Structural, Pro-Inflammatory and Calcium Handling Remodeling Underlies Spontaneous Onset of Paroxysmal Atrial Fibrillation in JDP2-Overexpressing Mice
Abstract
1. Introduction
2. Results
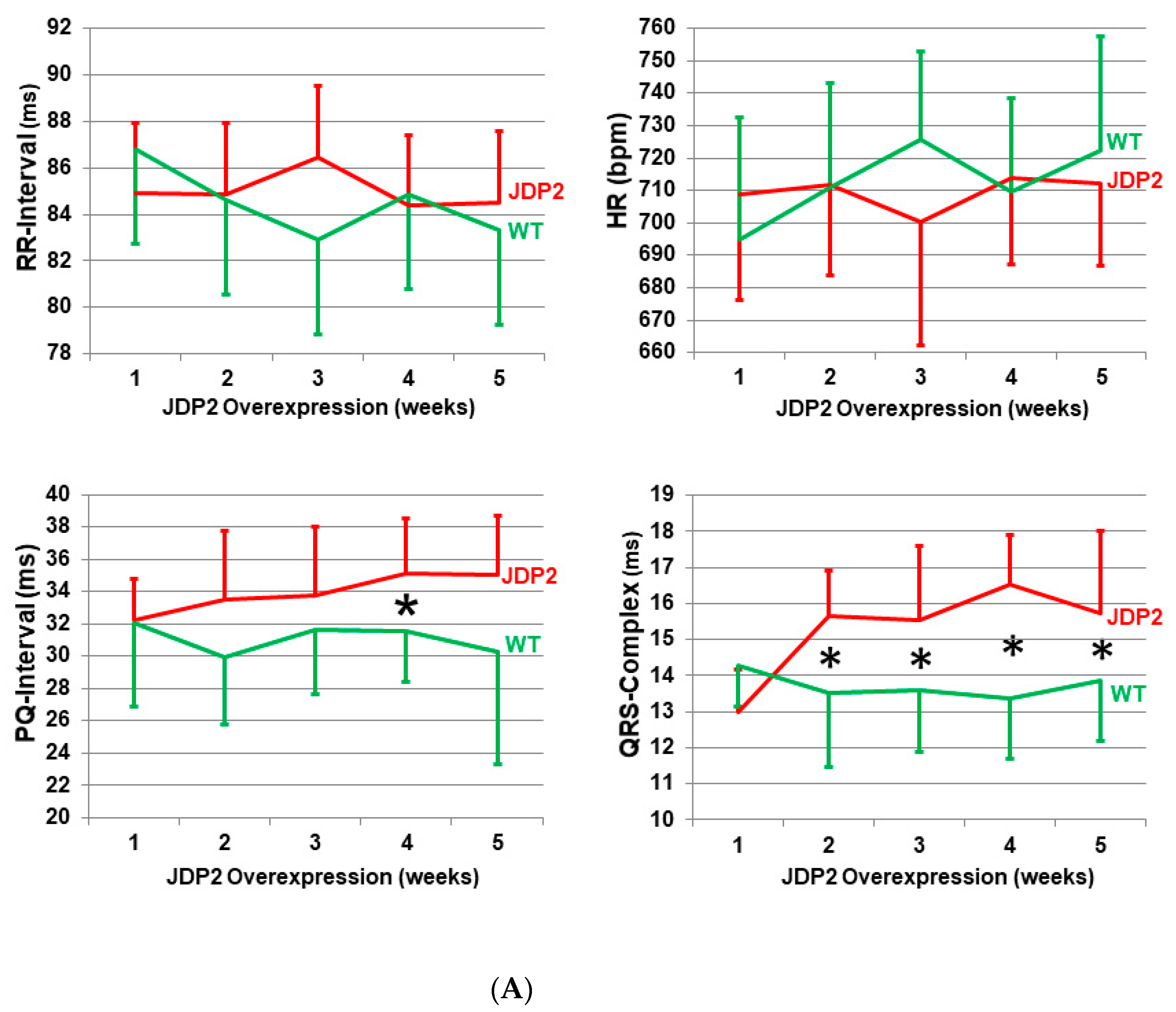
2.1. Conduction Defects and Arrhythmias in JDP2 Mice
2.2. Atrial Hypertrophy
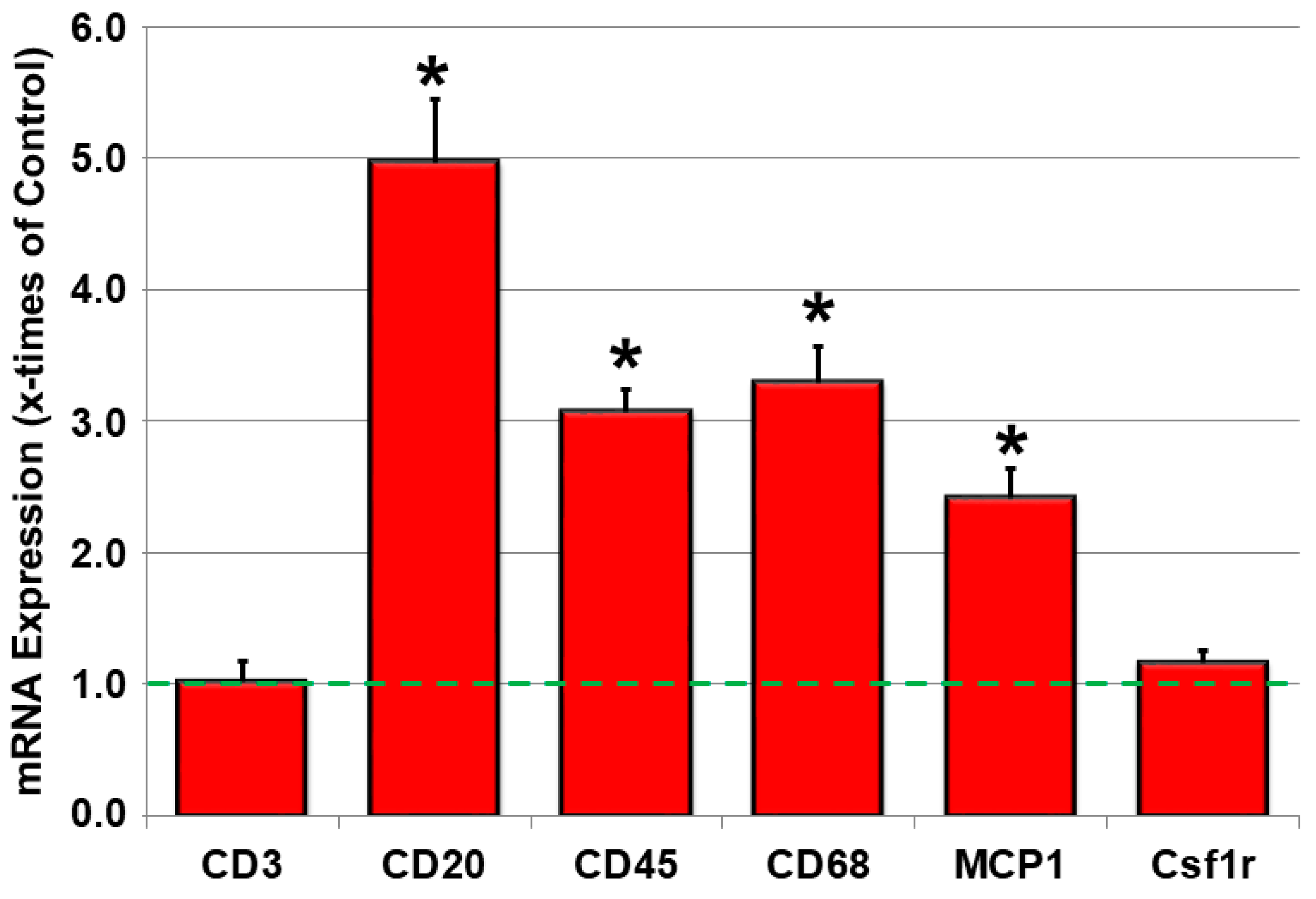
2.3. Inflammatory Parameters Are Increased in JDP2 Mice
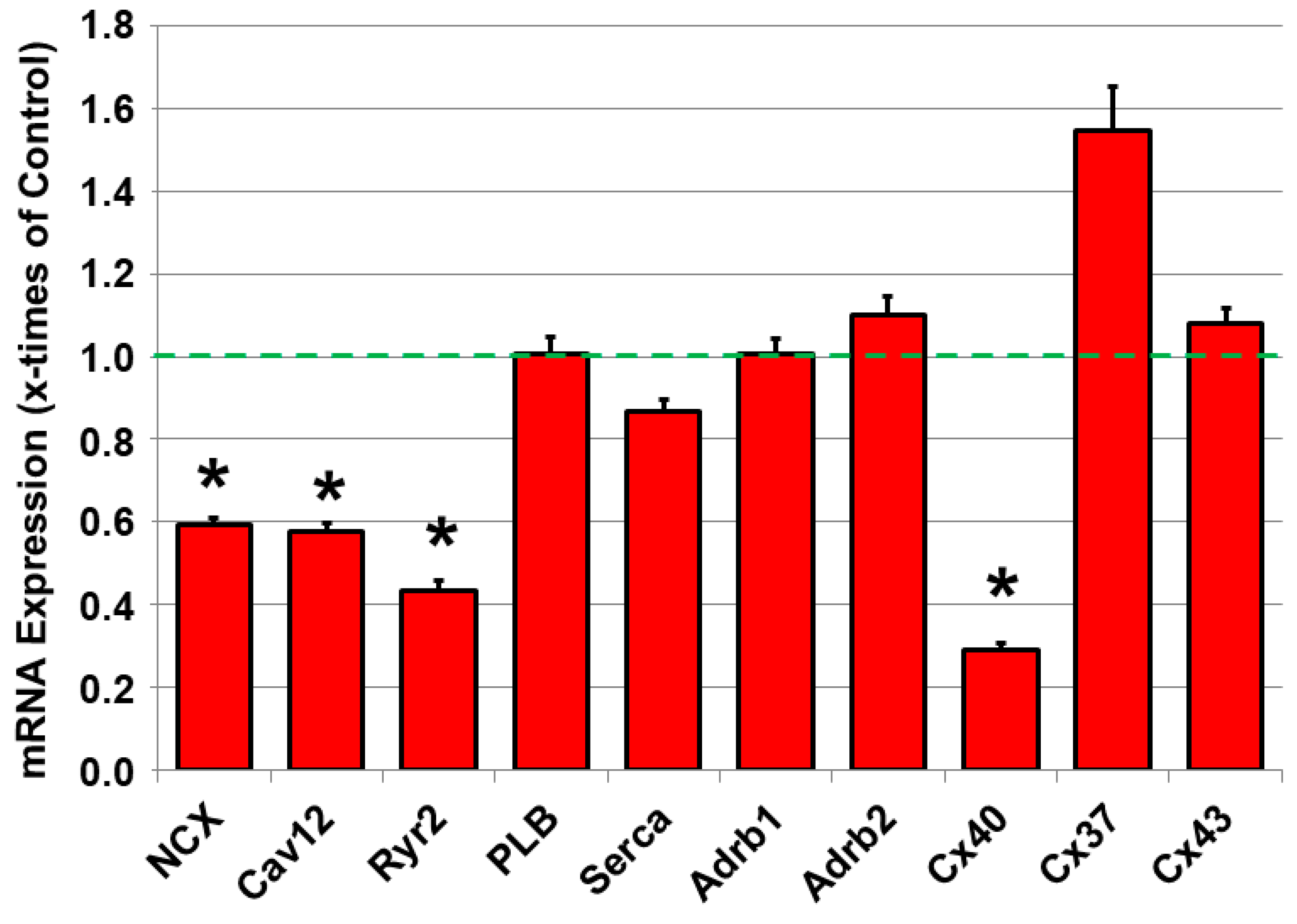
2.4. Dysregulated mRNA Expression of Calcium-Handling Proteins and Connexin40 in JDP2 Mice
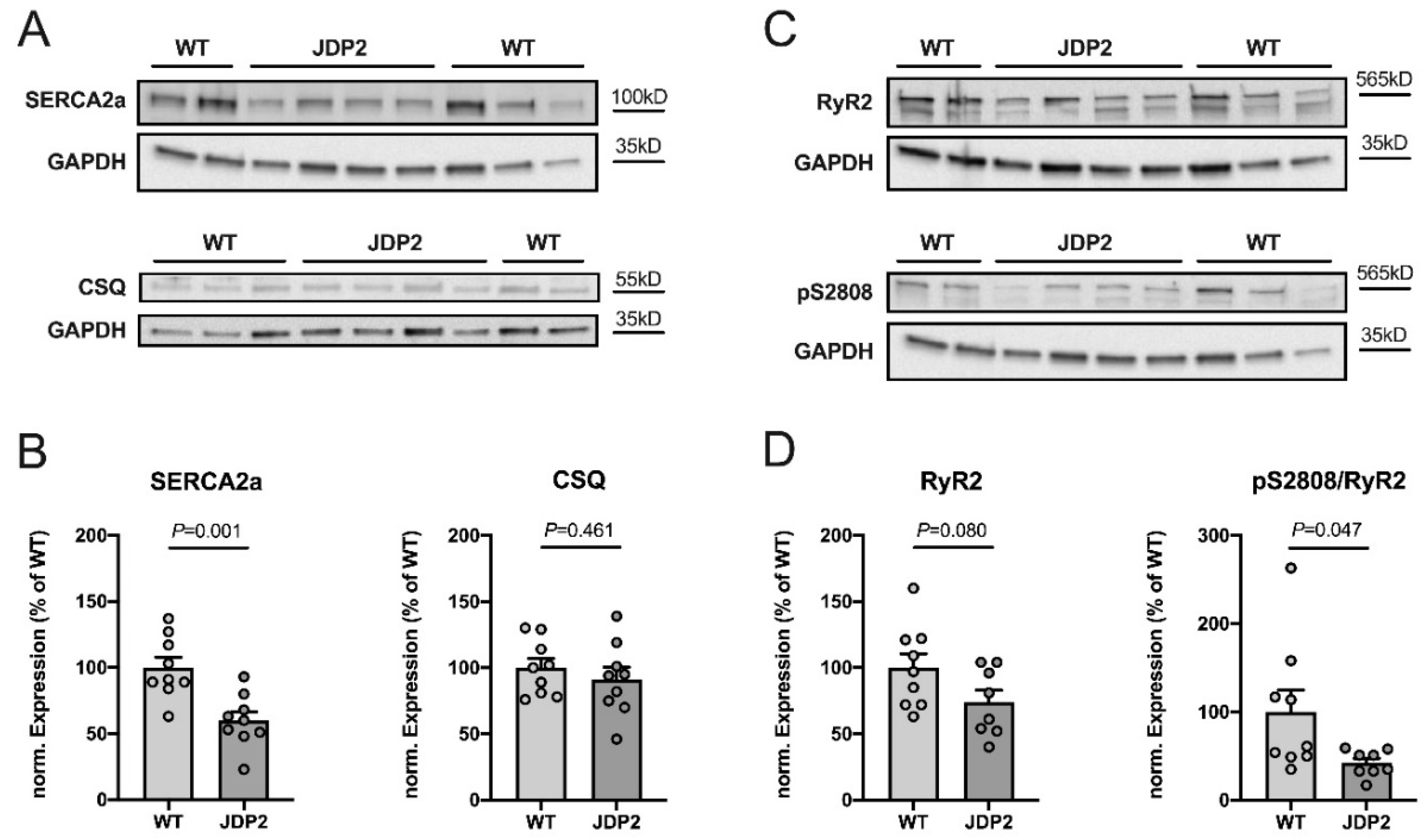
2.5. Remodeling of Sarcoplasmic Reticulum Calcium Handling in JDP2 Mice
3. Discussion
4. Limitations
5. Methods
5.1. JDP2-Overexpressing Mice
5.2. Electrocardiography Recordings
5.3. Atrial Tissue Preparation
5.4. Isolation of Atrial Cardiomyocytes and Determination of Cell Size
5.5. Real-Time RT-PCR
5.6. Western Blots
5.7. Analysis of Fibrosis
5.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart. J. 2020, 1–125. [Google Scholar] [CrossRef]
- Schotten, U.; Verheule, S.; Kirchhof, P.; Goette, A. Pathophysiological mechanisms of atrial fibrillation: A translational appraisal. Physiol. Rev. 2011, 91, 265–325. [Google Scholar] [CrossRef]
- Greiser, M.; Lederer, W.J.; Schotten, U. Alterations of atrial Ca2+ handling as cause and consequence of atrial fibrillation. Cardiovasc. Res. 2011, 89, 722–733. [Google Scholar] [CrossRef][Green Version]
- Xintarakou, A.; Tzeis, S.; Psarras, S.; Asvestas, D.; Vardas, P. Atrial fibrosis as a dominant factor for the development of atrial fibrillation: Facts and gaps. EP Eur. 2020, 22, 342–351. [Google Scholar] [CrossRef]
- Hu, Y.-F.; Chen, Y.-J.; Lin, Y.-J.; Chen, S.-A. Inflammation and the pathogenesis of atrial fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef]
- Riley, G.; Syeda, F.; Kirchhof, P.; Fabritz, L. An introduction to murine models of atrial fibrillation. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef]
- Kalfon, R.; Friedman, T.; Aronheim, A. JDP2 and ATF3—bZIP repressors in cardiac remodeling. Int. J. Cardiol. 2018, 257, 228. [Google Scholar] [CrossRef]
- Kehat, I.; Heinrich, R.; Ben-Izhak, O.; Miyazaki, H.; Gutkind, J.S.; Aronheim, A. Inhibition of basic leucine zipper transcription is a major mediator of atrial dilatation. Cardiovasc. Res. 2006, 70, 543–554. [Google Scholar] [CrossRef]
- Heger, J.; Bornbaum, J.; Würfel, A.; Hill, C.; Brockmann, N.; Gáspár, R.; Pálóczi, J.; Varga, Z.V.; Sárközy, M.; Bencsik, P.; et al. JDP2 overexpression provokes cardiac dysfunction in mice. Sci. Rep. 2018, 8, 7647–7657. [Google Scholar] [CrossRef]
- Fan, D.; Yang, Z.; Liu, F.Y.; Tang, N.; Deng, W.; Tang, Q.Z. A potential therapeutic approach to cardiac remodeling: JDP2. Int. J. Cardiol. 2018, 254, 283. [Google Scholar] [CrossRef]
- Ozcan, C.; Battaglia, E.; Young, R.; Suzuki, G. LKB1 knockout mouse develops spontaneous atrial fibrillation and provides mechanistic insights into human disease process. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef]
- Magnani, J.W.; Johnson, V.M.; Sullivan, L.M.; Gorodeski, E.Z.; Schnabel, R.B.; Lubitz, S.A.; Levy, D.; Ellinor, P.T.; Benjamin, E.J. P wave duration and risk of longitudinal atrial fibrillation in persons ≥ 60 years old (from the Framingham Heart Study). Am. J. Cardiol. 2001, 107, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.U.; Lewin, G.; Baba, H.A.; Bokník, P.; Fabritz, L.; Kirchhefer, U.; Kirchhof, P.; Loser, K.; Matus, M.; Neumann, J.; et al. Heart-directed expression of a human cardiac isoform of cAMP-response element modulator in transgenic mice. J. Biol. Chem. 2005, 280, 6906–6914. [Google Scholar] [CrossRef]
- Müller, F.U.; Neumann, J.; Schmitz, W. Transcriptional regulation by cAMP in the heart. Mol. Cell. Biochem. 2000, 212, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Darlyuk-Saadon, I.; Weidenfeld-Baranboim, K.; Yokoyama, K.K.; Hai, T.; Aronheim, A. The bZIP repressor proteins, c-Jun dimerization protein 2 and activating transcription factor 3, recruit multiple HDAC members to the ATF3 promoter. Biochim. Biophys. Acta 2012, 1819, 1142–1153. [Google Scholar] [CrossRef]
- Stümpel, F.T.; Stein, J.; Himmler, K.; Scholz, B.; Seidl, M.D.; Skryabin, B.V.; Müller, F.U. Homozygous CREM-IbΔC-X Overexpressing Mice Are a Reliable and Effective Disease Model for Atrial Fibrillation. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Nattel, S. How does fibrosis promote atrial fibrillation persistence: In silico findings, clinical observations, and experimental data. Cardiovasc. Res. 2016, 110, 295–297. [Google Scholar] [CrossRef]
- Voigt, N.; Li, N.; Wang, Q.; Wang, W.; Trafford, A.W.; Abu-Taha, I.; Sun, Q.; Wieland, T.; Ravens, U.; Nattel, S.; et al. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012, 125, 2059–2070. [Google Scholar] [CrossRef]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Wehrens, X.H.T.; Nattel, M.; Dobrev, D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef]
- Pluteanu, F.; Nikonova, Y.; Holzapfel, A.; Herzog, B.; Scherer, A.; Preisenberger, J.; Plačkić, J.; Scheer, K.; Ivanova, T.; Bukowska, A.; et al. Progressive Impairment of Atrial Myocyte Function During Left Ventricular Hypertrophy and Heart Failure. J. Mol. Cell. Cardiol. 2018, 114, 253–263. [Google Scholar] [CrossRef]
- Shahid, F.; Lip, G.Y.H.; Shantsila, E. Role of Monocytes in Heart Failure and Atrial Fibrillation. J. Am. Heart. Assoc. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Sekiguchi, A.; Iwasaki, Y.K.; Date, T.; Sagara, K.; Tanabe, H.; Suma, H.; Sawada, H.; Aizawa, T. Recruitment of immune cells across atrial endocardium in human atrial fibrillation. Circ. J. 2010, 74, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, N.; Walecki, M.; Schäfer, M.K.; Kessler, M.; Zobeiri, M.; Rinné, S.; Kiper, A.K.; Komadowski, M.A.; Vowinkel, K.S.; Wemhöner, K.; et al. The VAMP-associated protein VAPB is required for cardiac and neuronal pacemaker channel function. FASEB J. 2018, 32, 6159–6173. [Google Scholar] [CrossRef] [PubMed]
- Vignier, N.; Mougenot, N.; Bonne, G.; Muchir, A. Effect of genetic background on the cardiac phenotype in a mouse model of Emery-Dreifuss muscular dystrophy. Biochem. Biophys. Rep. 2019, 19. [Google Scholar] [CrossRef]
- Parahuleva, M.S.; Lipps, C.; Parviz, B.; Hölschermann, H.; Schieffer, B.; Schulz, R.; Euler, G. MicroRNA expression profile of human advanced coronary atherosclerotic plaques. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]


| ECG Recordings | 1 Week | 5 Weeks | ||
|---|---|---|---|---|
| WT | JDP2 | WT | JDP2 | |
| P-wave duration | 10.0 ± 0.9 ms | 10.6 ± 0.8 ms | 10.0 ± 1.5 ms | 11.6 ± 1.1 ms * |
| PQ-interval | 32.0 ± 5.1 ms | 32.3 ± 2.6 ms | 30.3 ± 7.0 ms | 35.1 ± 3.6 ms |
| QRS-complex | 14.3 ± 1.2 ms | 13.0 ± 1.2 ms | 13.9 ± 1.7 ms | 15.8 ± 2.3 ms * |
| RR-interval | 86.8 ± 5.0 ms | 84.9 ± 4.0 ms | 83.3 ± 4.1 ms | 84.5 ± 3.1 ms |
| HR-variability | 0.031 ± 0.013 | 0.042 ± 0.034 | 0.021 ± 0.01 | 0.027 ± 0.01 |
| QT-interval | 29.1 ± 0.7 ms | 29.1 ± 1.4 ms | 29.5 ± 1.8 ms | 31.3 ± 1.9 ms |
| n | 6 | 7 | 10 | 11 |
| Primer | Sequences of Primer |
|---|---|
| Adrb1 | Qiagen, QT00258692 |
| Adrb2 | 5′-TGGTACCGTGCCACCCACAA-3′ |
| 5′-AAGACCATCACCACCAGGGGCA-3′ | |
| ANP | 5′-CTGCTAATCAGCCATGCAAA-3′ |
| 5′-GATGGAGACCATCCTGGCTA-3′ | |
| Cav1.2 | 5′-CAGCCACTCTCCAGTCACTC-3′ |
| 5′-CTGGAGTAGGGATGTGCTCG-3′ | |
| CD3 | 5′-ATGCGGTGGAACACTTTCTGG-3′ |
| 5′-GCACGTCAACTCTACACTGGT-3′ | |
| CD11b | 5′-CTGAGACTGGAGGCAACCAT-3′ |
| 5′-GATATCTCCTTCGCGCAGAC-3′ | |
| CD20 | 5′-CCTTTCCCAGCAGAGCCTAC-3′ |
| 5′-TCATGATTTGGACAGCCCCC-3′ | |
| CD45 | 5′-ATGGTCCTCTGAATAAAGCCCA-3′ |
| 5′-TCAGCACTATTGGTAGGCTCC-3′ | |
| CD68 | 5′-ACTTCGGGCCATGTTTCTCT-3′ |
| 5′-GCTGGTAGGTTGATTGTCGT-3′ | |
| Collagen1 | 5′-TTCTCCTGGRAAAGATGGTGC-3′ |
| 5′-GGACCAGCATCACCTTTAACA-3′ | |
| Csf1r | 5′-TCCACCGGGACGTAGCA-3′ |
| 5′-CCAGTCCAAAGTCCCCAATCT-3′ | |
| Cx37 | 5′-ATAAAGGCACGAAGGGACCA-3′ |
| 5′-GTCAAGTTGGCCCAGTTCTG-3′ | |
| Cx40 | 5′-AGGGCTGAGCTTGCTTCTTA-3′ |
| 5′-TTAGTGCCAGTGTCGGGAAT-3′ | |
| Cx43 | 5′-GAAACAATTCCTCCTGCCGC-3′ |
| 5′-AGTTGGAGATGGTGCTTCCG-3′ | |
| Elastin | 5′-CTGCTGCTAAGGCTGCTAAG-3′ |
| 5′-CCACCAACACCAGGAATGC-3′ | |
| Fibronectin | 5′-ACAGAGCTCAACCTCCCTGA-3′ |
| 5′-TGTGCTCTCCTGGTTCTCCT-3′ | |
| JDP2 | 5′-ATGATGCCTGGGCAGATCCCA-3′ |
| 5′-TCACTTCTTGTCCAGCTGCTCC-3′ | |
| MCP1 | 5′-CCACAACCACCTCAAGCA-3′ |
| 5′-TGAAAGGGAATACCATAACATC-3′ | |
| NCX | 5′-CTACCAGGTCCTAAGTCAACAG-3′ |
| 5′-TGCGTGCCTCTTCAAGATG-3′ | |
| PLB | 5′-GCAATACCTCACTCGCTCGGCTATC-3′ |
| 5′-TGGAGATTCTGACGTGCTTGCTGAG-3′ | |
| RyR | 5′-AGCTGGAAGACCCTGCAATC-3′ |
| 5′-ACCAGGCTGAAATATCCCCG-3′ | |
| SERCA | 5′-TGACTGGTGATGGTGTGAATG-3′ |
| 5′-GATGAGGTAGCGGATGAACTG-3′ | |
| 18SrRNA | 5′-TTGACGGAAGGGCACCACCA-3′ |
| 5′-AGAACGGCCATGCACCACCA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parahuleva, M.S.; Kockskämper, J.; Heger, J.; Grimm, W.; Scherer, A.; Bühler, S.; Kreutz, J.; Schulz, R.; Euler, G. Structural, Pro-Inflammatory and Calcium Handling Remodeling Underlies Spontaneous Onset of Paroxysmal Atrial Fibrillation in JDP2-Overexpressing Mice. Int. J. Mol. Sci. 2020, 21, 9095. https://doi.org/10.3390/ijms21239095
Parahuleva MS, Kockskämper J, Heger J, Grimm W, Scherer A, Bühler S, Kreutz J, Schulz R, Euler G. Structural, Pro-Inflammatory and Calcium Handling Remodeling Underlies Spontaneous Onset of Paroxysmal Atrial Fibrillation in JDP2-Overexpressing Mice. International Journal of Molecular Sciences. 2020; 21(23):9095. https://doi.org/10.3390/ijms21239095
Chicago/Turabian StyleParahuleva, Mariana S., Jens Kockskämper, Jacqueline Heger, Wolfram Grimm, Anna Scherer, Sarah Bühler, Julian Kreutz, Rainer Schulz, and Gerhild Euler. 2020. "Structural, Pro-Inflammatory and Calcium Handling Remodeling Underlies Spontaneous Onset of Paroxysmal Atrial Fibrillation in JDP2-Overexpressing Mice" International Journal of Molecular Sciences 21, no. 23: 9095. https://doi.org/10.3390/ijms21239095
APA StyleParahuleva, M. S., Kockskämper, J., Heger, J., Grimm, W., Scherer, A., Bühler, S., Kreutz, J., Schulz, R., & Euler, G. (2020). Structural, Pro-Inflammatory and Calcium Handling Remodeling Underlies Spontaneous Onset of Paroxysmal Atrial Fibrillation in JDP2-Overexpressing Mice. International Journal of Molecular Sciences, 21(23), 9095. https://doi.org/10.3390/ijms21239095






