Sitagliptin Modulates the Response of Ovarian Cancer Cells to Chemotherapeutic Agents
Abstract
1. Introduction
2. Results
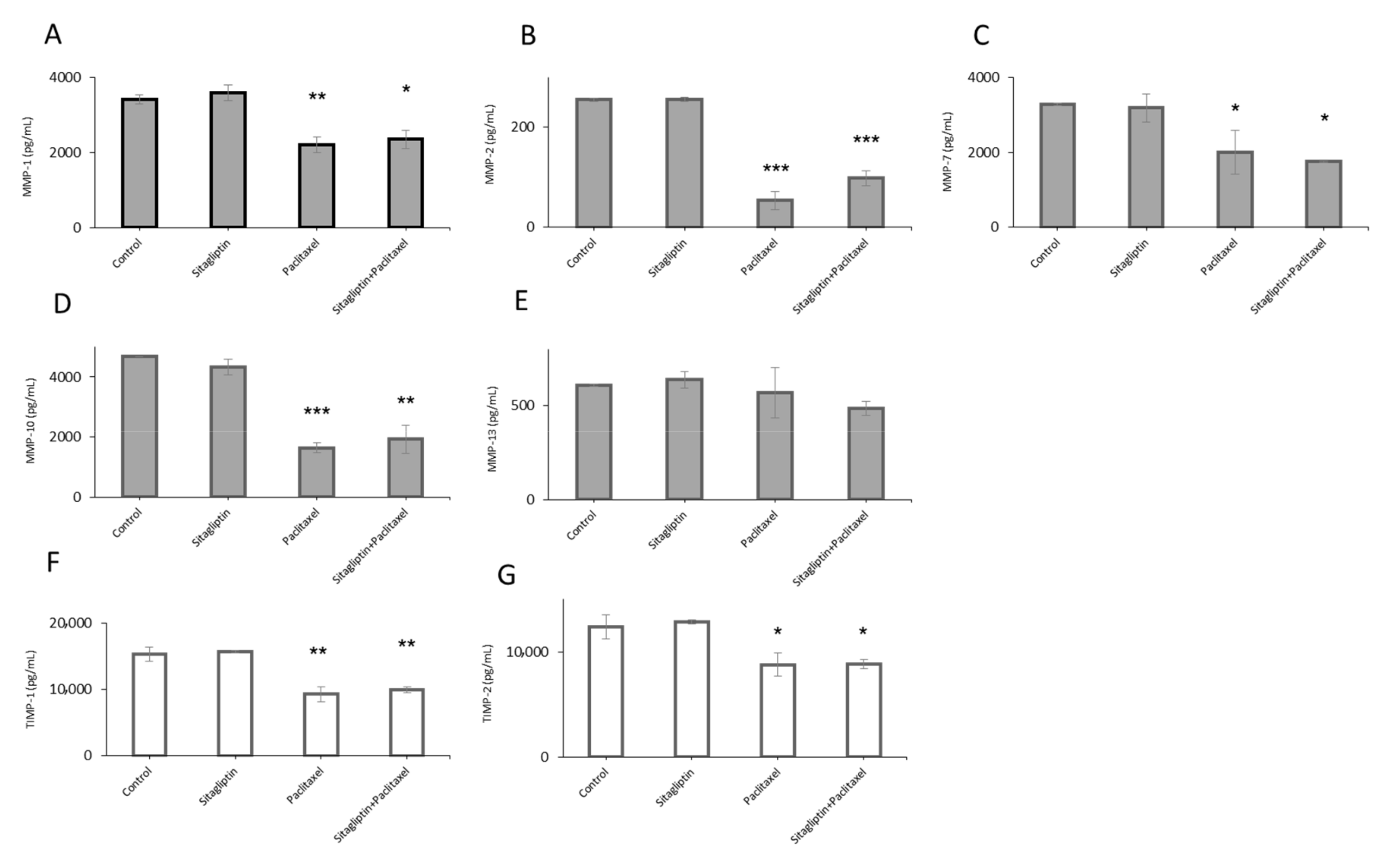
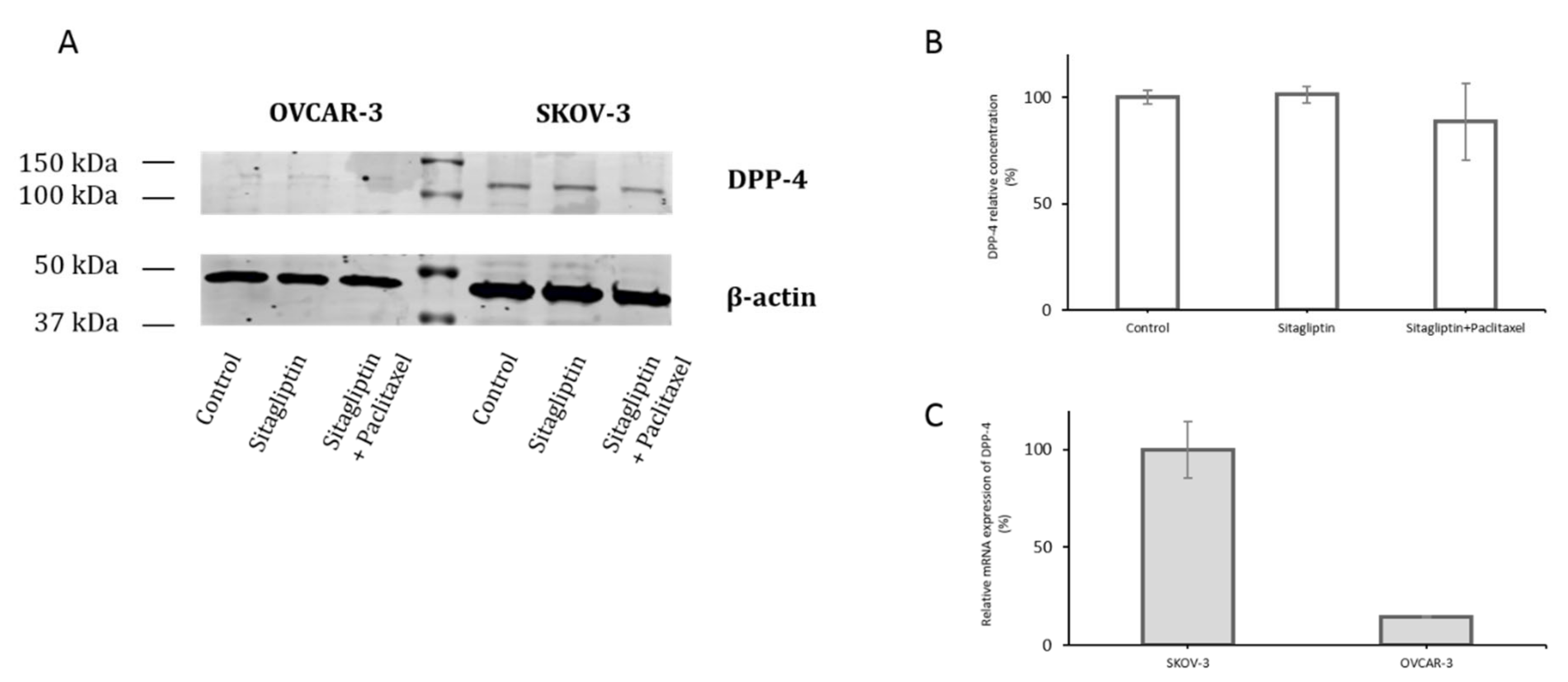
2.1. Distinct Metalloproteinases Production in Sitagliptin Stimulated Ovarian Cancer Cells
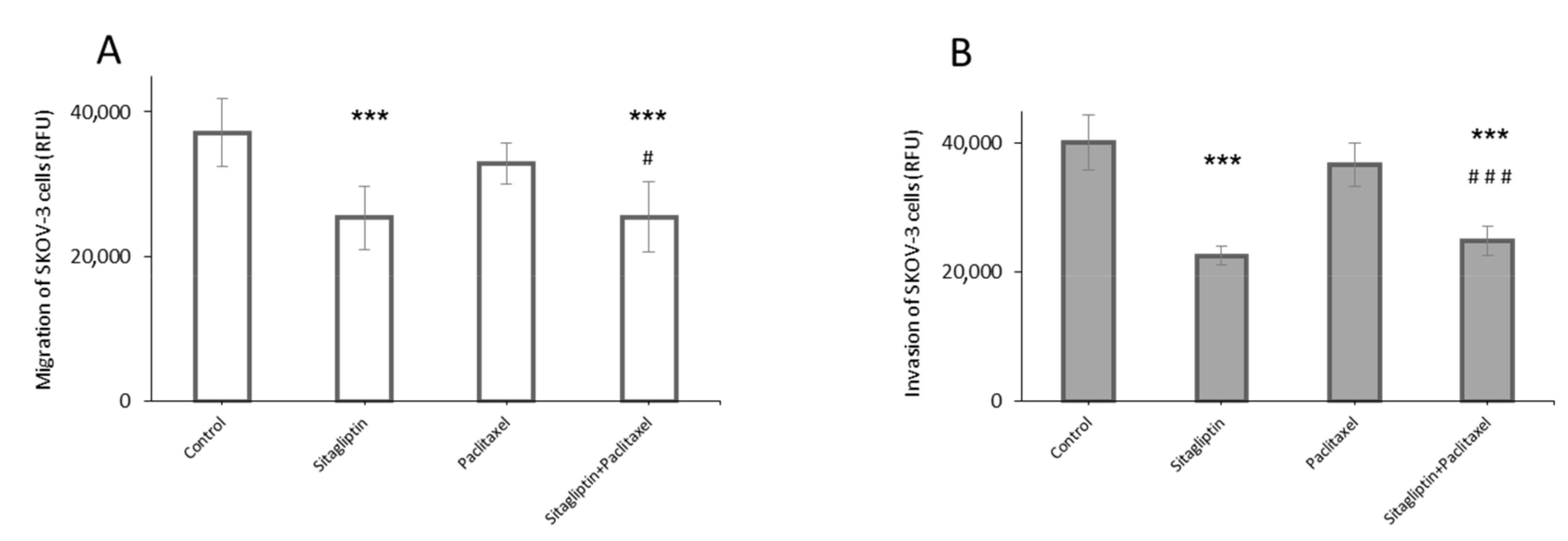
2.2. Sitagliptin Reduces Migration and Invasiveness of Ovarian Cancer Cells
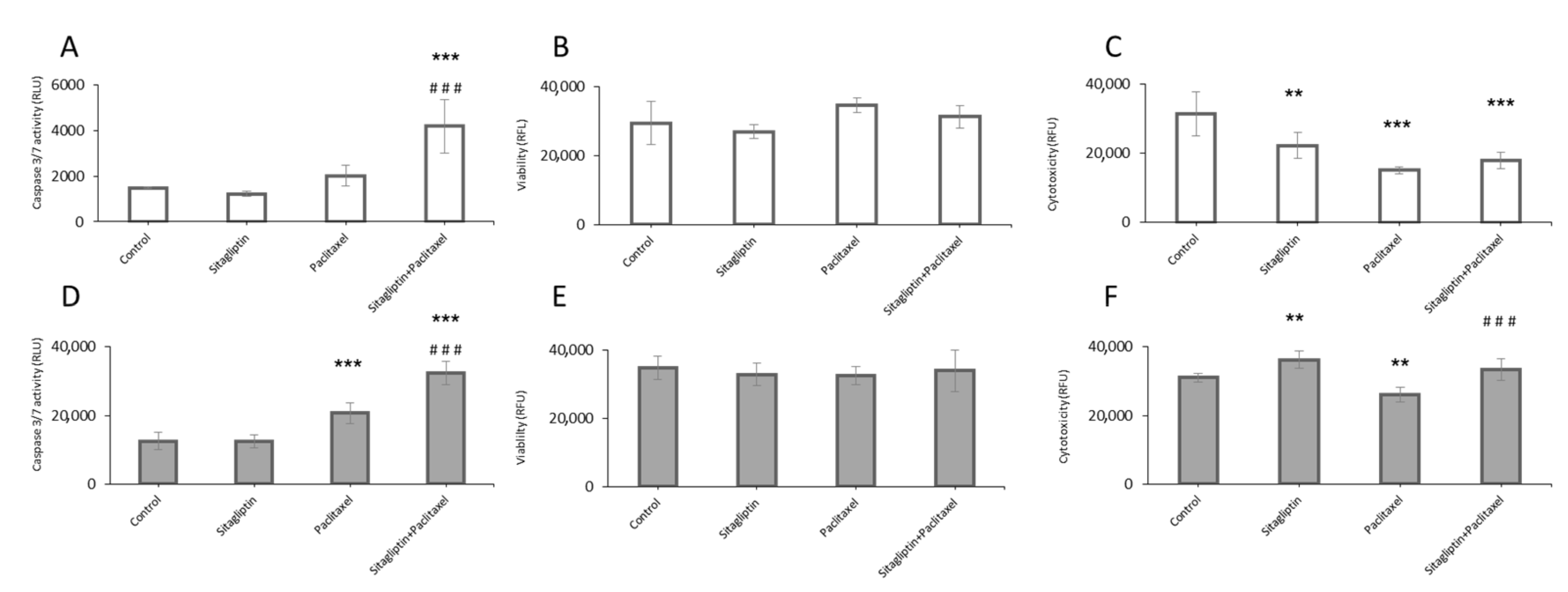
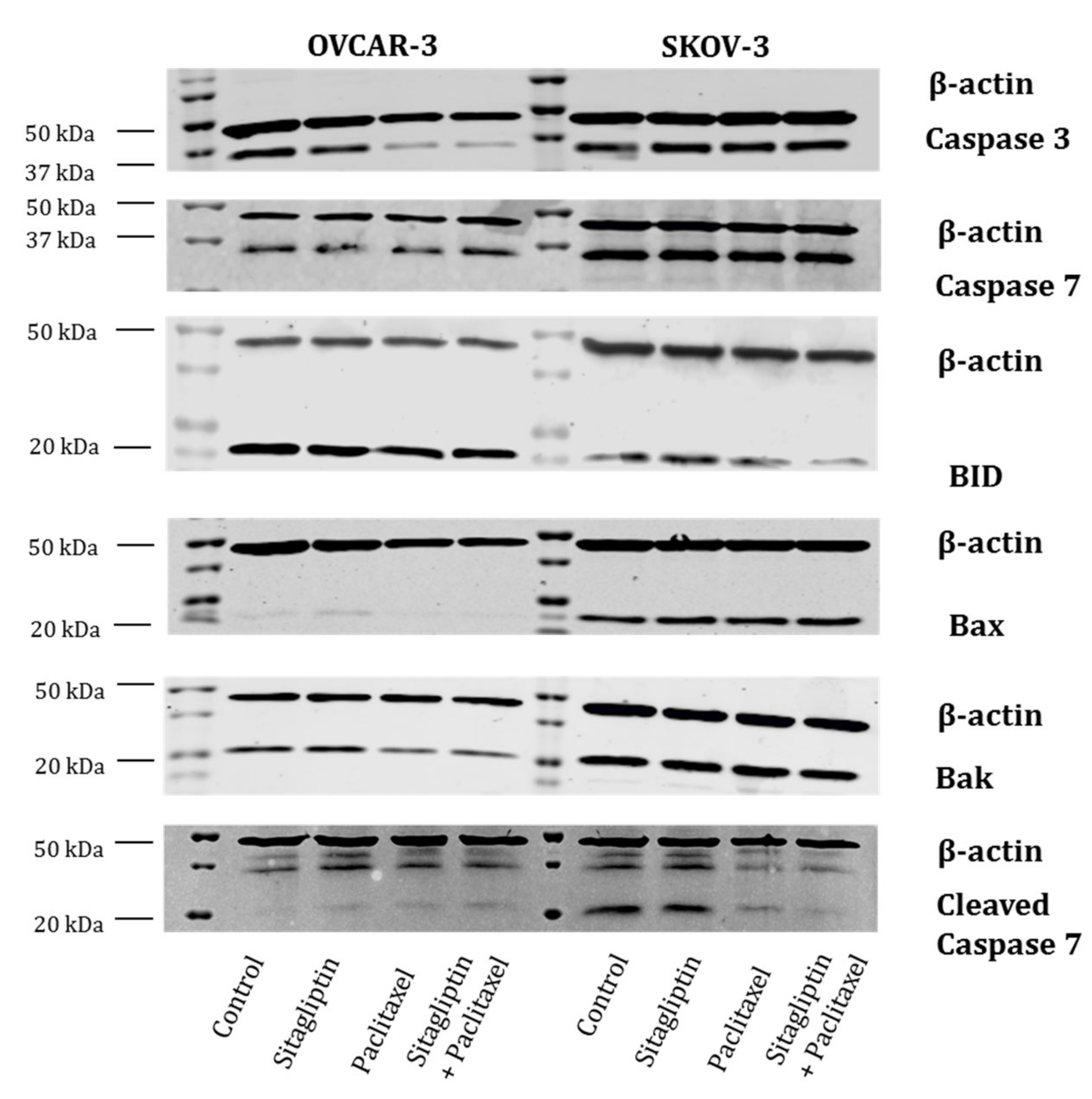
2.3. Sitagliptin Induces Caspase Activity in Paclitaxel Stimulated Ovarian Cancer Cells
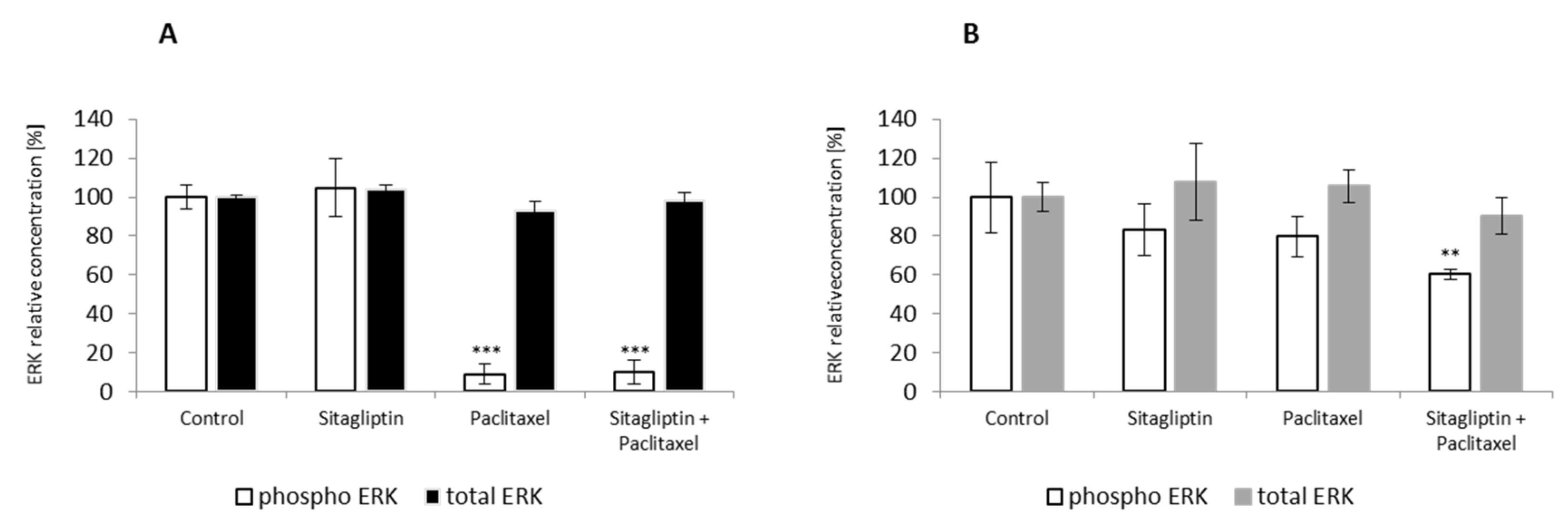
2.4. Sitagliptin Maintained Paclitaxel Influence on ERK Signaling Pathway by Inhibition of Phosphorylation on Residue Thr185 and Thr187
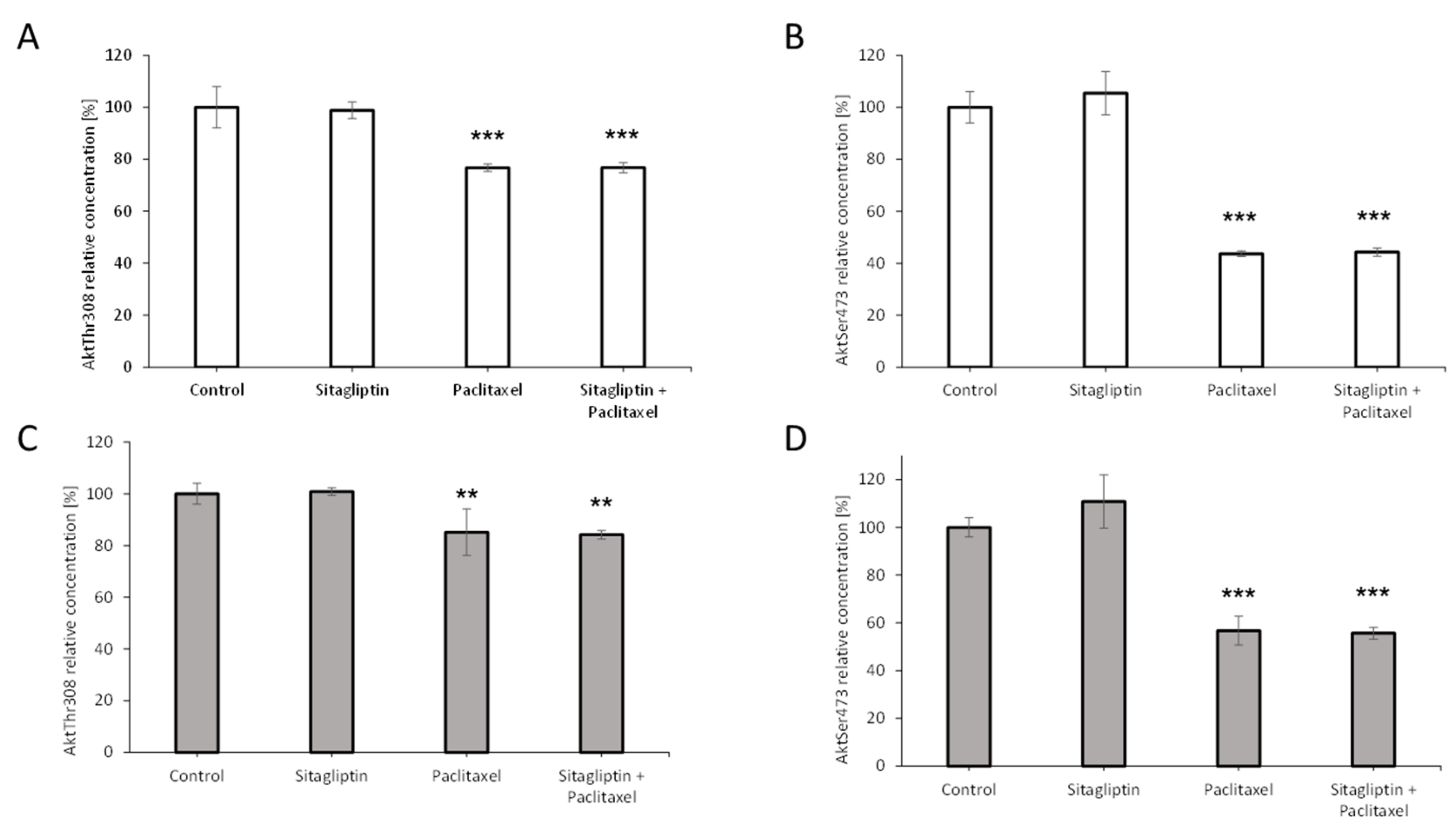
2.5. Sitagliptin Maintained Paclitaxel Influence on Akt Signaling Pathway by Inhibition of Phosphorylation on Residue Thr308 and Ser473
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Immunoblotting
4.3. Fluorescent Bead-Based Luminex Cytokine Assay and MAP Kit
4.4. Intracellular Signaling Assay
4.5. Viability, Cytotoxicity, and Apoptosis Assays
4.6. Transwell Migration Assay
4.7. Transwell Invasion Assay
4.8. RNA Isolation and Quantification
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, D.; Li, N.; Xi, Y.; Zhao, Y.; Wang, T. Diabetes mellitus and risk of ovarian cancer. A systematic review and meta-analysis of 15 cohort studies. Diabetes Res. Clin. Pract. 2017, 130, 43–52. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, F.B. The global implications of diabetes and cancer. Lancet 2014, 383, 1947–1948. [Google Scholar] [CrossRef]
- Nargis, T.; Chakrabarti, P. Significance of circulatory DPP4 activity in metabolic diseases. IUBMB Life 2018, 70, 112–119. [Google Scholar] [CrossRef]
- Prakash, A. Sitagliptin: A review in type 2 diabetes. Drugs 2017, 77, 209–224. [Google Scholar] [CrossRef]
- Amritha, C.; Kumaravelu, P.; Chellathai, D.D. Evaluation of anti cancer effects of D PP-4 inhibitors in colon cancer-An in vitro study. J. Clin. Diagn. Res. 2015, 9, FC14. [Google Scholar] [CrossRef]
- Pinheiro, M.M.; Stoppa, C.L.; Valduga, C.J.; Okuyama, C.E.; Gorjão, R.; Pereira, R.M.S.; Diniz, S.N. Sitagliptin inhibit human lymphocytes proliferation and Th1/Th17 differentiation in vitro. Eur. J. Pharm. Sci. 2017, 100, 17–24. [Google Scholar] [CrossRef]
- Peters, A. Incretin-based therapies: Review of current clinical trial data. Am. J. Med. 2010, 123, S28–S37. [Google Scholar] [CrossRef]
- Makdissi, A.; Ghanim, H.; Vora, M.; Green, K.; Abuaysheh, S.; Chaudhuri, A.; Dhindsa, S.; Dandona, P. Sitagliptin exerts an anti-inflammatory action. J. Clin. Endocrinol. Metab. 2012, 97, 3333–3341. [Google Scholar] [CrossRef]
- Del Carmen, M.G.; Birrer, M.; Schorge, J.O. Carcinosarcoma of the ovary: A review of the literature. Gynecol. Oncol. 2012, 125, 271–277. [Google Scholar] [CrossRef]
- Kaku, T.; Ogawa, S.; Kawano, Y.; Ohishi, Y.; Kobayashi, H.; Hirakawa, T.; Nakano, H. Histological classification of ovarian cancer. Med. Mol. Morphol. 2003, 36, 9–17. [Google Scholar] [CrossRef]
- Hudson, L.G.; Zeineldin, R.; Stack, M.S. Phenotypic plasticity of neoplastic ovarian epithelium: Unique cadherin profiles in tumor progression. Clin. Exp. Metastasis 2008, 25, 643–655. [Google Scholar] [CrossRef]
- Du Bois, A.; Lück, H.-J.; Meier, W.; Adams, H.-P.; Möbus, V.; Costa, S.; Bauknecht, T.; Richter, B.; Warm, M.; Schröder, W.; et al. A randomized clinical trial of Cisplatin/Paclitaxel versus Carboplatin/Paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003, 95, 1320–1329. [Google Scholar] [CrossRef]
- Kumar, A.; Hoskins, P.; Tinker, A. Dose-dense Paclitaxel in advanced ovarian cancer. Clin. Oncol. 2015, 27, 40–47. [Google Scholar] [CrossRef]
- Peng, J.; Hamanishi, J.; Matsumura, N.; Abiko, K.; Murat, K.; Baba, T.; Yamaguchi, K.; Horikawa, N.; Hosoe, Y.; Murphy, S.K.; et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-b to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015, 75, 5034–5045. [Google Scholar] [CrossRef]
- Lee, T.Y.; Martinez-Outschoorn, U.E.; Schilder, R.J.; Kim, C.H.; Richard, S.D.; Rosenblum, N.G.; Johnson, J.M. Metformin as a therapeutic target in endometrial cancers. Front. Oncol. 2018, 8, 341. [Google Scholar] [CrossRef]
- Kłych, A.; Kosowska, A. Matrix metalloproteinases and their inhibitors. The role in the development of diabetic microangiopathy. Diabet. Klin. 2012, 1, 114–120. [Google Scholar]
- Gallego-Colon, E.; Klych-Ratuszny, A.; Kosowska, A.; Garczorz, W.; Aghdam, M.R.F.; Wozniak, M.; Francuz, T. Exenatide modulates metalloproteinase expression in human cardiac smooth muscle cells via the inhibition of Akt signaling pathway. Pharmacol. Rep. 2018, 70, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.M.; Coleman, R.L.; Sood, A.K. Targeting the tumour microenvironment in ovarian cancer. Eur. J. Cancer 2016, 56, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Fossati, M.; Buzzonetti, A.; Monego, G.; Catzola, V.; Scambia, G.; Fattorossi, A.; Battaglia, A. Immunological changes in the ascites of cancer patients after intraperitoneal administration of the bispecific antibody catumaxomab (anti-EpCAM × anti-CD3). Gynecol. Oncol. 2015, 138, 343–351. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, Q.; Lau, W.B.; Lau, B.; Xu, L.; Zhao, L.; Yang, H.; Feng, M.; Xuan, Y.; Yang, Y.; et al. Tumor microenvironment: The culprit for ovarian cancer metastasis? Cancer Lett. 2016, 377, 174–182. [Google Scholar] [CrossRef]
- Kosowska, A.; Gallego-Colon, E.; Garczorz, W.; Kłych, A.; Aghdam, M.R.F.; Wozniak, M.; Witek, A.; Wróblewska-Czech, A.; Cygal, A.; Wojnar, J.; et al. Exenatide modulates tumor–endothelial cell interactions in human ovarian cancer cells. Endocr. Connect. 2017, 6, 856–865. [Google Scholar] [CrossRef]
- Röhrborn, D.; Wronkowitz, N.; Eckel, J. DPP4 in diabetes. Front. Immunol. 2015, 6, 386. [Google Scholar] [CrossRef]
- Wojciechowska, J.; Krajewski, W.; Bolanowski, M.; Kręcicki, T.; Zatoński, T. Diabetes and cancer: A review of current knowledge. Exp. Clin. Endocrinol. Diabetes 2016, 124, 263–275. [Google Scholar] [CrossRef]
- Wenjing, H.; Shuang, Y.; Weisong, L.; Haipeng, X. Exendin-4 does not modify growth or apoptosis of human colon cancer cells. Endocr. Res. 2017, 86, 1–10. [Google Scholar] [CrossRef]
- Corrado, G.; Salutari, V.; Palluzzi, E.; Distefano, M.G.; Scambia, G.; Ferrandina, G. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev. Anticancer Ther. 2017, 17, 1147–1158. [Google Scholar] [CrossRef]
- Shlomai, G.; Neel, B.; Leroith, D.; Leroith, D. Type 2 diabetes mellitus and cancer: The role of pharmacotherapy. J. Clin. Oncol. 2016, 34, 4261–4269. [Google Scholar] [CrossRef]
- Broekman, K.E.; Hof, M.A.J.; Touw, D.J.; Gietema, J.A.; Nijman, H.W.; Lefrandt, J.D.; Reyners, A.K.L.; Jalving, M. Phase I study of metformin in combination with carboplatin/paclitaxel chemotherapy in patients with advanced epithelial ovarian cancer. Investig. New Drugs 2020, 38, 1454–1462. [Google Scholar] [CrossRef]
- Erices, R.; Bravo, M.L.; Gonzalez, P.; Oliva, B.; Racordon, D.; Garrido, M.; Ibañez, C.; Kato, S.; Brañes, J.; Pizarro, J.; et al. Metformin, at concentrations corresponding to the treatment of diabetes, potentiates the cytotoxic effects of Carboplatin in cultures of ovarian cancer cells. Reprod. Sci. 2013, 20, 1433–1446. [Google Scholar] [CrossRef]
- Du, J.; Shi, H.; Ren, F.; Wang, J.-L.; Wu, Q.; Li, X.; Zhang, R. Inhibition of the IGF signaling pathway reverses cisplatin resistance in ovarian cancer cells. BMC Cancer 2017, 17, 851. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, H.; Du, J.; Wang, B.; Yang, L. Anticancer activity of metformin, an antidiabetic drug, against ovarian cancer cells involves inhibition of Cysteine-Rich 61 (Cyr61)/Akt/mammalian target of Rapamycin (mTOR) signaling pathway. Med. Sci. Monit. 2018, 24, 6093–6101. [Google Scholar] [CrossRef]
- Zhao, J.; Klausen, C.; Yi, Y.; Cheng, J.-C.; Chang, H.-M.; Leung, P.C.K. Betacellulin enhances ovarian cancer cell migration by up-regulating Connexin43 via MEK-ERK signaling. Cell. Signal. 2020, 65, 109439. [Google Scholar] [CrossRef]
- MacKenzie, R.; Talhouk, A.; Eshragh, S.; Lau, S.; Cheung, D.; Chow, C.; Le, N.; Cook, L.S.; Wilkinson, N.; McDermott, J.; et al. Morphologic and molecular characteristics of mixed epithelial ovarian cancers. Am. J. Surg. Pathol. 2015, 39, 1548–1557. [Google Scholar] [CrossRef]
- Kossaï, M.; Leary, A.; Scoazec, J.-Y.; Genestie, C. Ovarian cancer: A heterogeneous disease. Pathobiology 2017, 85, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Garziera, M.; Roncato, R.; Montico, M.; De Mattia, E.; Gagno, S.; Poletto, E.; Scalone, S.; Canzonieri, V.; Giorda, G.; Sorio, R.; et al. New challenges in tumor mutation heterogeneity in advanced ovarian cancer by a targeted Next-Generation Sequencing (NGS) approach. Cells 2019, 8, 584. [Google Scholar] [CrossRef]
- Winterhoff, B.; Talukdar, S.; Chang, Z.; Wang, J.; Starr, T.K. Single-cell sequencing in ovarian cancer. Curr. Opin. Obstet. Gynecol. 2019, 31, 49–55. [Google Scholar] [CrossRef]
- Kamat, A.A.; Fletcher, M.; Gruman, L.M.; Mueller, P.; Lopez, A.; Landen, C.N.; Han, L.; Gershenson, D.M.; Sood, A.K. The Clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin. Cancer Res. 2006, 12, 1707–1714. [Google Scholar] [CrossRef]
- Henderson, B.E.; Lee, N.H.; Seewaldt, V.L.; Shen, H. The influence of race and ethnicity on the biology of cancer. Nat. Rev. Cancer 2012, 12, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2014, e51046. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Magnotti, E.; Marasco, W.A. The latest animal models of ovarian cancer for novel drug discovery. Expert Opin. Drug Discov. 2018, 13, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Shen, S.-C.; Hou, W.-C.; Yang, L.-Y.; Chen, Y.-C. Heme oxygenase-1 inhibits breast cancer invasion via suppressing the expression of matrix metalloproteinase-9. Mol. Cancer Ther. 2008, 7, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosowska, A.; Garczorz, W.; Kłych-Ratuszny, A.; Aghdam, M.R.F.; Kimsa-Furdzik, M.; Simka-Lampa, K.; Francuz, T. Sitagliptin Modulates the Response of Ovarian Cancer Cells to Chemotherapeutic Agents. Int. J. Mol. Sci. 2020, 21, 8976. https://doi.org/10.3390/ijms21238976
Kosowska A, Garczorz W, Kłych-Ratuszny A, Aghdam MRF, Kimsa-Furdzik M, Simka-Lampa K, Francuz T. Sitagliptin Modulates the Response of Ovarian Cancer Cells to Chemotherapeutic Agents. International Journal of Molecular Sciences. 2020; 21(23):8976. https://doi.org/10.3390/ijms21238976
Chicago/Turabian StyleKosowska, Agnieszka, Wojciech Garczorz, Agnieszka Kłych-Ratuszny, Mohammad Reza F. Aghdam, Małgorzata Kimsa-Furdzik, Klaudia Simka-Lampa, and Tomasz Francuz. 2020. "Sitagliptin Modulates the Response of Ovarian Cancer Cells to Chemotherapeutic Agents" International Journal of Molecular Sciences 21, no. 23: 8976. https://doi.org/10.3390/ijms21238976
APA StyleKosowska, A., Garczorz, W., Kłych-Ratuszny, A., Aghdam, M. R. F., Kimsa-Furdzik, M., Simka-Lampa, K., & Francuz, T. (2020). Sitagliptin Modulates the Response of Ovarian Cancer Cells to Chemotherapeutic Agents. International Journal of Molecular Sciences, 21(23), 8976. https://doi.org/10.3390/ijms21238976




