count Agar | Pomorze | N 51.17100 E 021.95998 | Kraśnik |
| B48/18 | Pseudomonas sp. | MW255714 | wild raspberry rhizosphere | Plate Count Agar | Pomorze | N 51.17100 E 021.95998 | Kraśnik |
| B49/18 | Arthrobacter sp. | MW255715 | wild raspberry rhizosphere | Plate Count Agar | Bobowiska | N 51.41395 E 022.24402 | Puławy |
| B50/18 | Chryseobacterium balustinum | MW255716 | wild raspberry rhizosphere | Plate Count Agar | Śmiary | - | Siedlce |
| B51/18 | Pedobacter sp. | MW255717 | wild raspberry rhizosphere | Plate Count Agar | Śmiary | - | Siedlce |
| B52/18 | Luteibacter rhizovicinus | MW255718 | wild raspberry rhizosphere | Plate Count Agar | Śmiary | - | Siedlce |
| B53/18 | Burkholderia sp. | MW255719 | wild raspberry rhizosphere | Agar with soil extract | Śmiary | - | Siedlce |
| B54/18 | Janthinobacterium lividum | MW255720 | wild raspberry rhizosphere | Agar with soil extract | Śmiary | - | Siedlce |
| B56/18 | Burkholderia sp. | MW255721 | wild raspberry rhizosphere | Agar with soil extract | Chruślanki Józefowskie | N 51.00926 E 022.01120 | Kraśnik |
| B57/18 | Novosphingobium sp. | MW255722 | wild raspberry rhizosphere | Agar with soil extract | Pomorze | N 51.17137 E 021.95993 | Kraśnik |
| B58/18 | Arthrobacter globiformis | MW255652 | wild raspberry rhizosphere | Agar with soil extract | Wierzchowiska | - | Janów Lubelski |
| B59/18 | Arthrobacter sp. | - | wild raspberry rhizosphere | Agar with soil extract | Pomorze | N 51.17137 E 021.95993 | Kraśnik |
| B61/18 | Bacillus simplex | MW255723 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01083 E 022.01578 | Kraśnik |
| B62/18 | Chryseobacterium sp. | MW255724 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01083 E 022.01578 | Kraśnik |
| B63/18 | Pseudomonas sp. | MW255725 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01083 E 022.01578 | Kraśnik |
| B64/18 | Variovorax sp. | MW255726 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01083 E 022.01578 | Kraśnik |
| B65/18 | Pseudomonas sp. | MW255727 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.00926 E 022.01120 | Kraśnik |
| B66/18 | Flavobacterium sp. | - | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01083 E 022.01578 | Kraśnik |
| B67/18 | Burkholderia sp. | MW255728 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.00903 E 022.01139 | Kraśnik |
| B68/18 | Bacillus mycoides | MW255647 | wild raspberry roots | Plate Count Agar | Chruślanki Józefowskie | N 51.00903 E 022.01139 | Kraśnik |
| B69/18 | Bacillus simplex | MW255648 | wild raspberry roots | Plate Count Agar | Chruślanki Józefowskie | N 51.01083 E 022.01578 | Kraśnik |
| B70/18 | Shinella sp. | MW255729 | wild raspberry rhizosphere | Plate Count Agar | Wierzchowiska | - | Janów Lubelski |
| B71/18 | Roseomonas mucosa | MW255730 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.00926 E 022.01120 | Kraśnik |
| B72/18 | Microbacterium sp. | MW255731 | wild raspberry rhizosphere | Plate Count Agar | Śmiary | - | Siedlce |
| B73/18 | Rhodococcus erythropolis | MW255732 | wild raspberry rhizosphere | with soil extract | Bobowiska | N 51.41395 E 022.24402 | Puławy |
| B74/18 | Arthrobacter sp. | MW255733 | wild raspberry rhizosphere | Plate Count Agar | Pomorze | N 51.17137 E 021.95993 | Kraśnik |
| B75/18 | Arthrobacter sp. | MW255653 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.00926 E 022.01120 | Kraśnik |
| B76/18 | Pseudomonas sp. | MW255734 | wild raspberry rhizosphere | Plate Count Agar | Chruślanki Józefowskie | N 51.01083 E 022.01578 | Kraśnik |
| B77/18 | Bacillus sp. | MW255735 | wild raspberry rhizosphere | Plate Count Agar | Wierzchowiska | - | Janów Lubelski |
References
- Garrido, C.; Carbú, M.; Javier, F.; Cantoral, V.E.G.J.M. New Insights in the Study of Strawberry Fungal Pathogens. Genes Genomes Genom. 2011, 5, 24–39. [Google Scholar]
- Pohto, A. Survey for Phytophthora fragariae var. fragariae in Finland. EPPO Bull. 1999, 29, 159–162. [Google Scholar] [CrossRef]
- Parikka, P.; Lemmetty, A.; Sundelin, T.; Strømeng, G.; Stensvand, A. Survival of Colletotrichum acutatum in plant residue. Acta Hortic. 2016, 1117, 177–180. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Bio/Technol. 2019, 18, 597–616. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of Plant Growth-Promoting Bacteria for Biocontrol of Plant Diseases: Principles, Mechanisms of Action, and Future Prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef]
- Santhanam, R.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Natl. Acad. Sci. USA 2015, 112, E5013–E5020. [Google Scholar] [CrossRef]
- Afegbua, S.L.; Batty, L.C. Effect of plant growth promoting bacterium;Pseudomonas putidaUW4 inoculation on phytoremediation efficacy of monoculture and mixed culture of selected plant species for PAH and lead spiked soils. Int. J. Phytoremediat. 2019, 21, 200–208. [Google Scholar] [CrossRef]
- De Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Sanders, E.R.; Kaplan, D.; Hirsch, A.M. Antifungal Activity of Bacillus Species Against Fusarium and Analysis of the Potential Mechanisms Used in Biocontrol. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef] [PubMed]
- Fikri, A.S.I.; Rahman, I.A.; Nor, N.S.M.; Hamzah, A. Isolation and identification of local bacteria endophyte and screening of its antimicrobial property against pathogenic bacteria and fungi. AIP Conf. Proc. 2018, 1940, 020072. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Effects of pesticides on plant growth promoting traits of Mesorhizobium strain MRC4. J. Saudi Soc. Agric. Sci. 2012, 11, 63–71. [Google Scholar] [CrossRef]
- Oria, B. Television to the Rescue of Romantic Comedy: “Sex and the City’s” Revitalisation of the Genre at the Turn of the Millennium. Int. J. Interdiscip. Soc. Sci. Annu. Rev. 2011, 5, 127–138. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Augustin, J.; Merbach, W. Plant rhizodeposition—An important source for carbon turnover in soils. J. Plant Nutr. Soil Sci. 2002, 165, 397–407. [Google Scholar] [CrossRef]
- Jones, D.L. Organic acids in the rhizosphere—A critical review David. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Oszust, K.; Cybulska, J.; Frąc, M. How Do Trichoderma Genus Fungi Win a Nutritional Competition Battle against Soft Fruit Pathogens? A Report on Niche Overlap Nutritional Potentiates. Int. J. Mol. Sci. 2020, 21, 4235. [Google Scholar] [CrossRef]
- Waschina, S.; D’Souza, G.; Kost, C.; Kaleta, C. Metabolic network architecture and carbon source determine metabolite production costs. FEBS J. 2016, 283, 2149–2163. [Google Scholar] [CrossRef]
- Deng, H.; Ge, L.; Xu, T.; Zhang, M.; Wang, X.; Zhang, Y.; Peng, H. Analysis of the Metabolic Utilization of Carbon Sources and Potential Functional Diversity of the Bacterial Community in Lab-Scale Horizontal Subsurface-Flow Constructed Wetlands. J. Environ. Qual. 2011, 40, 1730–1736. [Google Scholar] [CrossRef]
- Da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Dernoling, F.; Figueroa, D.; Bååth, E. Comparison of factors limiting bacterial growth in different soils. Soil Biol. Biochem. 2007, 39, 2485–2495. [Google Scholar] [CrossRef]
- Latawiec, A.E. A reconnaissance-scale gis-based multicriteria decision analysis to support sustainable biochar use: Poland as a case study. J. Environ. Eng. Landsc. Manag. 2017, 25, 208–222. [Google Scholar] [CrossRef]
- Fabian, C.; Reimann, C.; Fabian, K.; Birke, M.; Baritz, R.; Haslinger, E. GEMAS: Spatial distribution of the pH of European agricultural and grazing land soil. Appl. Geochem. 2014, 48, 207–216. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef]
- Donmez, M.F.; Esitken, A.; Yildiz, H.; Ercisli, S. Biocontrol of Botrytis Cinerea on strawberry fruit by plant growth promoting bacteria. J. Anim. Plant Sci. 2011, 21, 758–763. [Google Scholar]
- Pierson, L.S.; Pierson, E.A. Metabolism and function of phenazines in bacteria: Impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010, 86, 1659–1670. [Google Scholar] [CrossRef]
- Haggag, W.M.; El Soud, M.A. Production and Optimization of Pseudomonas fluorescens Biomass and Metabolites for Biocontrol of Strawberry Grey Mould. Am. J. Plant Sci. 2012, 3, 836–845. [Google Scholar] [CrossRef]
- Gangwar, P.; Alam, S.I.; Singh, L. Metabolic Characterization of cold active Pseudomonas, Arthrobacter, Bacillus, and Flavobacterium spp. from Western Himalayas. Indian J. Microbiol. 2011, 51, 70–75. [Google Scholar] [CrossRef]
- Ahmad, I.; Pichtel, J.; Hayat, S. (Eds.) Plant-Bacteria Interactions: Strategies and Techniques to Promote Plant Growth; Wiley-VCH: Weinheim, Germany, 2008; Volume 1, ISBN 978-3-527-31901. [Google Scholar]
- De Saad, A.M.S.; De Nadra, M.C.M. Sugar and malic acid utilization and acetic acid formation byLeuconostoc oenos. World J. Microbiol. Biotechnol. 1992, 8, 280–283. [Google Scholar] [CrossRef]
- Barbey, C.; Crépin, A.; Bergeau, D.; Ouchiha, A.; Mijouin, L.; Taupin, L.; Orange, N.; Feuilloley, M.; Dufour, A.; Burini, J.-F.; et al. In Planta Biocontrol of Pectobacterium atrosepticum by Rhodococcus erythropolis Involves Silencing of Pathogen Communication by the Rhodococcal Gamma-Lactone Catabolic Pathway. PLoS ONE 2013, 8, e66642. [Google Scholar] [CrossRef]
- Corrêa, B.O.; Schafer, J.T.; Moura, A.B. Spectrum of biocontrol bacteria to control leaf, root and vascular diseases of dry bean. Biol. Control 2014, 72, 71–75. [Google Scholar] [CrossRef]
- Elsayed, Y.; Refaat, J.; Yasmin Elsayed, C.; Abdelmohsen, U.R.; Fouad, M.A. The Genus Rhodococcus as a source of novel bioactive substances: A review. J. Pharmacogn. Phytochem. 2017, 6, 83–92. [Google Scholar]
- Kitagawa, W.; Tamura, T. Three Types of Antibiotics Produced from Rhodococcus erythropolis Strains. Microbes Environ. 2008, 23, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D. Induced Systemic Resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-Dependent Signaling Pathway and Activates PAMP-Triggered Immunity in Arabidopsis. Front. Plant Sci. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Fira, D.; Dimkić, I.; Beric, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Berić, T.; Stević, T.; Pavlovic, S.; Šavikin, K.; Fira, D.; Stanković, S. Additive and synergistic effects of Bacillus spp. isolates and essential oils on the control of phytopathogenic and saprophytic fungi from medicinal plants and marigold seeds. Biol. Control 2015, 87, 6–13. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Allah, E.F.A. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Han, J.-H.; Shim, H.; Shin, J.-H.; Kim, K.S. Antagonistic Activities of Bacillus spp. Strains Isolated from Tidal Flat Sediment Towards Anthracnose Pathogens Colletotrichum acutatum and C. gloeosporioides in South Korea. Plant Pathol. J. 2015, 31, 165–175. [Google Scholar] [CrossRef]
- Moreira, R.R.; Nesi, C.N.; De Mio, L.L.M. Bacillus spp. and Pseudomonas putida as inhibitors of the Colletotrichum acutatum group and potential to control Glomerella leaf spot. Biol. Control 2014, 72, 30–37. [Google Scholar] [CrossRef]
- Shternshis, M.V.; Shpatova, T.; Belyaev, A. Effect of Two Biological Formulations Based on Bacillus subtilis and Pseudomonas fluorescens on Control of Didymella applanata, the Causal Agent of Red Raspberry Cane Spur Blight. Int. J. Agron. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Cabanás, C.G.-L.; Legarda, G.; Ruano-Rosa, D.; Pizarro-Tobías, P.; Valverde-Corredor, A.; Niqui, J.L.; Triviño, J.C.; Roca, A.; Mercado-Blanco, J. Indigenous Pseudomonas spp. Strains from the Olive (Olea europaea L.) Rhizosphere as Effective Biocontrol Agents against Verticillium dahliae: From the Host Roots to the Bacterial Genomes. Front. Microbiol. 2018, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Vallad, G.E.; Zhang, S.; Wen, A.; Balogh, B.; Figueiredo, J.F.L.; Behlau, F.; Jones, J.B.; Momol, M.T.; Olson, S.M. Effect of Application Frequency and Reduced Rates of Acibenzolar-S-Methyl on the Field Efficacy of Induced Resistance Against Bacterial Spot on Tomato. Plant Dis. 2012, 96, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Ozyilmaz, U.; Benlioglu, K. Enhanced Biological Control of Phytophthora Blight of Pepper by Biosurfactant-Producing Pseudomonas. Plant Pathol. J. 2013, 29, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Radkov, A.D.; Moe, L.A. Amino Acid Racemization in Pseudomonas putida KT2440. J. Bacteriol. 2013, 195, 5016–5024. [Google Scholar] [CrossRef]
- Valentini, M.; García-Mauriño, S.M.; Pérez-Martínez, I.; Santero, E.; Canosa, I.; Lapouge, K. Hierarchical management of carbon sources is regulated similarly by the CbrA/B systems in Pseudomonas aeruginosa and Pseudomonas putida. Microbiology 2014, 160, 2243–2252. [Google Scholar] [CrossRef]
- Eraqi, W.A.; Yassin, A.S.; Ali, A.E.; Amin, M.A. Utilization of Crude Glycerol as a Substrate for the Production of Rhamnolipid byPseudomonas aeruginosa. Biotechnol. Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Kaelbling, L.P.; Littman, M.L.; Moore, A.W. Reinforcement Learning: A Survey. J. Artif. Intell. Res. 1996, 4, 237–285. [Google Scholar] [CrossRef]
- Argüelles, J.C. Physiological roles of trehalose in bacteria and yeasts: A comparative analysis. Arch. Microbiol. 2000, 174, 217–224. [Google Scholar] [CrossRef]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar] [CrossRef]
- Peiren, J.; Buyse, J.; De Vos, P.; Lang, E.; Clermont, D.; Hamon, S.; Bégaud, E.; Bizet, C.; Pascual, J.; Ruvira, M.A.; et al. Improving survival and storage stability of bacteria recalcitrant to freeze-drying: A coordinated study by European culture collections. Appl. Microbiol. Biotechnol. 2015, 99, 3559–3571. [Google Scholar] [CrossRef]
- Wepking, C.; Badgley, B.; Barrett, J.E.; Knowlton, K.F.; Lucas, J.M.; Minick, K.J.; Ray, P.P.; Shawver, S.E.; Strickland, M.S. Prolonged exposure to manure from livestock-administered antibiotics decreases ecosystem carbon-use efficiency and alters nitrogen cycling. Ecol. Lett. 2019, 22, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the Soil Environment—Degradation and Their Impact on Microbial Activity and Diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.G.; Deforest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Kuske, C.R. Identification of Cellulose-Responsive Bacterial and Fungal Communities in Geographically and Edaphically Different Soils by Using Stable Isotope Probing. Appl. Environ. Microbiol. 2012, 78, 2316–2327. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Iñigo, M.; Pérez-Sanz, A.; Ortiz, I.; Alonso, J.; Alarcón, R.; García, P.; Lobo, M. Bulk soil and rhizosphere bacterial community PCR–DGGE profiles and β-galactosidase activity as indicators of biological quality in soils contaminated by heavy metals and cultivated with Silene vulgaris (Moench) Garcke. Chemosphere 2009, 75, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Comerford, N.B.; Franzluebbers, A.J.; Stromberger, M.E.; Morris, L.; Markewitz, D.; Moore, R. Assessment and Evaluation of Soil Ecosystem Services. Soil Horizons 2013, 54, 1–14. [Google Scholar] [CrossRef]
- HLPE. Agroecological and Other Innovative Approaches for Sustainable Agriculture and Food Systems that Enhance Food Security and Nutrition; A Report by High Level Panel Experters on Food Security Nutrtion; Communicate World Food Security; HLPE: Rome, Italy, 2019; pp. 1–162. [Google Scholar]
- Ji, S.-H.; Kim, J.-S.; Lee, C.-H.; Seo, H.-S.; Chun, S.-C.; Oh, J.; Choi, E.-H.; Park, G. Enhancement of vitality and activity of a plant growth-promoting bacteria (PGPB) by atmospheric pressure non-thermal plasma. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Menéndez, E.; Garcia-Fraile, P. Plant probiotic bacteria: Solutions to feed the world. AIMS Microbiol. 2017, 3, 747–748. [Google Scholar] [CrossRef]
- Oszust, K.; Frąc, M. First report on the microbial communities of the wild and planted raspberry rhizosphere—A statement on the taxa, processes and a new indicator of functional diversity. Ecol. Indic. 2020, 107117, 107117. [Google Scholar] [CrossRef]
- Jezierska, T.S.; Frąc, M. Microbiological indices of soil quality fertilized with dairy sewage sludge. Int. Agrophys. 2008, 22, 215–219. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G. Building Phylogenetic Trees from Molecular Data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Malarczyk, D.G.; Panek, J.; Frąc, M. Triplex Real-Time PCR Approach for the Detection of Crucial Fungal Berry Pathogens—Botrytis spp., Colletotrichum spp. and Verticillium spp. Int. J. Mol. Sci. 2020, 21, 8469. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Ricciuti, P. A Standardized Method for Estimating the Functional Diversity of Soil Bacterial Community by Biolog® EcoPlatesTM Assay—The Case Study of a Sustainable Olive Orchard. Appl. Sci. 2019, 9, 4035. [Google Scholar] [CrossRef]
- Pinzari, F.; Ceci, A.; Abu-Samra, N.; Canfora, L.; Maggi, O.; Persiani, A.M. Phenotype MicroArray™ system in the study of fungal functional diversity and catabolic versatility. Res. Microbiol. 2016, 167, 710–722. [Google Scholar] [CrossRef]
- Pinzari, F.; Maggi, O.; Lunghini, D.; Di Lonardo, D.P.; Persiani, A.M. A simple method for measuring fungal metabolic quotient and comparing carbon use efficiency of different isolates: Application to Mediterranean leaf litter fungi. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2017, 151, 371–376. [Google Scholar] [CrossRef][Green Version]
- Atlas, R. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439804087. [Google Scholar]
| Examined Isolates of Bacteria | Phytopathogens | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colletotrichum spp. | Verticillium spp. | Phytophthora spp. | Botrytis spp. | ||||||||||
| G172/18 | G371/18 | G166/18 | G293/18 | G296/18 | G297/18 | G368/18 | G373/18 | G369/18 | G275/16 | G277/18 | G276/18 | ||
| Rhodococcus sp. | B12/18 | + | + | ++ | + | ++ | ++ | +++ | + | ++ | − | ++ | +++ |
| Pseudomonas sp. | B25/18 | ++ | ++ | +++ | +++ | ++ | ++ | ++ | + | +++ | − | − | − |
| Pseudomonas sp. | B37/18 | − | − | ++ | +++ | +++ | ++ | +++ | + | ++ | − | − | − |
| Bacillus sp. | B39/18 | − | ++ | ++ | ++ | ++ | ++ | ++ | + | +++ | ++ | − | ++ |
| Bacillus sp. | B40/18 | − | − | − | ++ | + | ++ | + | + | + | − | − | − |
| Arthrobacter sp. | B49/18 | − | − | ++ | ++ | ++ | ++ | +++ | + | ++ | − | +++ | − |
| Arthrobacter sp. | B58/18 | ++ | ++ | ++ | ++ | + | +++ | + | + | − | − | ++ | − |
| Rhodococcus sp. | B73/18 | − | − | − | ++ | ++ | ++ | ++ | + | ++ | − | ++ | − |
| Isolate Names | Isolate Number | Amino Acids, Peptides and Polypeptides | Carboxylic Acids and Esters | Polyols | Sugar Acids and Their Compounds | Sugar Derivatives | Sugars |
|---|---|---|---|---|---|---|---|
| Arthrobacter sp. | B49/18 | 0.54 | 0.84 | 0.76 | 0.00 | 1.43 | 0.54 |
| Arthrobacter sp. | B58/18 | 2.15 | 4.76 | 1.44 | 3.19 | 1.47 | 2.31 |
| Bacillus sp. | B39/18 | 2.45 | 2.31 | 2.18 | 0.77 | 1.66 | 0.85 |
| Bacillus sp. | B40/18 | 2.26 | 2.01 | 2.19 | 0.76 | 1.14 | 0.56 |
| Pseudomonas sp. | B25/18 | 1.81 | 1.49 | 1.79 | 0.58 | 1.26 | 0.51 |
| Pseudomonas sp. | B37/18 | 2.19 | 2.17 | 2.41 | 0.80 | 1.39 | 0.62 |
| Rhodococcus sp. | B73/18 | 2.44 | 2.20 | 1.91 | 0.77 | 1.32 | 0.61 |
| Rhodococcus sp. | B12/18 | 1.28 | 1.41 | 0.89 | 0.25 | 1.75 | 0.81 |
| Metabolic Abilities | Substrate Additive | Rhodococcus sp. B12/18 | Pseudomonas sp. B37/18 | Arthrobacter sp. B58/18 | |||
|---|---|---|---|---|---|---|---|
| 24 h | 168 h | 24 h | 168 h | 24 h | 168 h | ||
| Proteolytic | 4% skim milk | − | − | + | +++ | − | − |
| 4% gelatin | − | − | +++ | +++ | − | + | |
| Amylolytic | 1% starch | − | − | ++ | +++ | + | ++ |
| Amonification | 4% skim milk | + | + | +++ | +++ | ++ | ++ |
| 4% urea | ++ | ++ | − | − | + | + | |
| Denitryfication | 0.1% KNO3 | + | + | − | − | + | ++ |
| Nitrogen fixation | Medium without nitrogen | − | − | − | − | − | − |
| Cellulolytic | Shredded straw | − | + | + | +++ | ++ | +++ |
| Nitryfication | Nitryfication medium | − | − | − | − | − | − |
| Phosphate solubilization | Pikovska medium | − | − | − | − | − | − |
| Enzymatic activity | Substrate | Rhodococcus sp. B12/18 | Pseudomonas sp. B37/18 | Arthrobacter sp. B58/18 | |||
| Alkaline phosphatase | 2-naphthyl phosphate | ++ | − | − | |||
| Esterase (C 4) | 2-naphthyl butyrate | ++ | ++ | + | |||
| Lipase esterase (C 8) | 2-naphthyl caprylate | +++ | + | + | |||
| Lipase (C 14) | 2-naphthyl myristate | − | − | + | |||
| Leucine arrylamidase | L-leucyl-2-naphthyllamide | +++ | ++ | +++ | |||
| Valine arrylamidase | L-valyl-2-naphthlamide | ++ | − | ++ | |||
| Cystine arrylamidase | L-cystyl-2-naphthlamide | + | − | ++ | |||
| Trypsin | N-benzoyl-DL-arginine-2-naphthyllamide | − | − | − | |||
| α-chymotrypsin | N-glutaryl-phenylalanine-2-naphthyllamide | + | − | − | |||
| Acid phosphatase | 2-naphthyl phosphate | ++ | + | + | |||
| Naphthyl-AS-BI phosphohydrolase | Naphthyl AS-BI-phosphate | +++ | ++ | ++ | |||
| α-galactosidase | 6-Br-2-naphthyl-αD-galactopyranoside | − | − | ++ | |||
| ß-galactosidase | 2-naphthyl-ßD-galactopyranoside | − | − | − | |||
| ß-glucuronidase | Naphthyl-AS-BI-ßD-glucuronide | − | − | − | |||
| α-glucosidase | 2-naphthyl-αD-glucopyranoside | +++ | − | ++ | |||
| ß-glucosidase | 6-Br-2-naphthyl-ßD-glucopyranoside | +++ | − | − | |||
| N-acetyl-ß-glucosaminidase | 1-naphthyl-N-acetyl-ßD-glucosaminide | − | − | − | |||
| α-mannosidase | 6-Br-2-naphthyl-αD-mannopyranoside | − | − | + | |||
| α-fucosidase | 2-naphthyl-αL-fucopyranoside | − | − | − | |||
| Amino Acids, Peptideds and Polypeptides | Polyols and Others | Sugars | Sugar Acids and Their Compounds | Carboxylic Acids and Esters | Sugar Derivatives |
|---|---|---|---|---|---|
| D-Aspartic Acid | D-Sorbitol | Dextrin | D-Galacturonic Acid | p-Hydroxyphenylacetic Acid | β-Methyl-D-glucoside |
| D-Serine | D-Mannitol | D-Maltose | L-Galactonic Acid lactone | L-Lactic Acid | D-Salicin |
| Glycyl-L-proline | D-Arabitol | D-Trehalose | D-Gluconic Acid | Citric Acid | N-Acetyl-D-glucosamine |
| L-Alanine | Myo-inositol | D-Cellobiose | D-Glucuronic Acid | α-Ketoglutaric Acid | N-Acetyl-β-D-mannosamine |
| L-Arginine | Glycerol | Gentiobiose | Glucuronamide | D-Malic Acid | N-Acetyl-D-galactosamine |
| L-Aspartic Acid | Tween 40 | Sucrose | Mucic Acid | L-Malic Acid | Inosine |
| L-Glutamic Acid | D-Turanose | Quinic Acid | Bromosuccinic Acid | D-Glucose-6-PO4 | |
| L-Histidine | Stachyose | D-Saccharic Acid | α-Hydroxybutyric Acid | D-Fructose-6-PO4 | |
| L-Pyroglutamic Acid | D-Raffinose | β-Hydroxy-D | |||
| L-Serine | α-D-Lactose | α-Ketobutyric Acid | |||
| γ-Amino-butyric Acid | D-Melibiose | Acetoacetic Acid | |||
| Gelatin | N-Acetylneuraminic Acid | Propionic Acid | |||
| α-D-Glucose | Acetic Acid | ||||
| D-Mannose | Formic Acid | ||||
| D-Fructose | Methyl Pyruvate | ||||
| D-Galactose | D-Lactic Acid Methyl Ester | ||||
| 3-Methylglucose | L-Butyric Acid | ||||
| D-Fucose | |||||
| L-Fucose | |||||
| L-Rhamnose | |||||
| Pectin |
| Antibiotics | Organic Compounds | Chemical Soil Properties | Toxic Substances | |
|---|---|---|---|---|
| pH | Salinity | |||
| Fusidic Acid | 1% Sodium Lactate | pH 6 | 1% NaCl | Guanidine HCl |
| Troleandomycin | D-serine | pH 5 | 4% NaCl | Niaproof 4 |
| Rifamycin SV | Sodium Butyrate | 8% NaCl | Tetrazolium Violet | |
| Minocycline | Sodium Bromate | Tetrazolium Blue | ||
| Lincomycin | Lithium Chloride | |||
| Vancomycin | Potassium tellurite | |||
| Nalidixic Acid | ||||
| Aztreonam | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pylak, M.; Oszust, K.; Frąc, M. Searching for New Beneficial Bacterial Isolates of Wild Raspberries for Biocontrol of Phytopathogens-Antagonistic Properties and Functional Characterization. Int. J. Mol. Sci. 2020, 21, 9361. https://doi.org/10.3390/ijms21249361
Pylak M, Oszust K, Frąc M. Searching for New Beneficial Bacterial Isolates of Wild Raspberries for Biocontrol of Phytopathogens-Antagonistic Properties and Functional Characterization. International Journal of Molecular Sciences. 2020; 21(24):9361. https://doi.org/10.3390/ijms21249361
Chicago/Turabian StylePylak, Michał, Karolina Oszust, and Magdalena Frąc. 2020. "Searching for New Beneficial Bacterial Isolates of Wild Raspberries for Biocontrol of Phytopathogens-Antagonistic Properties and Functional Characterization" International Journal of Molecular Sciences 21, no. 24: 9361. https://doi.org/10.3390/ijms21249361
APA StylePylak, M., Oszust, K., & Frąc, M. (2020). Searching for New Beneficial Bacterial Isolates of Wild Raspberries for Biocontrol of Phytopathogens-Antagonistic Properties and Functional Characterization. International Journal of Molecular Sciences, 21(24), 9361. https://doi.org/10.3390/ijms21249361






