Application of the RBBP9 Serine Hydrolase Inhibitor, ML114, Decouples Human Pluripotent Stem Cell Proliferation and Differentiation
Abstract
1. Introduction
2. Results
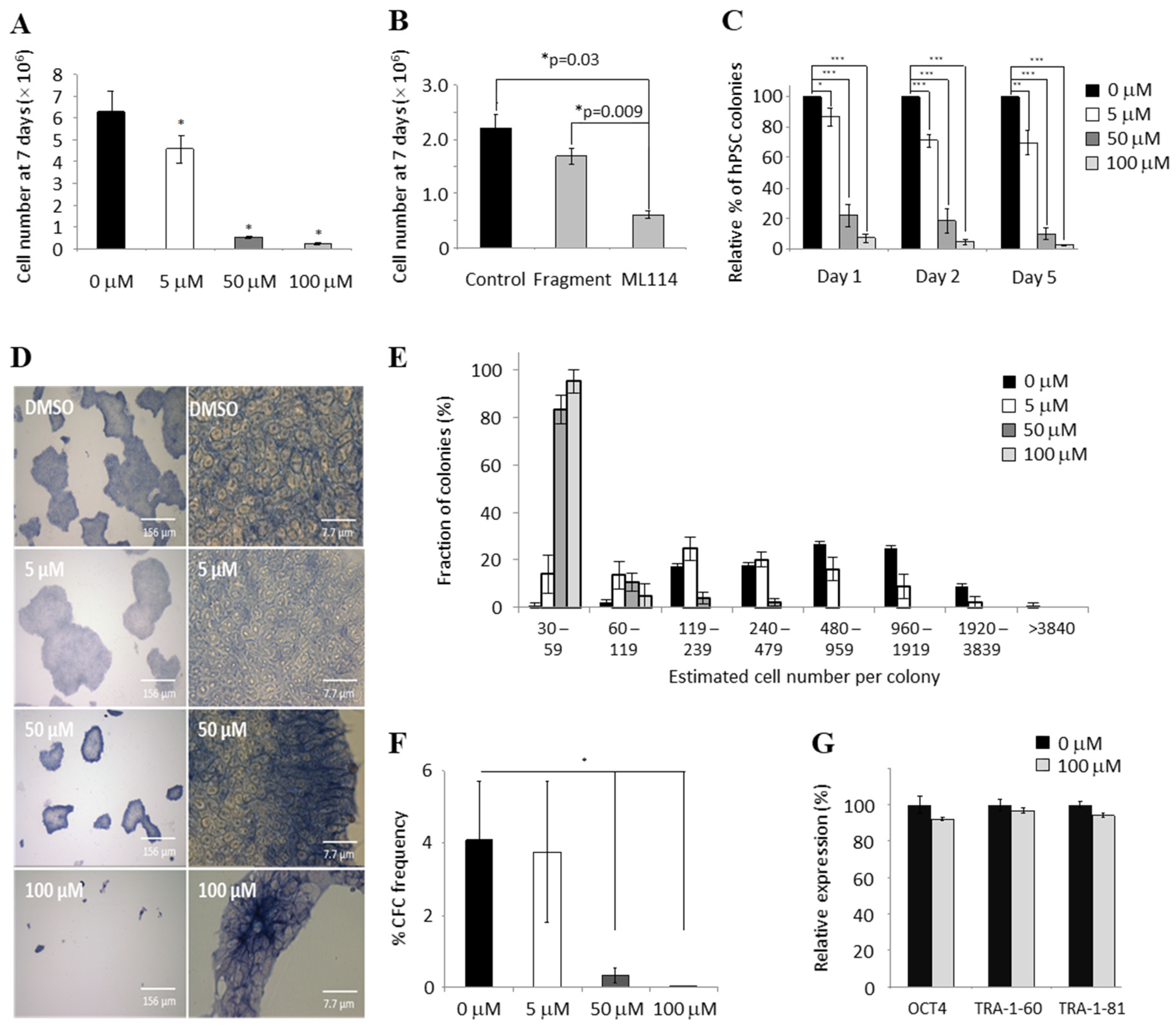
2.1. ML114 Reduces hPSC Population Growth Rate without Inducing Differentiation
2.2. ML114 Reduces Pluripotent CFC Number and Colony Size without Inducing Differentiation
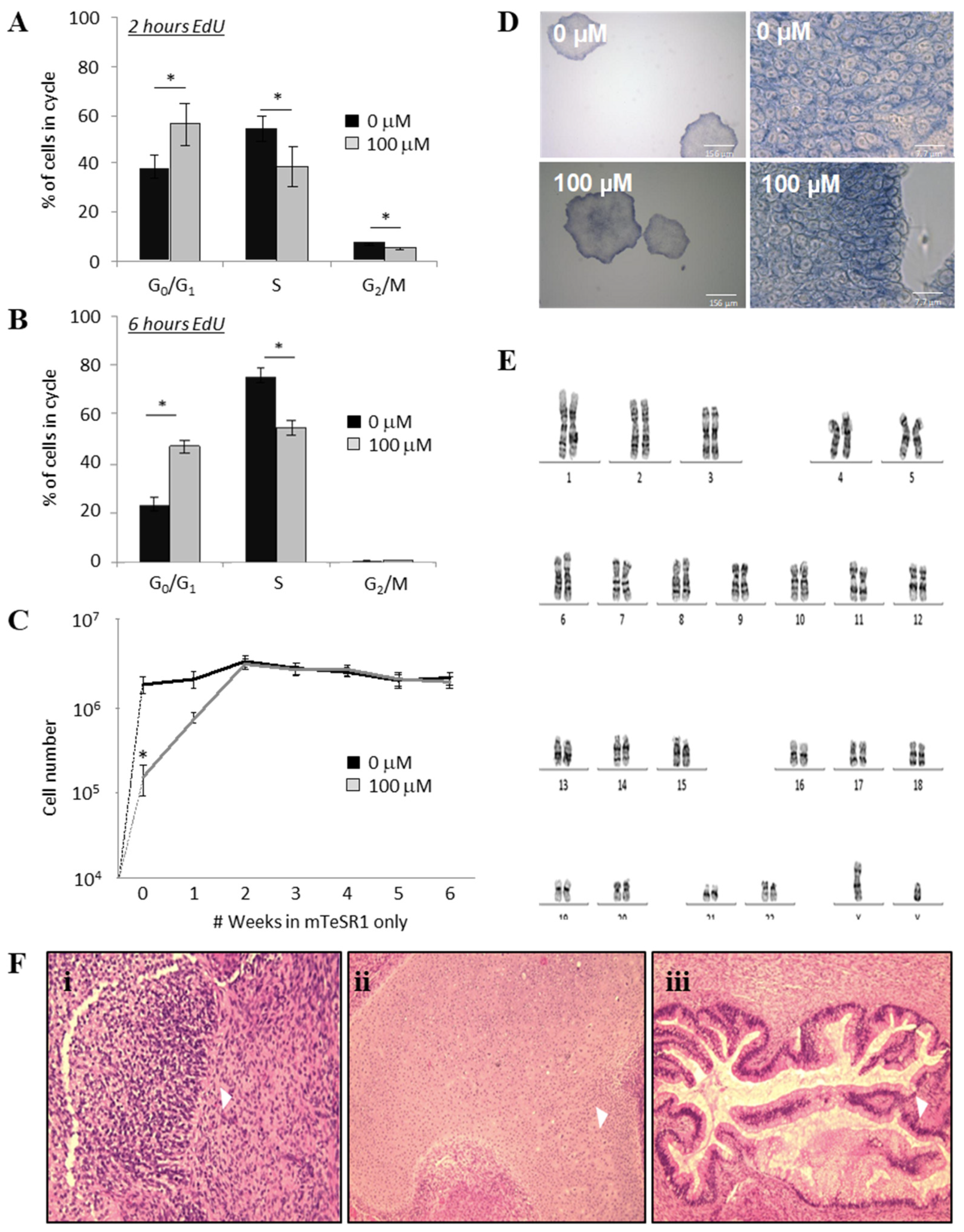
2.3. ML114 Slows Progression from G0/G1 into S-Phase without Differentiation or Karyotype Changes
2.4. The Effects of ML114 on hPSCs are Reversible
2.5. ML114 Does Not Induce Genomic Instability or Remove Differentiation Capacity
2.6. hPSC-Expressed Genes Affected by ML114 Treatment Are Involved in Protein Modification Processes
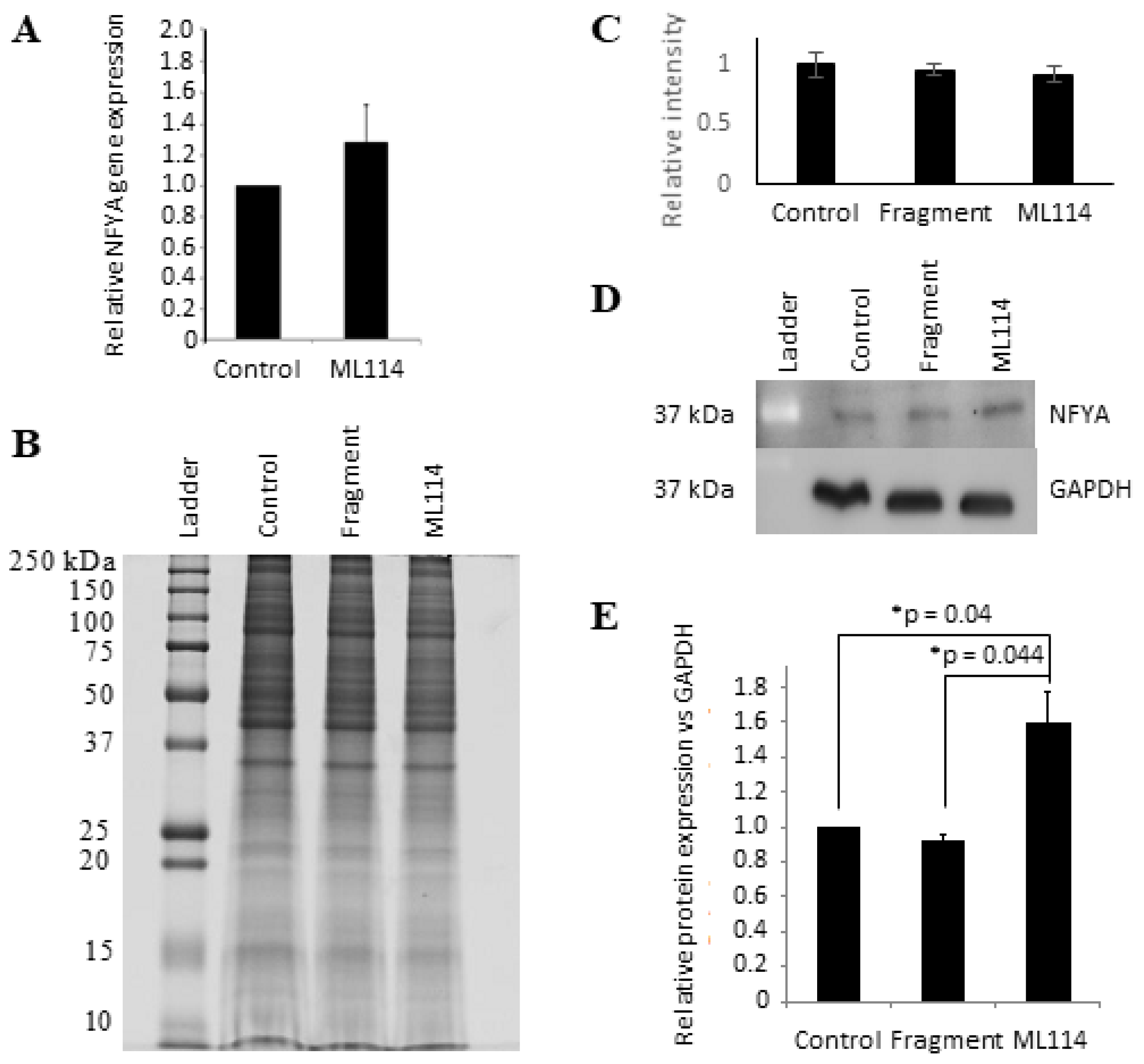
2.7. NFYA Is a Predicted Target of RBBP9 SH Activity
2.8. Western Blot Analysis of ML114-Treated hPSCs Supports NFYA as an Effector of RBBP9 SH Activity
3. Discussion
3.1. Inhibition of RBBP9 SH Activity Decouples Decreased hPSC Proliferation from Differentiation
3.2. DEAF1 and NFYA: Pluripotency Regulators as Candidate Effectors of RBBP9 SH Activity
3.3. Summary
4. Materials and Methods
4.1. Cell Culture
4.2. Colony-Forming Cell Assay
4.3. Dead Cell Counting
4.4. Flow Cytometry Analysis of Pluripotency Markers
4.5. Cell Proliferation Assay
4.6. Teratoma and Karyotyping Analyses
4.7. Affymetrix, Gene Ontology/Promoter Analyses, and Real-Time PCR
4.8. SDS-PAGE and Western Blotting
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| hPSC | Human pluripotent stem cell |
| RBBP9 | Retinoblastoma binding protein 9 |
| SH | Serine hydrolase |
| siRNA | Small interfering RNA |
| CFC | Colony-forming cell |
References
- Woitach, J.T.; Zhang, M.; Niu, C.H.; Thorgeirsson, S.S. A retinoblastoma-binding protein that affects cell-cycle control and confers transforming ability. Nat. Genet. 1998, 19, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Woitach, J.T.; Hong, R.; Keck, C.L.; Zimonjic, D.B.; Popescu, N.C.; Thorgeirsson, S.S. Assignment of the Bog gene (RBBP9) to syntenic regions of mouse chromosome 2G1-H1 and human chromosome 20p11.2 by fluorescence in situ hybridization. Cytogenet Genome Res [Internet]. Cytogenet. Cell Genet. 1999, 85, 252. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; Yang, Q.S.; Wang, S.; Meng, X.F.; Ying, K.; Xie, Y.; Ma, Y.M. Cloning and expression of a novel retinoblastoma binding protein CDNA, RBBP10. Biochem. Genet. 2002, 40, 273. [Google Scholar] [CrossRef] [PubMed]
- Shields, D.J.; Niessen, S.; Murphy, E.A.; Mielgo, A.; Desgrosellier, J.S.; Lau, S.K.M.; Barnes, L.A.; Lesperance, J.; Bouvet, M.; Tarin, D.; et al. RBBP9: A tumor-associated serine hydrolase activity required for pancreatic neoplasia. Proc. Natl. Acad. Sci. USA 2010, 107, 2189–2194. [Google Scholar] [CrossRef]
- Shenoy, V.M.; Thompson, B.R.; Shi, J.; Zhu, H.-J.; Smith, D.E.; Amidon, G.L. Chemoproteomic Identification of Serine Hydrolase RBBP9 as a Valacyclovir-Activating Enzyme. Mol. Pharm. Am. Chem. Soc. (ACS) 2020, 17, 1706–1714. [Google Scholar] [CrossRef]
- Hirst, M.; Delaney, A.; Rogers, S.A.; Schnerch, A.; Persaud, D.R.; O’Connor, M.D.; Zeng, T.; Moksa, M.; Fichter, K.; Mah, D.; et al. LongSAGE profiling of nine human embryonic stem cell lines. Genome Biol. BioMed. Cent. 2007, 8, R113. [Google Scholar] [CrossRef]
- Vorobiev, S.M.; Su, M.; Seetharaman, J.; Huang, Y.J.; Chen, C.X.; Maglaqui, M.; Janjua, H.; Proudfoot, M.; Yakunin, A.; Xiao, R.; et al. Crystal structure of human retinoblastoma binding protein Proteins Struct Funct Bioinforma. NIH Public Access 2009, 74, 526–529. [Google Scholar]
- Aoyama, A.; Chen, W.T. A 170-kDa membrane-bound protease is associated with the expression of invasiveness by human malignant melanoma cells. Proc. Natl. Acad. Sci. USA 1990, 87, 8296–8300. [Google Scholar] [CrossRef]
- Goldstein, L.A.; Ghersi, G.; Piñeiro-Sánchez, M.L.; Salamone, M.; Yeh, Y.; Flessate, D.; Chen, W.T. Molecular cloning of seprase: A serine integral membrane protease from human melanoma. Biochim. Biophys. Acta Mol. Basis Dis. Elsevier B.V. 1997, 1361, 11–19. [Google Scholar] [CrossRef]
- Bachovchin, D.A.; Speers, A.E.; Brown, S.J.; Cravatt, B.F.; Spicer, T.; Mercer, B.A.; Ferguson, J.; Hodder, P.; Rosen, H.R. Probe Report for RBBP9 Inhibitors—Probe; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. [Google Scholar] [PubMed]
- Bachovchin, D.A.; Wolfe, M.R.; Masuda, K.; Brown, S.J.; Spicer, T.P.; Fernandez-Vega, V.; Chase, P.; Hodder, P.S.; Rosen, H.; Cravatt, B.F. Oxime esters as selective, covalent inhibitors of the serine hydrolase retinoblastoma-binding protein 9 (RBBP9). Bioorg. Med. Chem. Lett. 2010, 20, 2254–2258. [Google Scholar] [CrossRef][Green Version]
- O’Connor, M.D.; Wederell, E.; Robertson, G.; Delaney, A.; Morozova, O.; Poon, S.S.S.; Yap, D.; Fee, J.; Zhao, Y.; McDonald, H. Retinoblastoma-binding proteins 4 and 9 are important for human pluripotent stem cell maintenance. Exp. Hematol. 2011, 39, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.A.; Ghule, P.N.; Therrien, J.A.; Lian, J.B.; Stein, J.L.; Van Wijnen, A.J.; Stein, G.S. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J. Cell Physiol. 2006, 209, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.; Roth-Albin, I.; Bhatia, S.; Pilquil, C.; Lee, J.H.; Bhatia, M.; Levadoux-Martin, M.; McNicol, J.; Russell, J.; Collins, T.; et al. Lengthened G1 phase indicates differentiation status in human embryonic stem cells. Stem Cells Dev. 2013, 22, 279–295. [Google Scholar] [CrossRef]
- O’Connor, M.D.; Kardel, M.D.; Eaves, C.J. Functional assays for human embryonic stem cell pluripotency. Methods Mol. Biol. 2011, 690, 67–80. [Google Scholar] [PubMed]
- Manni, I.; Mazzaro, G.; Gurtner, A.; Mantovani, R.; Haugwitz, U.; Krause, K.; Engeland, K.; Sacchi, A.; Soddu, S.; Piaggio, S.G. NF-Y Mediates the Transcriptional Inhibition of the cyclin B1, cyclin B2, and cdc25C Promoters upon Induced G2 Arrest. J. Biol. Chem. 2001, 276, 5570–5576. [Google Scholar] [CrossRef]
- Shi, Z.; Chiang, C.-I.; Labhart, P.; Zhao, Y.; Yang, J.; Mistretta, T.-A.; Henning, S.J.; Maity, S.N.; Mori-Akiyama, Y. Context-specific Role of SOX9 in NF-Y Mediated Gene Regulation in Colorectal Cancer Cells—PubMed. Nucleic Acid. Res. 2015, 43, 6257. [Google Scholar] [CrossRef] [PubMed]
- Dolfini, D.; Minuzzo, M.; Pavesi, G.; Mantovani, R. The short isoform of NF-YA belongs to the embryonic stem cell transcription factor circuitry. Stem Cells 2012, 30, 2450–2459. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Deng, J.M.; Zhang, Z.; Behringer, R.; de Crombrugghe, B.; Maity, S.N. The B Subunit of the CCAAT Box Binding Transcription Factor Complex (CBF/NF-Y) Is Essential for Early Mouse Development and Cell Proliferation. Cancer Res. 2003, 63, 8167. [Google Scholar]
- Gu, B.; Zhang, J.; Chen, Q.; Tao, B.; Wang, W.; Zhou, Y.; Chen, M.; Liu, Y.; Zhang, M. Aire regulates the expression of differentiation-associated genes and self-renewal of embryonic stem cells. Biochem. Biophys. Res. Commun. NIH Public Access 2010, 394, 418–423. [Google Scholar] [CrossRef]
- Gerloff, A.; Dittmer, A.; Oerlecke, I.; Holzhausen, H.J.; Dittmer, J. Protein expression of the Ets transcription factor Elf-1 in breast cancer cells is negatively correlated with histological grading, but not with clinical outcome. Oncol. Rep. 2011, 26, 1121–1125. [Google Scholar]
- Davie, J.R.; He, S.; Li, L.; Sekhavat, A.; Espino, P.; Drobic, B.; Dunn, K.L.; Sun, J.M.; Chen, H.Y.; Yu, J.; et al. Nuclear organization and chromatin dynamics—Sp1, Sp3 and histone deacetylases. Adv. Enzyme Regul. 2008, 48, 189–208. [Google Scholar] [CrossRef]
- Chuang, J.Y.; Wu, C.H.; Lai, M.D.; Chang, W.C.; Hung, J.J. Overexpression of Sp1 leads to p53-dependent apoptosis in cancer cells. Int. J. Cancer 2009, 125, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Da, Y.W.; Yao, Z. Functional analysis of two Sp1/Sp3 binding sites in murine Nanog gene promoter. Cell Res. 2006, 16, 319–322. [Google Scholar]
- Singh, A.M.; Chappell, J.; Trost, R.; Lin, L.; Wang, T.; Tang, J.; Matlock, B.K.; Weller, K.P.; Wu, H.; Zhao, S.; et al. Cell-cycle control of developmentally regulated transcription factors accounts for heterogeneity in human pluripotent cells. Stem. Cell Rep. 2013, 1, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Filipczyk, A.A.; Laslett, A.L.; Mummery, C.; Pera, M.F. Differentiation is coupled to changes in the cell cycle regulatory apparatus of human embryonic stem cells. Stem. Cell Res. 2007, 1, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Michowski, W.; Kolodziejczyk, A.; Sicinski, P. The cell cycle in stem cell proliferation, pluripotency and differentiation [Internet]. Nat. Cell Biol. 2019, 21, 1060–1067. [Google Scholar] [CrossRef]
- Bain, G.; Ray, W.J.; Yao, M.; Gottlieb, D.I. Retinoic acid promotes neural and represses mesodermal gene expression in mouse embryonic stem cells in culture. Biochem. Biophys. Res. Commun. 1996, 223, 691–694. [Google Scholar] [CrossRef]
- O’Connor, M.D.; Kardel, M.D.; Iosfina, I.; Youssef, D.; Lu, M.; Li, M.M.; Vercauteren, S.; Andras Nagy, A.; Eaves, C.J. Alkaline Phosphatase-Positive Colony Formation Is a Sensitive, Specific, and Quantitative Indicator of Undifferentiated Human Embryonic Stem Cells. Stem Cells 2008, 26, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, M.; Yanuka, O.; Itskovitz-Eldor, J.; Melton, D.A.; Benvenisty, N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2000, 97, 11307–11312. [Google Scholar] [CrossRef]
- Suzuki, D.E.; Nakahata, A.M.; Okamoto, O.K. Knockdown of E2F2 inhibits tumorigenicity, but preserves stemness of human embryonic stem cells. Stem Cells Dev. 2014, 23, 1266–1274. [Google Scholar] [CrossRef]
- Gkountela, S.; Li, Z.; Chin, C.J.; Lee, S.A.; Clark, A.T. PRMT5 is required for human embryonic stem cell proliferation but not pluripotency. Stem Cell Rev. Rep. 2014, 10, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Impens, F.; Timmerman, E.; Staes, A.; Moens, K.; Ariën, K.K.; Verhasselt, B.; Vandekerckhove, J.; Gevaert, K. A catalogue of putative HIV-1 protease host cell substrates. Biol. Chem. 2012, 393, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, F.; Wasner, M.; Dohna, C.L.Z.; Gurtner, A.; Ronchi, A.; Muller, H.; Manni, I.; Mossner, J.; Piaggio, G.; Mantovani, R.; et al. The cyclin B2 promoter depends on NF-Y, a trimer whose CCAAT-binding activity is cell-cycle regulated. Oncogene 1999, 18, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Manni, I.; Fontemaggi, G.; Tiainen, M.; Cenciarelli, C.; Bellorini, M.; Mantovani, R.; Sacchi, A.; Piaggio, G. Down-regulation of cyclin B1 gene transcription in terminally differentiated skeletal muscle cells is associated with loss of functional CCAAT-binding NF-Y complex. Oncogene 1999, 18, 2818–2827. [Google Scholar] [CrossRef][Green Version]
- Grskovic, M.; Chaivorapol, C.; Gaspar-Maia, A.; Li, H.; Ramalho-Santos, M. Systematic identification of cis-regulatory sequences active in mouse and human embryonic stem cells. PLoS Genet. 2007, 3, 1524–1540. [Google Scholar] [CrossRef]
- Mojsin, M.; Topalovic, V.; Marjanovic Vicentic, J.; Stevanovic, M. Transcription factor NF-Y inhibits cell growth and decreases SOX2 expression in human embryonal carcinoma cell line NT2/D1. Biochemistry 2015, 80, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, A.J.; Yang, P.; Conway, A.E.; Cinghu, S.; Freudenberg, J.M.; Yellaboina, S.; Jothi, R. Histone-Fold Domain Protein NF-Y Promotes Chromatin Accessibility for Cell Type-Specific Master Transcription Factors. Mol. Cell 2014, 55, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Veraksa, A.; Kennison, J.; McGinnis, W. DEAF-1 function is essential for the early embryonic development of Drosophila. Genesis 2002, 33, 67–76. [Google Scholar] [CrossRef]
- Ceribelli, M.; Dolfini, D.; Merico, D.; Gatta, R.; Viganò, A.M.; Pavesi, G.; Mantovani, R. The Histone-Like NF-Y Is a Bifunctional Transcription Factor. Mol. Cell Biol. 2008, 28, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Sun, Y.; Zhang, Y.; Luo, X.; Hou, W.; Yan, L.; Chen, Y.; Tian, E.; Han, J.; Zhang, H. Transcription factor NFY globally represses the expression of the C. elegans Hox gene Abdominal-B homolog egl-5. Dev. Biol. 2007, 308, 583–592. [Google Scholar] [CrossRef]
- Peng, Y.; Jahroudi, N. The NFY transcription factor functions as a repressor and activator of the von Willebrand factor promoter. Blood 2002, 99, 2408–2417. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Jahroudi, N. The NFY transcription factor inhibits von Willebrand factor promoter activation in non-endothelial cells through recruitment of histone deacetylases. J. Biol. Chem. 2003, 278, 8385–8394. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, S.; Maeda, M.; Yamanaka, S. The homeoprotein Nanog Is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003, 13, 631. [Google Scholar] [CrossRef]
- Lucibello, F.C.; Truss, M.; Zwicker, J.; Ehlert, F.; Beato, M.; Müller, R. Periodic cdc25C transcription is mediated by a novel cell cycle-regulated repressor element (CDE). EMBO J. 1995, 14, 132. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.; Lucibello, F.C.; Wolfraim, L.A.; Gross, C.; Truss, M.; Engeland, K.; Müller, R. Cell cycle regulation of the cyclin A, cdc25C and cdc2 genes is based on a common mechanism of transcriptional repression. EMBO J. 1995, 14, 4514. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, X.; Wang, M.; Zhang, R.; Fu, Y.; Xing, M.; Hua, Q.; Xie, X. Proliferation rate of somatic cells affects reprogramming efficiency. J. Biol. Chem. 2013, 288, 9767–9778. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.H.; Mu, L.; Li, X.L.; Hu, Y.B.; Liu, H.; Han, L.T.; Gong, J.P. Characterization and functional analysis of a slow-cycling subpopulation in colorectal cancer enriched by cell cycle inducer combined chemotherapy. Oncotarget 2017, 8, 78466. [Google Scholar] [CrossRef]
- Adewumi, O.; Aflatoonian, B.; Ahrlund-Richter, L.; Amit, M.; Andrews, P.W.; Beighton, G.; Bello, P.A.; Benvenisty, N.; Berry, L.S.; Bevan, S.; et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat. Biotechnol. 2007, 25, 803–816. [Google Scholar]
- Ludwig, T.E.; Levenstein, M.E.; Jones, J.M.; Berggren, W.T.; Mitchen, E.R.; Frane, J.L.; Crandall, L.J.; Daigh, C.A.; Conard, K.R.; Piekarczyk, M.S.; et al. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006, 24, 185–187. [Google Scholar] [CrossRef]
- Ungrin, M.; O’Connor, M.; Eaves, C.; Zandstra, P.W. Phenotypic analysis of human embryonic stem cells. Curr. Protoc. Stem Cell Biol. 2007. Chapter 1:Unit 1B.3. [Google Scholar] [CrossRef]
- Reich, M.; Liefeld, T.; Gould, J.; Lerner, J.; Tamayo, P.; Mesirov, J.P. GenePattern 2.0. Nat. Genet. 2006, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1. [Google Scholar] [CrossRef]
- Kabir, M.H.; Patrick, R.; Ho, J.W.K.; O’Connor, M.D. Identification of active signaling pathways by integrating gene expression and protein interaction data. BMC Syst. Biol. 2018, 120. [Google Scholar] [CrossRef] [PubMed]
- Roider, H.G.; Manke, T.; O’Keeffe, S.; Vingron, M.; Haas, S.A. PASTAA: Identifying transcription factors associated with sets of co-regulated genes. Bioinformatics 2009, 25, 435. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29. [Google Scholar] [CrossRef]
- Gauci, V.J.; Padula, M.P.; Coorssen, J.R. Coomassie blue staining for high sensitivity gel-based proteomics. J. Proteom. 2013, 90, 96–106. [Google Scholar] [CrossRef]
- Noaman, N.; Abbineni, P.S.; Withers, M.; Coorssen, J.R. Coomassie staining provides routine (sub)femtomole in-gel detection of intact proteoforms: Expanding opportunities for genuine Top-down Proteomics. Electrophoresis 2017, 38, 3086. [Google Scholar] [CrossRef]
| Matrix | Transcription Factor | Association Score | p-Value |
|---|---|---|---|
| ARNT_02 | Arnt | 10.876 | 0.00 × 10 |
| STRA13_01 | Stra13 | 9.882 | 0.00 × 10 |
| NFY_01 | N/A | 7.035 | 5.00 × 10−6 |
| TFIII_Q6 | Tfii-i | 6.654 | 1.70 × 10−5 |
| MAZR_01 | Mazr | 6.294 | 4.20 × 10−5 |
| NFKAPPAB65_01 | Rela | 6.156 | 5.40 × 10−5 |
| USF_Q6 | Usf1, Usf2a | 6.139 | 5.40 × 10−5 |
| MZF1_01 | Mzf-1 | 6.09 | 6.70 × 10−5 |
| DEAF1_01 | Deaf-1 | 5.999 | 8.00 × 10−5 |
| USF_Q6_01 | Usf-1, Usf1 | 5.802 | 1.27 × 10−4 |
| ATF1_Q6 | Atf-1 | 5.652 | 1.63 × 10−4 |
| NERF_Q2 | Nerf-1a | 5.422 | 2.79 × 10−4 |
| DEAF1_02 | Deaf-1 | 5.354 | 3.16 × 10−4 |
| MTF1_Q4 | Mtf-1 | 5.266 | 3.98 × 10−4 |
| MOVOB_01 | Movo-b | 5.161 | 4.91 × 10−4 |
| EGR2_01 | Egr-2 | 5.155 | 4.94 × 10-4 |
| CETS1P54_03 | C-ets-1 | 4.928 | 8.23 × 10−4 |
| NRF2_01 | N/A | 4.768 | 1.17 × 10−3 |
| ARNT_01 | Arnt | 4.584 | 1.72 × 10−3 |
| EGR3_01 | Egr-3 | 4.565 | 1.80 × 10−3 |
| YY1_Q6_02 | Yy1 | 4.563 | 1.80 × 10−3 |
| Matrix | Transcription Factor | Association Score | p-Value |
|---|---|---|---|
| NFY_01 | N/A | 4.681 | 1.37 × 10−3 |
| ETS_Q4 | Erf, Elf-1 | 4.194 | 3.76 × 10−3 |
| NFKAPPAB50_01 | N/A | 4.147 | 4.05 × 10−3 |
| PR_02 | N/A | 4.059 | 4.91 × 10−3 |
| NFY_Q6 | Cbf-a, Cbf-b | 3.616 | 1.21 × 10−2 |
| SZF11_01 | N/A | 3.597 | 1.26 × 10−2 |
| NFY_Q6_01 | Cbf-a, Cbf-b | 3.515 | 1.47 × 10−2 |
| DEAF1_02 | Deaf-1 | 3.393 | 1.88 × 10−2 |
| NFY_C | Cbf-a, Cbf-b | 3.314 | 2.15 × 10−2 |
| CRX_Q4 | Crx, Rx | 3.311 | 2.17 × 10−2 |
| ETS2_B | C-ets-1, C-ets-2 | 3.293 | 2.27 × 10−2 |
| MAZR_01 | Mazr | 3.288 | 2.34 × 10−2 |
| OCT1_01 | Pou2f1, Pou2f1a | 3.19 | 2.82 × 10−2 |
| E2F_Q4_01 | Dp-1, E2f-1 | 3.149 | 3.07 × 10−2 |
| CDP_02 | Cutl1 | 3.037 | 3.71 × 10−2 |
| CLOX_01 | Cutl | 3.037 | 3.71 × 10−2 |
| YY1_02 | Yy1 | 3.014 | 3.98 × 10−2 |
| HNF4_Q6_02 | Hnf-4, Hnf-4alpha | 2.98 | 4.20 × 10−2 |
| HSF1_Q6 | Hsf1, Hsf1long | 2.898 | 4.81 × 10−2 |
| Matrix | Transcription Factor | Association Score | p-Value |
|---|---|---|---|
| LBP1_Q6 | N/A | 5.55 | 1.12 × 10−4 |
| PAX4_03 | Pax-4a | 4.392 | 1.32 × 10−3 |
| MAZR_01 | Mazr | 4.348 | 1.43 × 10−3 |
| HEN1_01 | N/A | 4.133 | 2.10 × 10−3 |
| PAX9_B | Pax-9a | 4.133 | 2.10 × 10−3 |
| ETF_Q6 | N/A | 4.132 | 2.10 × 10−3 |
| HEB_Q6 | Heb | 3.898 | 3.45 × 10−3 |
| E2_Q6 | N/A | 3.806 | 4.25 × 10−3 |
| AP4_Q6_01 | Ap-4 | 3.804 | 4.25 × 10−3 |
| GC_01 | N/A | 3.556 | 7.05 × 10−3 |
| DEAF1_01 | Deaf-1 | 3.535 | 7.34 × 10−3 |
| SP1_Q4_01 | Sp1, Sp2 | 3.468 | 8.12 × 10−3 |
| SP1_Q6 | Sp1 | 3.468 | 8.12 × 10−3 |
| SP1_Q6_01 | Sp1, Sp3 | 3.468 | 8.12 × 10−3 |
| PAX5_01 | Pax-5 | 3.425 | 9.26 × 10−3 |
| SP1_01 | Sp1 | 3.357 | 1.03 × 10−2 |
| MYB_Q6 | C-myb | 3.352 | 1.03 × 10−2 |
| GATA1_01 | Gata-1 | 3.349 | 1.03 × 10−2 |
| E2_01 | N/A | 3.259 | 1.25 × 10−2 |
| CHCH_01 | Chch | 3.126 | 1.62 × 10−2 |
| Matrix | Transcription Factor | Association Score | p-Value |
|---|---|---|---|
| POU1F1_Q6 | Pou1f1, Pou1f1a | 4.809 | 5.40 × 10−4 |
| YY1_02 | Yy1 | 3.697 | 5.30 × 10−4 |
| AHR_01 | Ahr | 3.413 | 9.58 × 10−3 |
| PU_Q6 | Pu.1 | 3.365 | 1.01 × 10−2 |
| LXR_DR4_Q3 | N/A | 3.153 | 1.55 × 10−2 |
| CDP_02 | Cut1 | 3.061 | 1.83 × 10−2 |
| GATA3_03 | Gata-3 | 3.043 | 1.92 × 10−2 |
| NFKAPPAB50_01 | N/A | 3.017 | 2.07 × 10−2 |
| STAT3_02 | Stat3 | 2.990 | 2.20 × 10−2 |
| ROAZ_01 | Roaz | 2.969 | 2.27 × 10−2 |
| COUP_DR1_Q6 | Coup-tf1, Coup-tf2 | 2.931 | 2.41 × 10−2 |
| GATA1_02 | Gata-1 | 2.893 | 2.59 × 10−2 |
| NFE2_01 | Nf-e2 | 2.799 | 3.09 × 10−2 |
| OLFA_01 | Olf-1 | 2.772 | 2.23 × 10−2 |
| HFH4_01 | Foxf1, Foxj1 | 2.750 | 3.34 × 10−2 |
| LYF1_01 | N/A | 2.748 | 3.35 × 10−2 |
| E2_Q6 | N/A | 2.720 | 3.67 × 10−2 |
| NFMUE1_Q6 | N/A | 2.699 | 3.82 × 10−2 |
| PBX1_02 | Pbx1a | 2.646 | 4.14 × 10−2 |
| CBF_01 | N/A | 2.645 | 4.14 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.; Shparberg, R.A.; Coorssen, J.R.; O’Connor, M.D. Application of the RBBP9 Serine Hydrolase Inhibitor, ML114, Decouples Human Pluripotent Stem Cell Proliferation and Differentiation. Int. J. Mol. Sci. 2020, 21, 8983. https://doi.org/10.3390/ijms21238983
Lim S, Shparberg RA, Coorssen JR, O’Connor MD. Application of the RBBP9 Serine Hydrolase Inhibitor, ML114, Decouples Human Pluripotent Stem Cell Proliferation and Differentiation. International Journal of Molecular Sciences. 2020; 21(23):8983. https://doi.org/10.3390/ijms21238983
Chicago/Turabian StyleLim, Seakcheng, Rachel A. Shparberg, Jens R. Coorssen, and Michael D. O’Connor. 2020. "Application of the RBBP9 Serine Hydrolase Inhibitor, ML114, Decouples Human Pluripotent Stem Cell Proliferation and Differentiation" International Journal of Molecular Sciences 21, no. 23: 8983. https://doi.org/10.3390/ijms21238983
APA StyleLim, S., Shparberg, R. A., Coorssen, J. R., & O’Connor, M. D. (2020). Application of the RBBP9 Serine Hydrolase Inhibitor, ML114, Decouples Human Pluripotent Stem Cell Proliferation and Differentiation. International Journal of Molecular Sciences, 21(23), 8983. https://doi.org/10.3390/ijms21238983






