Intravitreal Injection of Liposomes Loaded with a Histone Deacetylase Inhibitor Promotes Retinal Ganglion Cell Survival in a Mouse Model of Optic Nerve Crush
Abstract
1. Introduction
2. Results
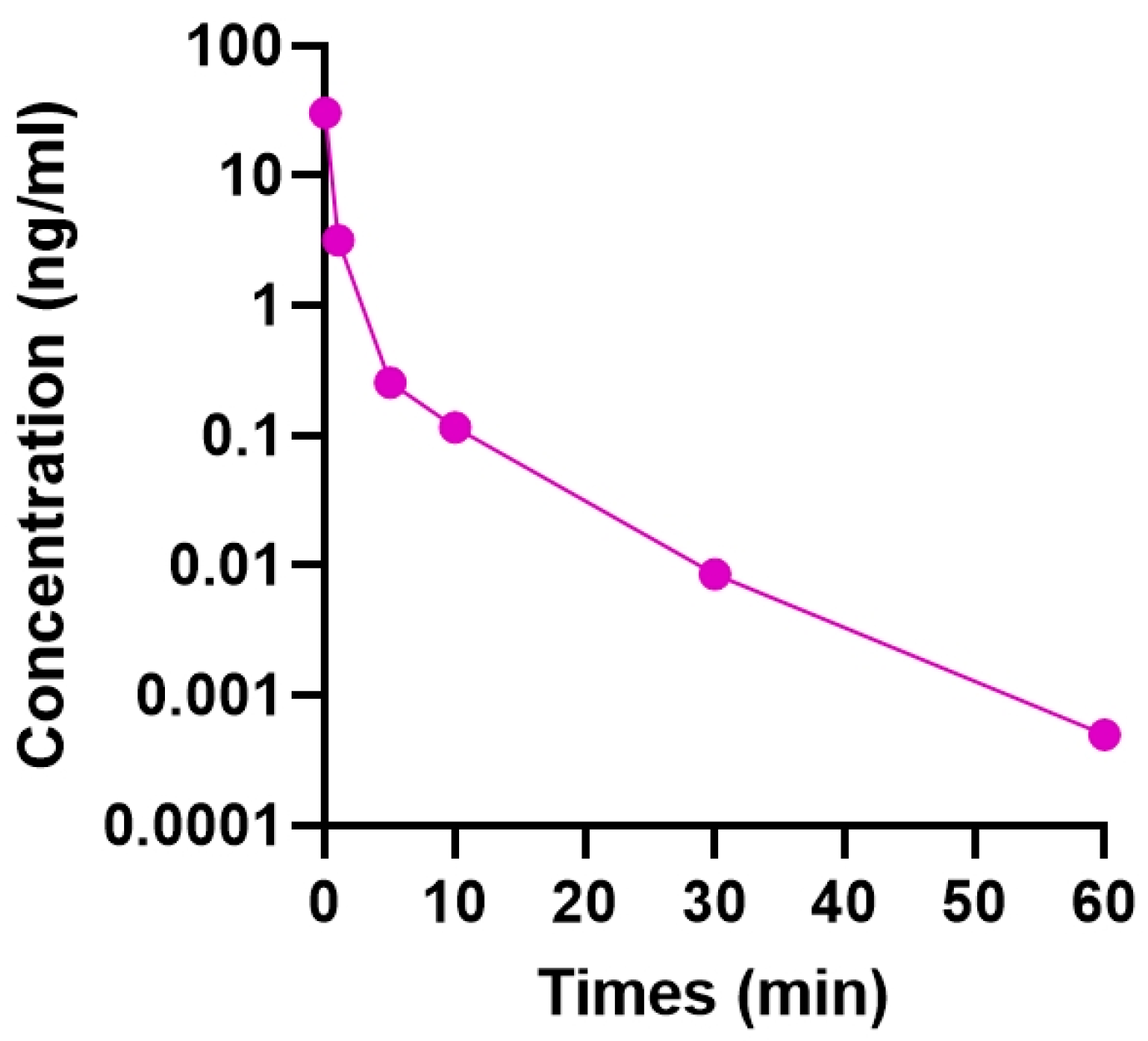
2.1. Residence Time of Intravitreally Injected TSA
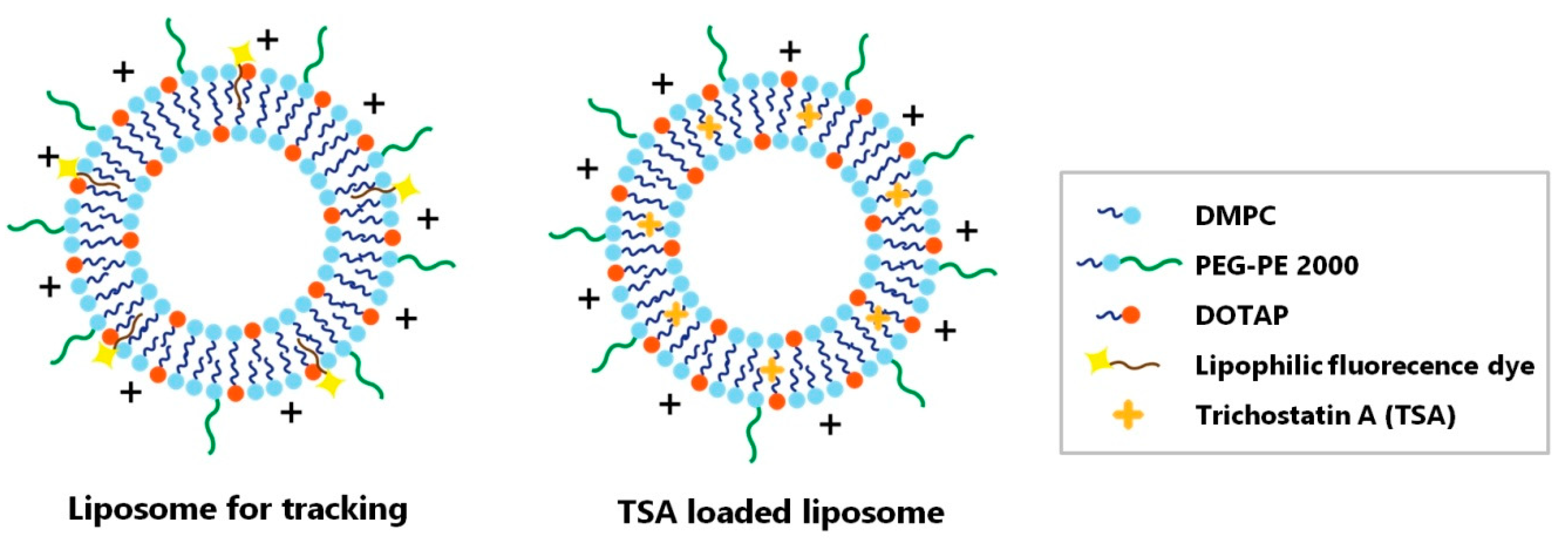
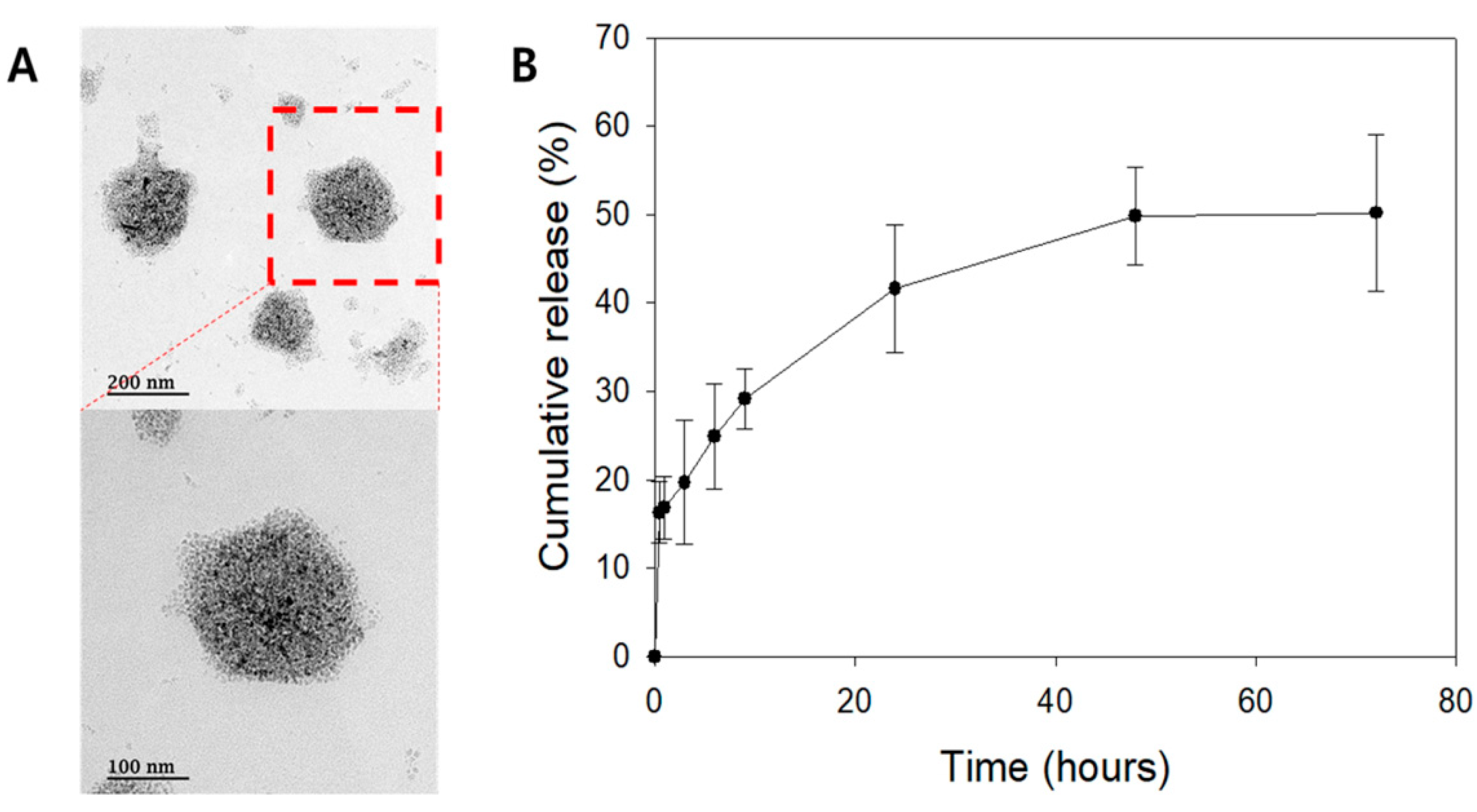
2.2. Liposomal Characterization
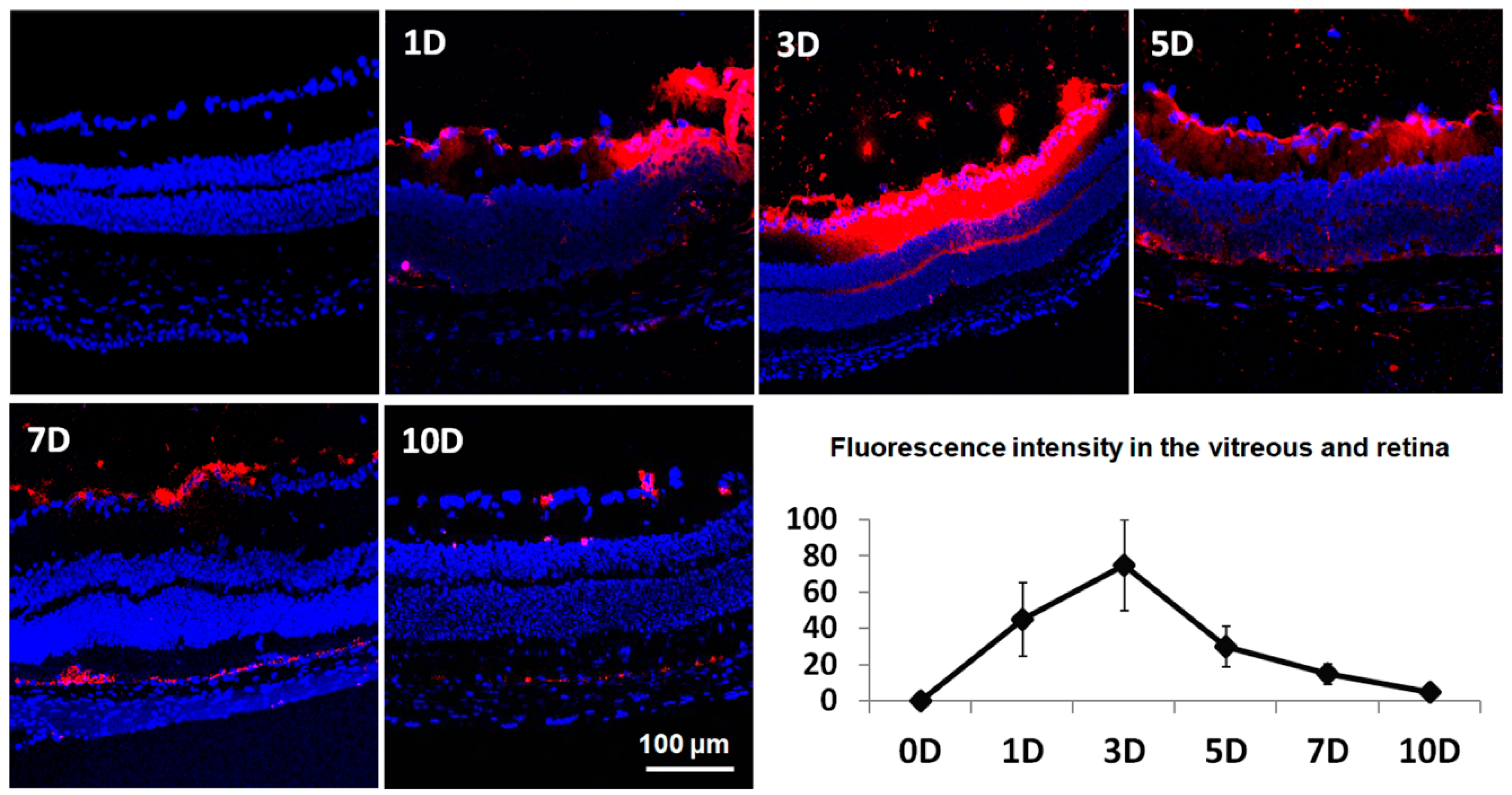
2.3. Temporal Distribution of Intravitreally Injected Liposomes
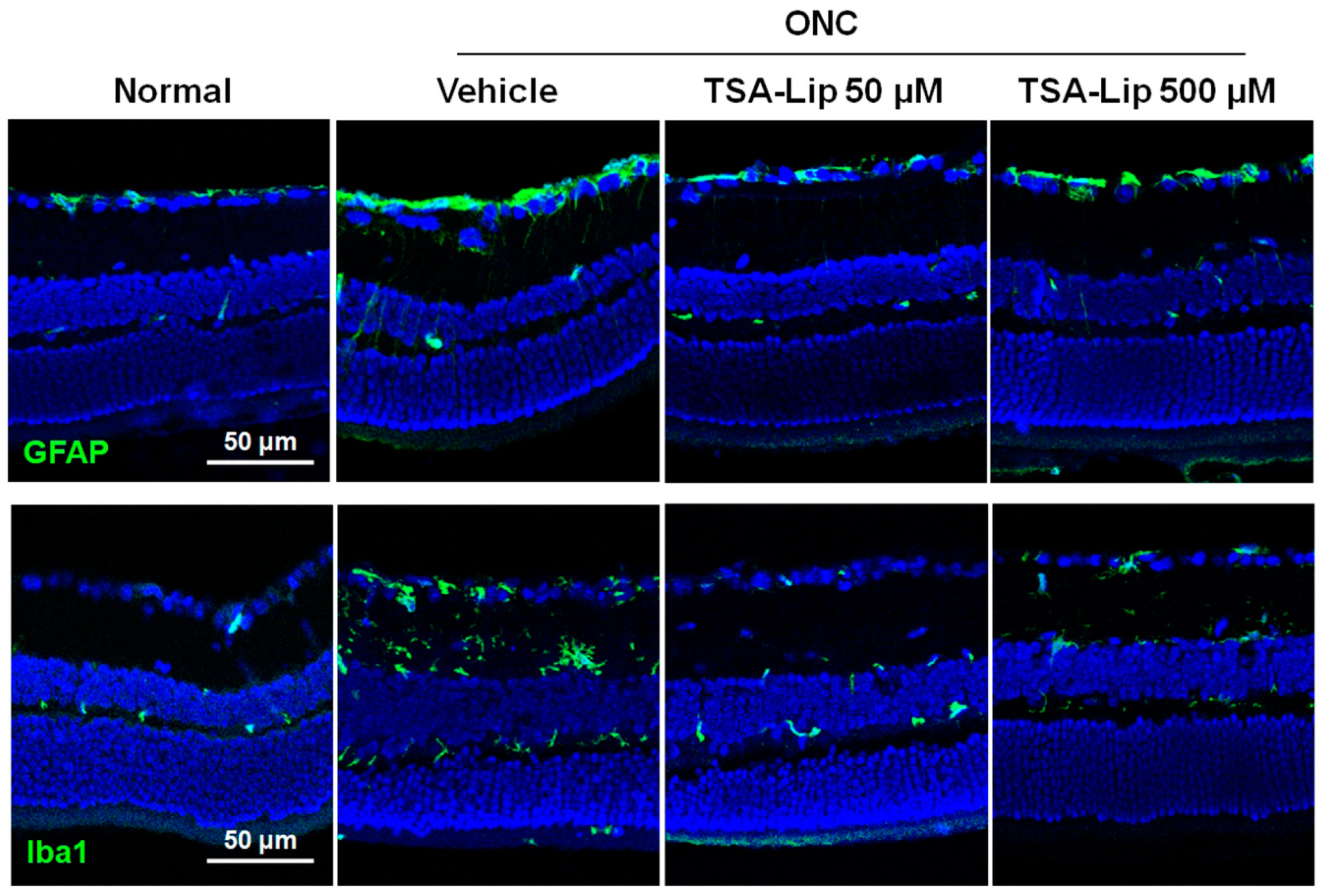
2.4. Intravitreal Injection of TSA-Loaded Liposomes Reduces Glial Cell Activation after ONC
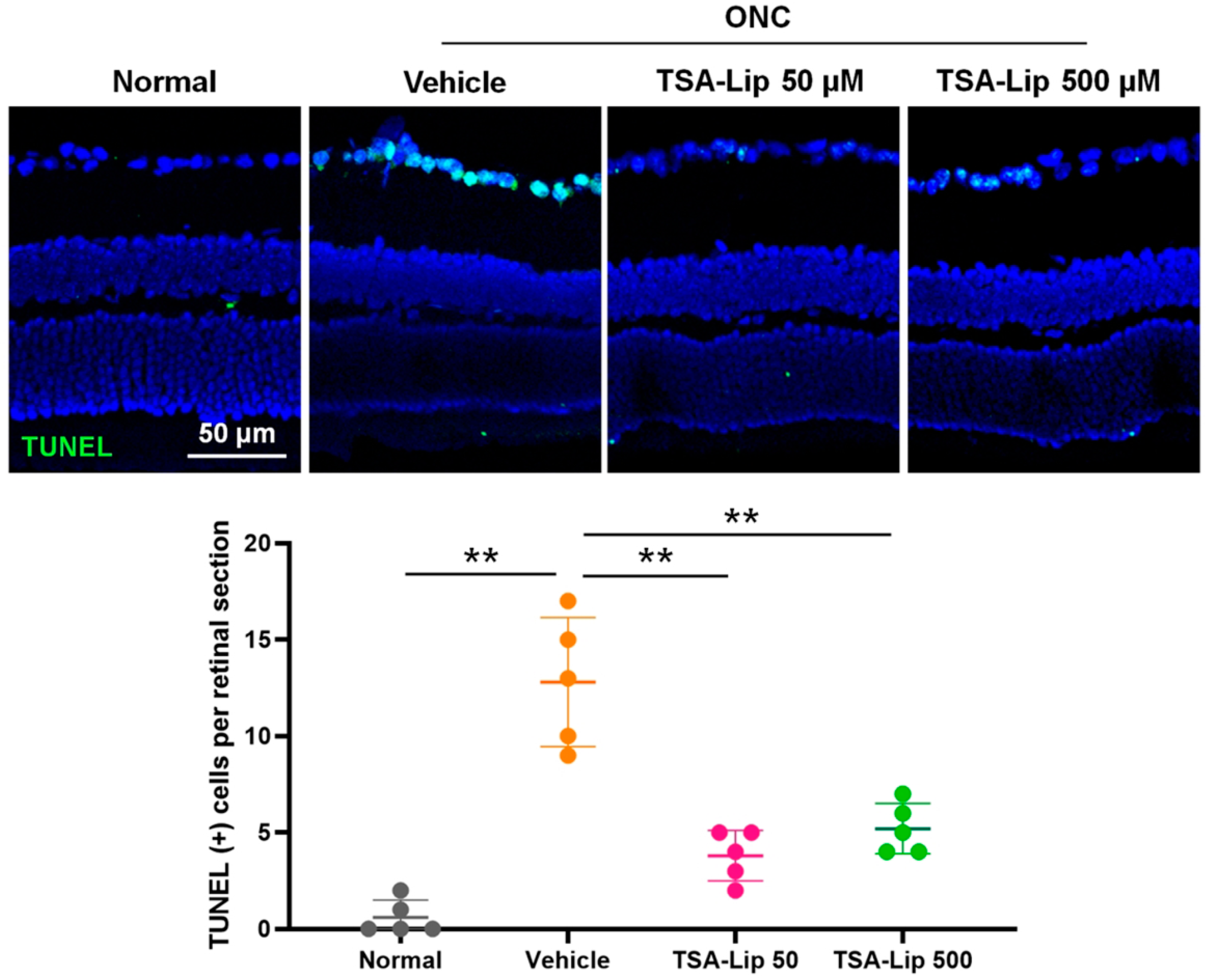
2.5. Intravitreal Injection of TSA-Loaded Liposomes Suppresses RGC Apoptosis after ONC
2.6. Effect of Intravitreally Injected TSA-Loaded Liposomes on RGC Survival after ONC
3. Discussion
4. Materials and Methods
4.1. Animal Use
4.2. Determination of Intravitreal Residence Time of TSA
4.3. Study Design
4.4. Liposome Preparation and Trichostatin A Loading
4.5. Optic Nerve Crush Model
4.6. Intravitreal Injection
4.7. Western Blot Analysis
4.8. Immunohistochemical Analysis
4.9. TUNEL Assay
4.10. Retinal Wholemounts and Brn3a Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kolb, H.; Linberg, K.A.; Fisher, S.K. Neurons of the human retina: A Golgi study. J. Comp. Neurol. 1992, 318, 147–187. [Google Scholar] [CrossRef]
- Hughes, W.F. Quantitation of ischemic damage in the rat retina. Exp. Eye. Res. 1991, 53, 573–582. [Google Scholar] [CrossRef]
- Abu-El-Asrar, A.M.; Dralands, L.; Missotten, L.; Al-Jadaan, I.A.; Geboes, K. Expression of apoptosis markers in the retinas of human subjects with diabetes. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2760–2766. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Mac Nair, C.E.; Schlamp, C.L.; Montgomery, A.D.; Shestopalov, V.I.; Nickells, R.W. Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J. Neuroinflammation 2016, 13, 93. [Google Scholar] [CrossRef]
- Xue, L.P.; Lu, J.; Cao, Q.; Kaur, C.; Ling, E.A. Nestin expression in Muller glial cells in postnatal rat retina and its upregulation following optic nerve transection. Neuroscience 2006, 143, 117–127. [Google Scholar] [CrossRef]
- Li, Y.; Schlamp, C.L.; Nickells, R.W. Experimental induction of retinal ganglion cell death in adult mice. Invest. Ophthalmol. Vis. Sci. 1999, 40, 1004–1008. [Google Scholar]
- Wu, Y.; Pang, Y.; Wei, W.; Shao, A.; Deng, C.; Li, X.; Chang, H.; Hu, P.; Liu, X.; Zhang, X. Resveratrol protects retinal ganglion cell axons through regulation of the SIRT1-JNK pathway. Exp. Eye. Res. 2020, 200, 108249. [Google Scholar] [CrossRef]
- Park, J.W.; Sung, M.S.; Ha, J.Y.; Guo, Y.; Piao, H.; Heo, H.; Park, S.W. Neuroprotective effect of Brazilian green propolis on retinal ganglion cells in ischemic mouse retina. Curr. Eye. Res. 2020, 45, 955–964. [Google Scholar] [CrossRef]
- Geroski, D.H.; Edelhauser, H.F. Drug delivery for posterior segment eye disease. Invest. Ophthalmol. Vis. Sci. 2000, 41, 961–964. [Google Scholar]
- Gaudana, R.; Jwala, J.; Boddu, S.H.S.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197–1216. [Google Scholar] [CrossRef]
- Holland, G.N.; Sakamoto, M.J.; Hardy, D.; Sidikaro, Y.; Kreiger, A.E.; Frenkel, L.M. Treatment of cytomegalovirus retinopathy in patients with acquired immunodeficiency syndrome. Use of the experimental drug 9-[2-hydroxy-1-(hydroxymethyl)ethoxymethyl]guanine. Arch. Ophthalmol. 1986, 104, 1794–1800. [Google Scholar] [CrossRef]
- del Amo, E.M.; Vellonen, K.S.; Kidron, H.; Urtti, A. Intravitreal clearance and volume of distribution of compounds in rabbits: In silico prediction and pharmacokinetic simulations for drug development. Eur. J. Pharm. Biopharm. 2015, 95, 215–226. [Google Scholar] [CrossRef]
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retinal. Eye. Res. 2010, 29, 596–609. [Google Scholar] [CrossRef]
- Souto, E.B.; Doktorovova, S.; Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L. Feasibility of lipid nanoparticles for ocular delivery of anti-inflammatory drugs. Curr. Eye. Res. 2010, 35, 537–552. [Google Scholar] [CrossRef]
- Ngwa, W.; Makrigiorgos, G.M.; Berbeco, R.I. Gold nanoparticle enhancement of stereotactic radiosurgery for neovascular age-related macular degeneration. Phys. Med. Biol. 2012, 57, 6371–6380. [Google Scholar] [CrossRef]
- Lee, J.; Goh, U.; Lee, H.J.; Kim, J.; Jeong, M.; Park, J.H. Effective retinal penetration of lipophilic and lipid-conjugated hydrophilic agents delivered by engineered liposomes. Mol. Pharm. 2017, 14, 423–430. [Google Scholar] [CrossRef]
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Oku, N. Anticancer therapy using glucuronate modified long-circulating liposomes. Adv. Drug. Deliv. Rev. 1999, 40, 63–73. [Google Scholar] [CrossRef]
- Sapra, P.; Tyagi, P.; Allen, T.M. Ligand-targeted liposomes for cancer treatment. Curr. Drug. Deliv. 2005, 2, 369–381. [Google Scholar] [CrossRef]
- van Rooijen, N.; van Nieuwmegen, R. Liposomes in immunology: Multilamellar phosphatidylcholine liposomes as a simple, biodegradable and harmless adjuvant without any immunogenic activity of its own. Immunol. Commun. 1980, 9, 243–256. [Google Scholar] [CrossRef]
- Lopez-Berestein, G.; Mehta, R.; Hopfer, R.; Mehta, K.; Hersh, E.M.; Juliano, R. Effects of sterols on the therapeutic efficacy of liposomal amphotericin B in murine candidiasis. Cancer. Drug. Deliv. 1983, 1, 37–42. [Google Scholar] [CrossRef]
- Sung, M.S.; Heo, H.; Eom, G.H.; Kim, S.Y.; Piao, H.; Guo, Y.; Park, S.W. HDAC2 regulates glial cell activation in ischemic mouse retina. Int. J. Mol. Sci. 2019, 20, 5159. [Google Scholar] [CrossRef]
- Schmitt, M.; Hippeläinen, E.; Raviña, M.; Arango-Gonzalez, B.; Antopolsky, M.; Vellonen, K.S.; Airaksinen, A.J.; Urtti, A. Intravitreal pharmacokinetics in mice: SPECT/CT imaging and scaling to rabbits and humans. Mol. Pharm. 2019, 16, 4399–4404. [Google Scholar] [CrossRef]
- Trifunović, D.; Arango-Gonzalez, B.; Comitato, A.; Barth, M.; Del Amo, E.M.; Kulkarni, M.; Sahaboglu, A.; Hauck, S.M.; Urtti, A.; Arsenijevic, Y.; et al. HDAC inhibition in the cpfl1 mouse protects degenerating cone photoreceptors in vivo. Hum. Mol. Genet. 2016, 25, 4462–4472. [Google Scholar]
- Yuan, L.; Neufeld, A.H. Tumor necrosis factor-alpha: A potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia 2000, 32, 42–50. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye. Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Saha, R.N.; Pahan, K. HATs and HDACs in neurodegeneration: A tale of disconcerted acetylation homeostasis. Cell. Death. Differ. 2006, 13, 539–550. [Google Scholar] [CrossRef]
- Kukucka, J.; Wyllie, T.; Read, J.; Mahoney, L.; Suphioglu, C. Human neuronal cells: Epigenetic aspects. Biomol. Concepts. 2013, 4, 319–333. [Google Scholar] [CrossRef]
- Zalewska, T.; Jaworska, J.; Sypecka, J.; Ziemka-Nalecz, M. Impact of a Histone deacetylase inhibitor-Trichostatin A on neurogenesis after hypoxia-ischemia in immature rats. Int. J. Mol. Sci. 2020, 21, 3808. [Google Scholar] [CrossRef]
- Gupta, R.; Ambasta, R.K.; Kumar, P. Pharmacological intervention of histone deacetylase enzymes in the neurodegenerative disorders. Life. Sci. 2020, 243, 117278. [Google Scholar] [CrossRef]
- Lebrun-Julien, F.; Suter, U. Combined HDAC1 and HDAC2 depletion promotes retinal ganglion cell survival after injury through reduction of p53 target gene expression. ASN. Neuro 2015, 7, 1759091415593066. [Google Scholar] [CrossRef]
- Bishop, P.N. Structural macromolecules and supramolecular organisation of the vitreous gel. Prog. Retin. Eye. Res. 2000, 19, 323–344. [Google Scholar] [CrossRef]
- Koo, H.; Moon, H.; Han, H.; Na, J.H.; Huh, M.S.; Park, J.H.; Woo, S.J.; Park, K.H.; Kwon, I.C.; Kim, K.; et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials 2012, 33, 3485–3493. [Google Scholar] [CrossRef]
- Kim, H.; Robinson, S.B.; Csaky, K.G. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharm. Res. 2009, 26, 329–337. [Google Scholar] [CrossRef]
- Baltan, S.; Bachleda, A.; Morrison, R.S.; Murphy, S.P. Expression of histone deacetylases in cellular compartments of the mouse brain and the effects of ischemia. Transl. Stroke. Res. 2011, 2, 411–423. [Google Scholar] [CrossRef]
- Shukla, S.; Tekwani, B.L. Histone deacetylases inhibitors in neurodegenerative diseases, neuroprotection and neuronal differentiation. Front. Pharmacol. 2020, 11, 537. [Google Scholar] [CrossRef]
- Sánchez-Migallón, M.C.; Valiente-Soriano, F.J.; Nadal-Nicolás, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Apoptotic retinal ganglion cell death after optic nerve transection or crush in mice: Delayed RGC loss with BDNF or a Caspase 3 inhibitor. Invest. Ophthalmol. Vis. Sci. 2016, 57, 81–93. [Google Scholar] [CrossRef]
- Pelzel, H.R.; Schlamp, C.L.; Nickells, R.W. Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis. BMC. Neurosci. 2010, 11, 62. [Google Scholar] [CrossRef]
- Stankowska, D.L.; Dibas, A.; Li, L.; Zhang, W.; Krishnamoorthy, V.R.; Chavala, S.H.; Nguyen, T.P.; Yorio, T.; Ellis, D.Z.; Acharya, S. Hybrid compound SA-2 is neuroprotective in animal models of retinal ganglion cell death. Invest. Ophthalmol. Vis. Sci. 2019, 60, 3064–3073. [Google Scholar] [CrossRef]
- Zahavi, A.; Weiss, S.; Vieyra, M.; Nicholson, J.D.; Muhsinoglu, O.; Barinfeld, O.; Zadok, D.; Goldenberg-Cohen, N. Ocular effects of sildenafil in naïve mice and a mouse model of optic nerve crush. Invest. Ophthalmol. Vis. Sci. 2019, 60, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, H.; Fang, F.; Liu, L.; Sun, Y.; Hu, Y. Longitudinal morphological and functional assessment of RGC neurodegeneration after optic nerve crush in mouse. Front. Cell. Neurosci. 2020, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Puyang, Z.; Feng, L.; Duan, L.; Liang, P.; Backman, V.; Liu, X.; Zhang, H.F. Optical detection of early damage in retinal ganglion cells in a mouse model of partial optic nerve crush injury. Invest. Ophthalmol. Vis. Sci. 2016, 57, 5665–5671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skeie, J.M.; Tsang, S.H.; Mahajan, V.B. Evisceration of mouse vitreous and retina for proteomic analyses. J. Vis. Exp. 2011, 50, 2795. [Google Scholar] [CrossRef] [PubMed]
- Levkovitch-Verbin, H.; Harris-Cerruti, C.; Groner, Y.; Wheeler, L.A.; Schwartz, M.; Yoles, E. RGC death in mice after optic nerve crush injury: Oxidative stress and neuroprotection. Invest. Ophthalmol. Vis. Sci. 2000, 41, 4169–4174. [Google Scholar]
- Tezel, G.; Yang, X.; Yang, J.; Wax, M.B. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain. Res. 2004, 996, 202–212. [Google Scholar] [CrossRef]
- Gabriele, M.L.; Ishikawa, H.; Schuman, J.S.; Ling, Y.; Bilonick, R.A.; Kim, J.S.; Kagemann, L.; Wollstein, G. Optic nerve crush mice followed longitudinally with spectral domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2011, 52, 2250–2254. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, M.S.; Moon, M.J.; Thomas, R.G.; Kim, S.Y.; Lee, J.S.; Jeong, Y.Y.; Park, I.-K.; Park, S.W. Intravitreal Injection of Liposomes Loaded with a Histone Deacetylase Inhibitor Promotes Retinal Ganglion Cell Survival in a Mouse Model of Optic Nerve Crush. Int. J. Mol. Sci. 2020, 21, 9297. https://doi.org/10.3390/ijms21239297
Sung MS, Moon MJ, Thomas RG, Kim SY, Lee JS, Jeong YY, Park I-K, Park SW. Intravitreal Injection of Liposomes Loaded with a Histone Deacetylase Inhibitor Promotes Retinal Ganglion Cell Survival in a Mouse Model of Optic Nerve Crush. International Journal of Molecular Sciences. 2020; 21(23):9297. https://doi.org/10.3390/ijms21239297
Chicago/Turabian StyleSung, Mi Sun, Myeong Ju Moon, Reju George Thomas, So Young Kim, Jun Sung Lee, Yong Yeon Jeong, In-Kyu Park, and Sang Woo Park. 2020. "Intravitreal Injection of Liposomes Loaded with a Histone Deacetylase Inhibitor Promotes Retinal Ganglion Cell Survival in a Mouse Model of Optic Nerve Crush" International Journal of Molecular Sciences 21, no. 23: 9297. https://doi.org/10.3390/ijms21239297
APA StyleSung, M. S., Moon, M. J., Thomas, R. G., Kim, S. Y., Lee, J. S., Jeong, Y. Y., Park, I.-K., & Park, S. W. (2020). Intravitreal Injection of Liposomes Loaded with a Histone Deacetylase Inhibitor Promotes Retinal Ganglion Cell Survival in a Mouse Model of Optic Nerve Crush. International Journal of Molecular Sciences, 21(23), 9297. https://doi.org/10.3390/ijms21239297





