Coping with DNA Double-Strand Breaks via ATM Signaling Pathway in Bovine Oocytes
Abstract
1. Introduction
2. Results
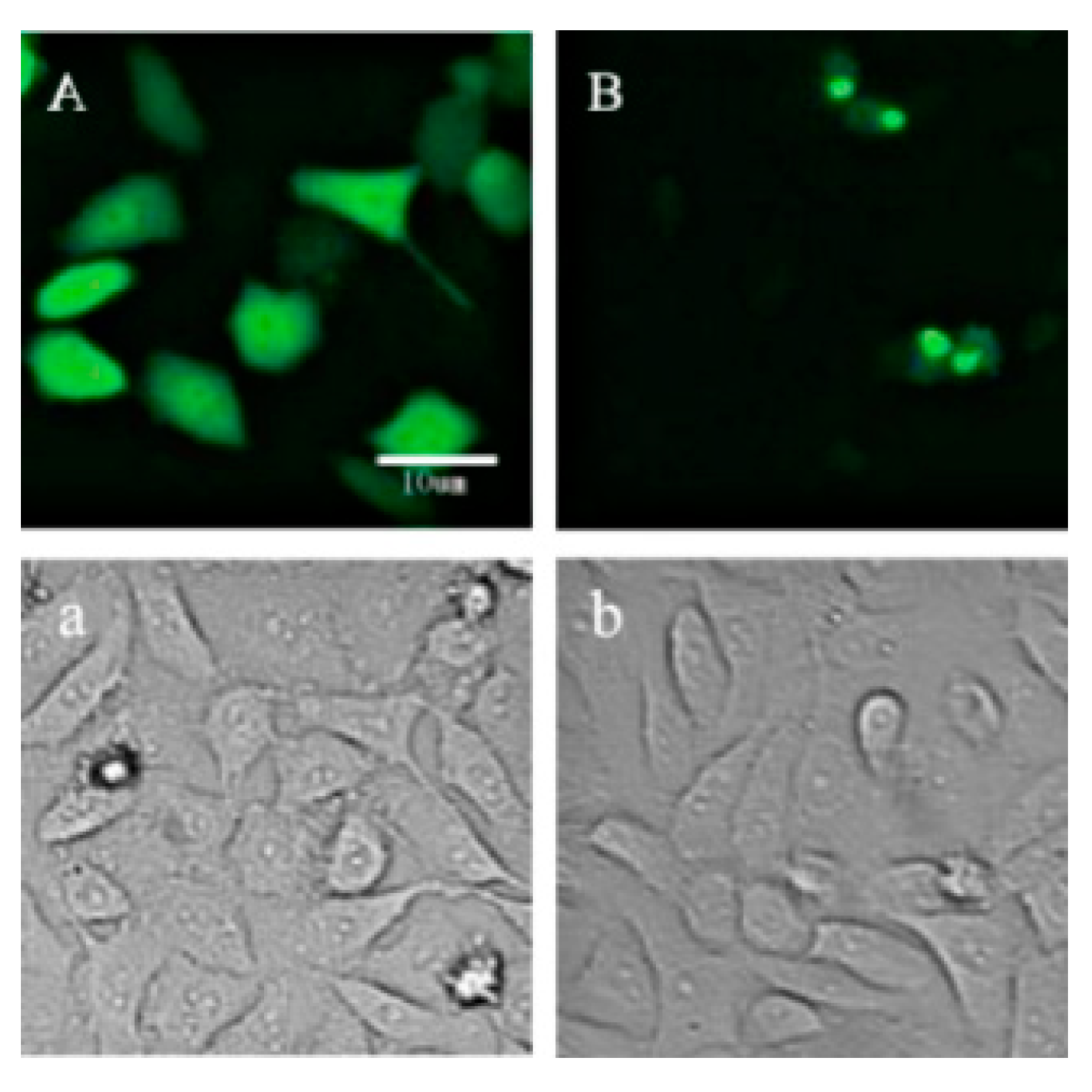
2.1. Location of p21-Venus in HeLa Cells
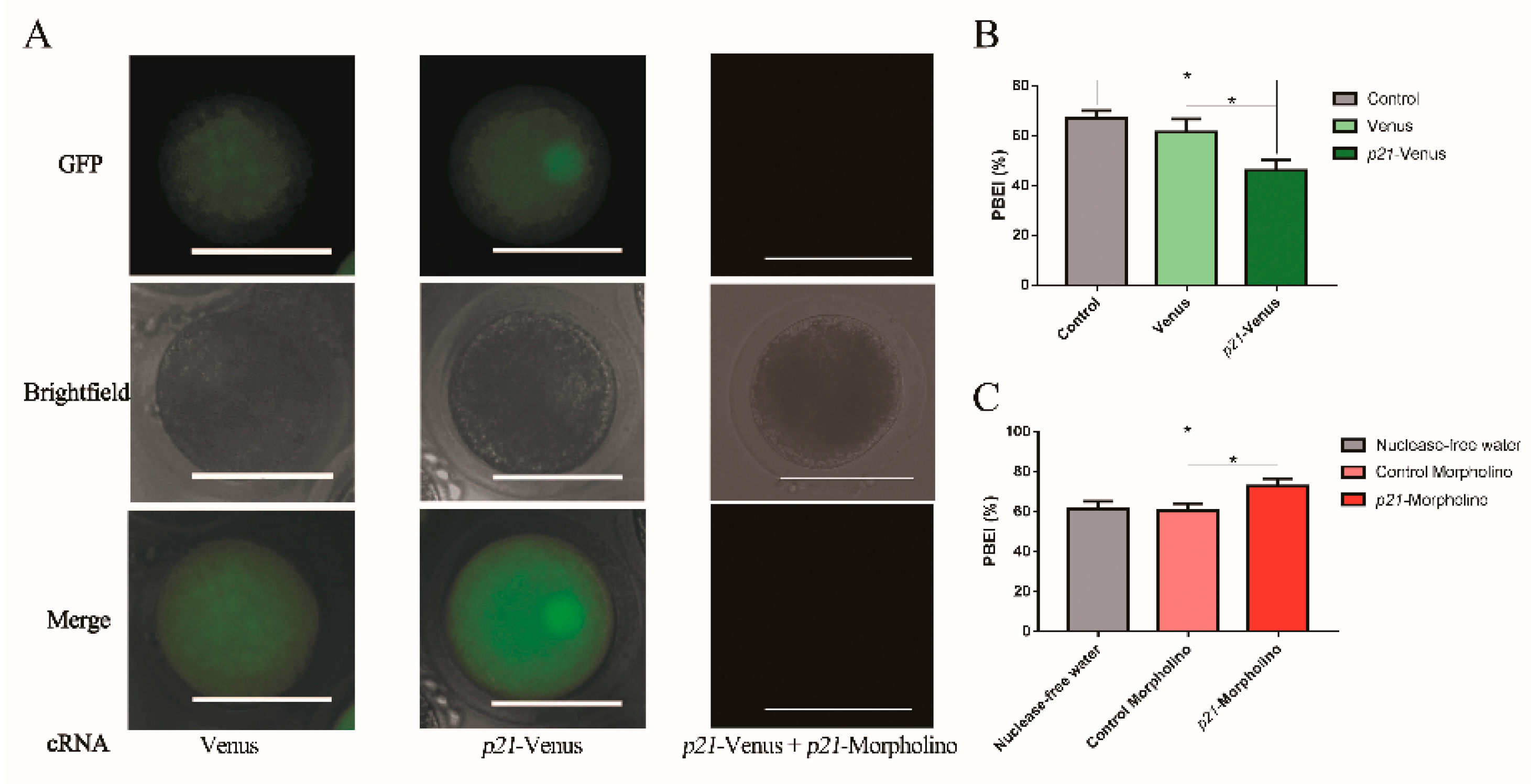
2.2. Effects of p21 on Oocyte IVM
2.3. Effects of DNA DSBs Induced by Zeocin on Bovine Oocyte IVM
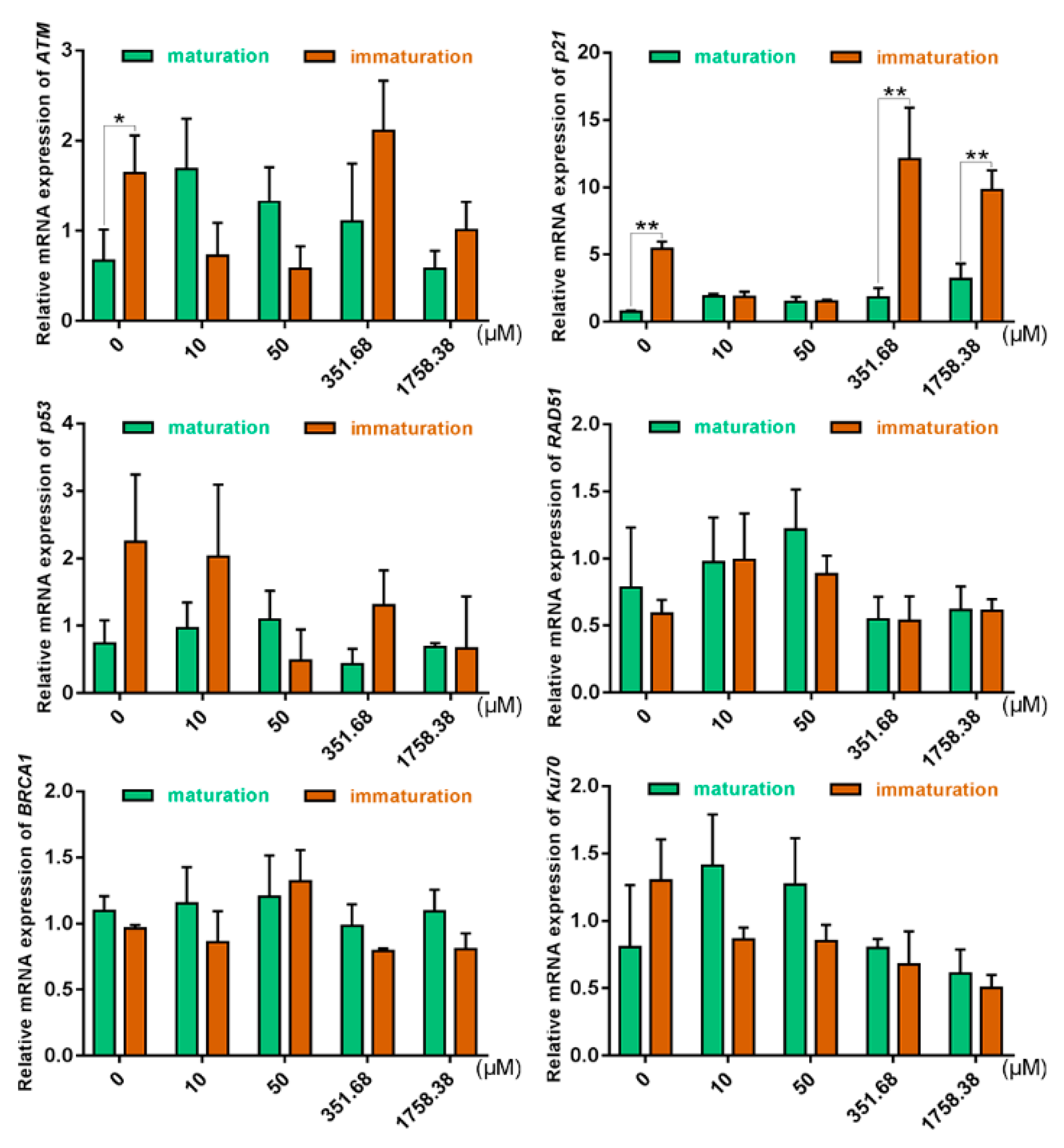
2.4. Expression of DSB Repair Genes in Oocytes Was Altered under Different Zeocin Concentrations
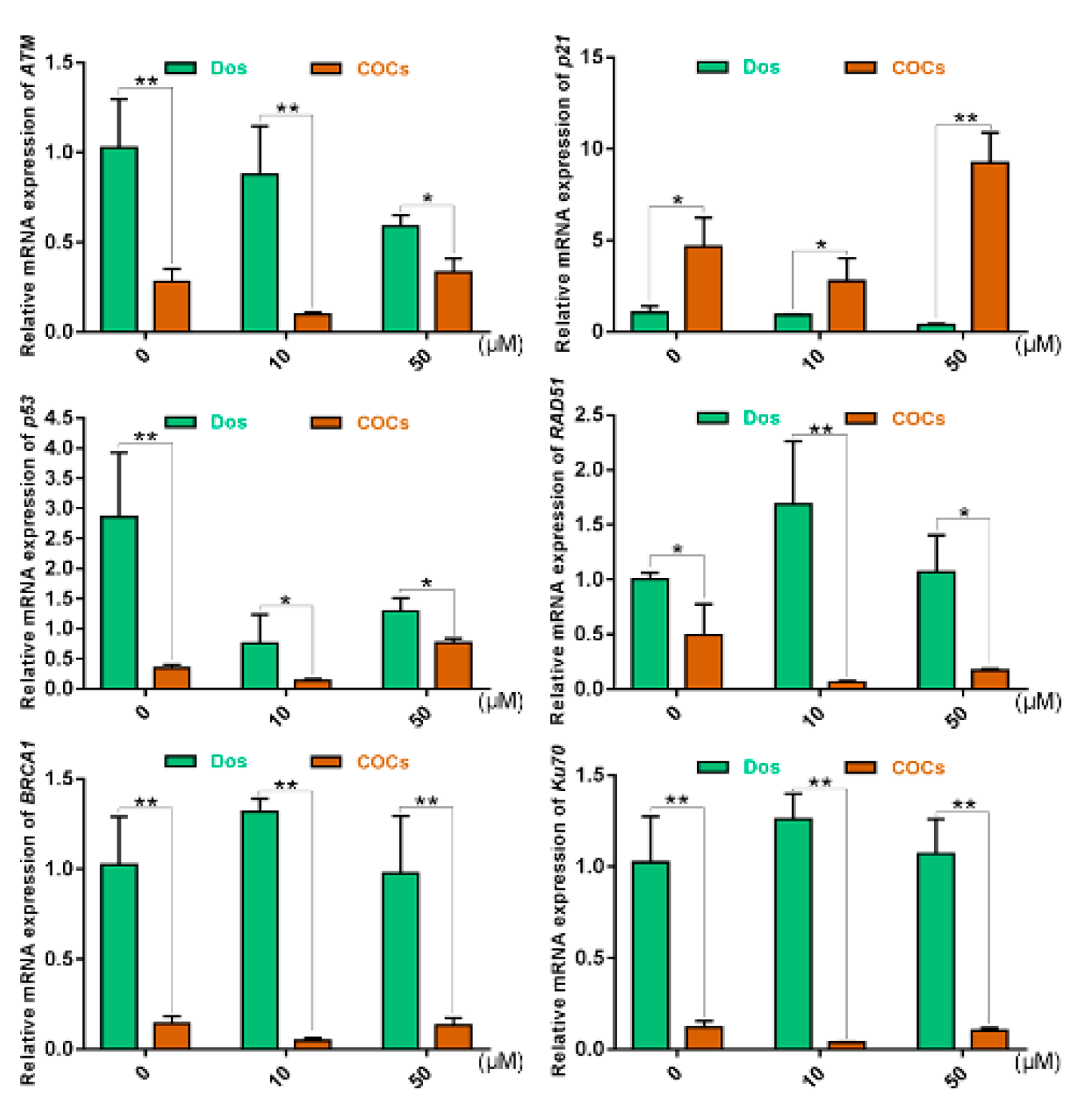
2.5. The Effects of Cumulus Cells on the Response of COCs to DNA DSBs in Prophase of Meiosis I
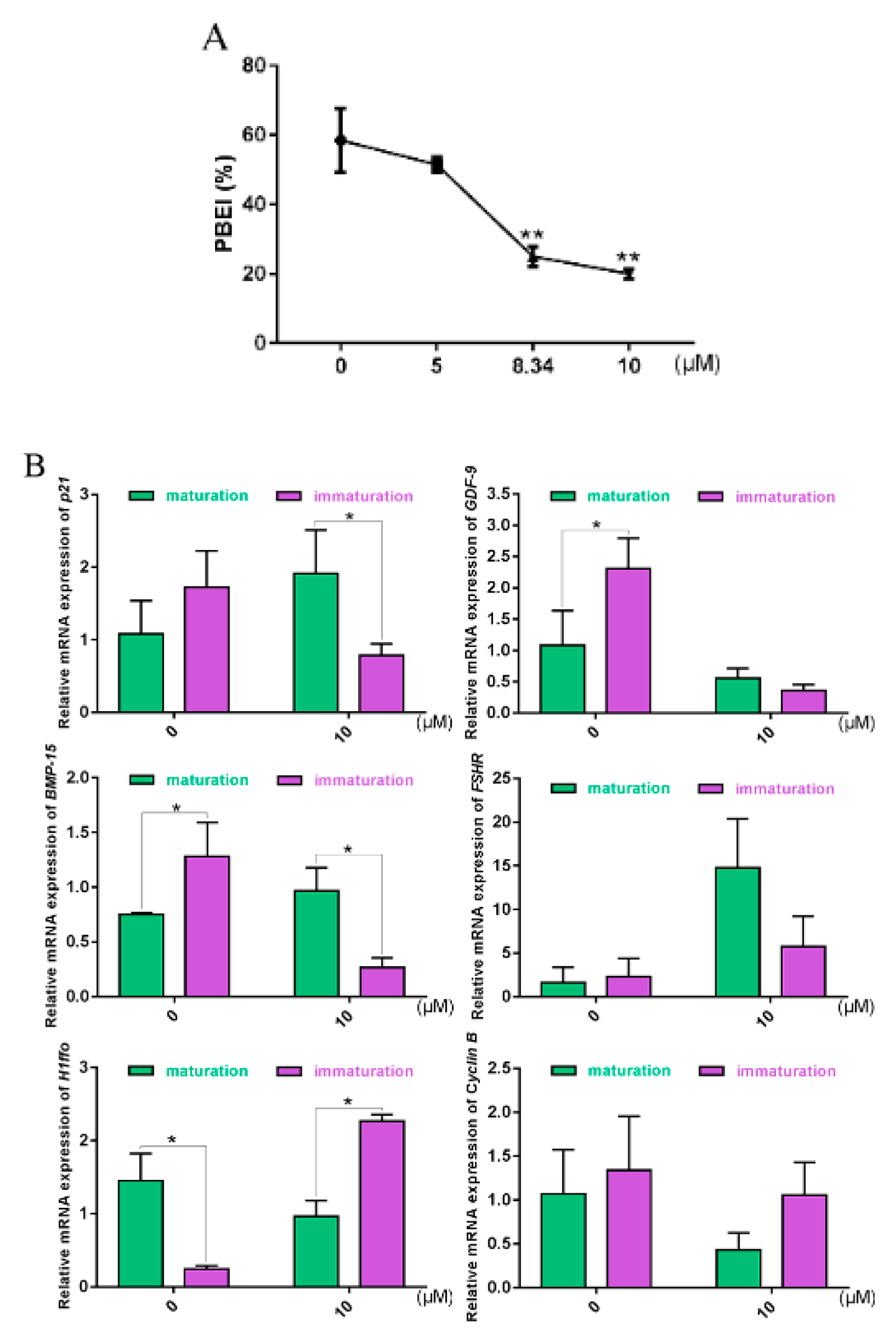
2.6. Oocyte Maturation and Gene Expression Altered with ATM-Specific Inhibitor
3. Discussion
Preconception
- (1)
- During meiosis I, Ku70, a key gene of nonhomologous recombination, was involved in coping with DNA damage when it did not reach the threshold of damage to arrest the cell cycle; after the damage reached the threshold and could not be dealt with completely by the repair function, the cycle process was arrested and the completion of meiosis I was prevented.
- (2)
- When GVBD, physiological DNA SSBs, and DSBs form during the displacement and separation of chromosomes, the fractures of damaged DNA need to be repaired quickly in order to ensure the successful completion of the subsequent maturation process in oocytes. Carroll et al. also confirmed that the expression of the DNA damage marker protein γH2AX increased 6-fold after GVBD, compared with the GV phase [22]. When abnormal conditions lead to repair obstacles by physiological DNA SSBs or DSBs damage, is there a DNA damage check mechanism in oocytes at this time?
4. Materials and Methods
4.1. Reagents
4.2. Overexpression or Interference Experiments of p21 at Germinal Vesicle (GV) Stage in Bovine Oocytes
4.2.1. Amplification of the Expression Vector for p21 and Transfection of Plasmids into Hela Cells
4.2.2. Oocyte Collection and In Vitro Maturation (IVM)
4.2.3. Preparation of p21-Venus cRNA and p21-Morpholino
4.2.4. Microinjection into GV-Stage Oocytes
4.3. Zeocin-Induced DNA Damage in Bovine Oocytes
4.3.1. HeLa Cell Viability Assay
4.3.2. Immunofluorescence Labeling
4.3.3. RNA Extraction and qRT-PCR
4.4. IVM and Gene Expression in Oocytes Treated with ATM-Specific Inhibitor
4.4.1. IVM of Oocytes with ATM-Specific Inhibitor
4.4.2. RNA Extraction and qRT-PCR
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Balaban, B.; Ata, B.; Isiklar, A.; Yakin, K.; Urman, B. Severe cytoplasmic abnormalities of the oocyte decrease cryosurvival and subsequent embryonic development of cryopreserved embryos. Hum. Reprod. 2008, 23, 1778–1785. [Google Scholar] [CrossRef]
- Chamayou, S.; Ragolia, C.; Alecci, C.; Storaci, G.; Guglielmino, A. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: A study of 967 transferred embryos. Reprod. Biomed. Online 2006, 13, 661–667. [Google Scholar] [CrossRef]
- Ciotti, P.M. First polar body morphology before ICSI is not related to embryo quality or pregnancy rate. Hum. Reprod. 2004, 19, 2334–2339. [Google Scholar] [CrossRef]
- Madaschi, C.; Aoki, T.; de Almeida Ferreira Braga, D.P.; de Cássia Sávio Figueira, R.; Francisco, L.S.; Iaconelli, A., Jr.; Borges, E., Jr. Zona pellucida birefringence score and meiotic spindle visualization in relation to embryo development and ICSI outcomes. Reprod. Biomed. Online 2009, 18, 681–686. [Google Scholar] [CrossRef]
- Madaschi, C.; de Souza Bonetti, T.C.; de Almeida Ferreira Braga, D.P.; Pasqualotto, F.F.; Iaconelli, A., Jr.; Borges, E., Jr. Spindle imaging: A marker for embryo development and implantation. Fertil. Steril. 2008, 90, 194–198. [Google Scholar] [CrossRef]
- Luberda, Z. The role of glutathione in mammalian gametes. Reprod. Biol. 2005, 5, 5–17. [Google Scholar]
- Pauli, S.A.; Session, D.R.; Shang, W.; Easley, K.; Sidell, N. Analysis of Follicular Fluid Retinoids in Women Undergoing In Vitro Fertilization: Retinoic Acid Influences Embryo Quality and Is Reduced in Women With Endometriosis. Reprod. Sci. 2013, 20, 1116–1124. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Nadal-Desbarats, L.; Gerard, N.; Dubois, A.; Holyoake, P.K.; Grupen, C.G. Differences in the metabolomic signatures of porcine follicular fluid collected from environments associated with good and poor oocyte quality. Reproduction 2013, 146, 221–231. [Google Scholar] [CrossRef]
- El Sheikh, M.; Mesalam, A.; Mesalam, A.A.; Idrees, M.; Lee, K.-L.; Kong, I.-K. Melatonin Abrogates the Anti-Developmental Effect of the AKT Inhibitor SH6 in Bovine Oocytes and Embryos. Int. J. Mol. Sci. 2019, 20, 2956. [Google Scholar] [CrossRef]
- Garcia, P.; Aspee, K.; Ramirez, G.; Dettleff, P.; Palomino, J.; Peralta, O.A.; Parraguez, V.H.; De los Reyes, M. Influence of growth differentiation factor 9 and bone morphogenetic protein 15 on in vitro maturation of canine oocytes. Reprod. Domest. Anim. 2019, 54, 373–380. [Google Scholar] [CrossRef]
- Ruso, H.; Dayanir, D.; Kalem, Z.; Tural, R.; Saribas, S.; Dincel, A.S.; Ozogul, C.; Gurgan, T. Cumulus cells and follicular fluid show alterations in bone morphogenic protein 15 (BMP-15), growth differentiation factor 9 (GDF-9), and oxidative status in patients with endometriosis. Hum. Reprod. 2019, 34, 289–290. [Google Scholar]
- D’Occhio, M.J.; Campanile, G.; Baruselli, P.S. Transforming growth factor-beta superfamily and interferon-tau in ovarian function and embryo development in female cattle: Review of biology and application. Reprod. Fertil. Dev. 2020, 32, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.; Palomino, J.; Aspee, K.; De Los Reyes, M. GDF-9 and BMP-15 mRNA Levels in Canine Cumulus Cells Related to Cumulus Expansion and the Maturation Process. Animals 2020, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Kalafut, J.; Przybyszewska, A.; Paziewska, B.; Adamczuk, G.; Kielbus, M.; Rivero-Mueller, A. FSHR Trans-Activation and Oligomerization. Front. Endocrinol. 2018, 9, 760. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Kountourakis, P.; Kottorou, A.E.; Antonacopoulou, A.G.; Rolfo, C.; Peeters, M.; Kalofonos, H.P. Follicle-Stimulating Hormone Receptor (FSHR): A Promising Tool in Oncology? Mol. Diagn. Ther. 2016, 20, 523–530. [Google Scholar] [CrossRef]
- Mamoru, T.; Maki, K.; Hennebold, J.D.; Eppig, J.J.; Viveiros, M.M.; Emery, B.R.; Carrell, D.T.; Kirkman, N.J.; Blazej, M.; Jian, Z. H1FOO Is Coupled to the Initiation of Oocytic Growth. Biol. Reprod. 2005, 72, 135–142. [Google Scholar]
- Mcgraw, S.; Vigneault, C.; Tremblay, K.; Sirard, M.A. Characterization of linker histone H1FOO during bovine in vitro embryo development. Mol. Reprod. Dev. 2006, 73, 692–699. [Google Scholar] [CrossRef]
- Eini, F.; Bidadkosh, A.; Nazarian, H.; Piryaei, A.; Novin, M.G.; Joharchi, K. Thymoquinone reduces intracytoplasmic oxidative stress and improves epigenetic modification in polycystic ovary syndrome mice oocytes, during in-vitro maturation. Mol. Reprod. Dev. 2019, 86, 1053–1066. [Google Scholar] [CrossRef]
- Bischof, J.; Brand, C.A.; Somogyi, K.; Májer, I.; Thome, S.; Mori, M.; Schwarz, U.S.; Lénárt, P. A cdk1 gradient guides surface contraction waves in oocytes. Nat. Commun. 2017, 8, 849. [Google Scholar] [CrossRef]
- Tuppi, M.; Kehrloesser, S.; Coutandin, D.W.; Rossi, V.; Dötsch, V. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat. Struct. Mol. Biol. 2018, 25, 261–269. [Google Scholar] [CrossRef]
- Horta, F.; Catt, S.; Ramachandran, P.; Vollenhoven, B.; Temple-Smith, P. Female ageing affects the DNA repair capacity of oocytes in IVF using a controlled model of sperm DNA damage in mice. Hum. Reprod. 2020, 35, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Marangos, P.; Carroll, J. Oocytes Progress beyond Prophase in the Presence of DNA Damage. Curr. Biol. 2012, 22, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Meulmeester, E.; Pereg, Y.; Shiloh, Y.; Jochemsen, A.G. ATM-Mediated Phosphorylations Inhibit Mdmx/Mdm2 Stabilization by HAUSP in Favor of p53 Activation. Cell Cycle 2005, 4, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Falck, J.; Petrini, J.H.J.; Williams, B.R.; Lukas, J.; Bartek, J. The DNA damage-dependent intra?S phase checkpoint is regulated by parallel pathways. Nat. Genet. 2002, 30, 290–294. [Google Scholar] [CrossRef]
- Gu, Y.; Turck, C.W.; Morgan, D.O. Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature 1993, 366, 707–710. [Google Scholar] [CrossRef]
- Soleimani, R.; Heytens, E.; Darzynkiewicz, Z.; Oktay, K. Mechanisms of chemotherapy-induced human ovarian aging: Double strand DNA breaks and microvascular compromise. Aging 2011, 3, 782–793. [Google Scholar] [CrossRef]
- Hickson, I.; Yan, Z.; Richardson, C.J.; Green, S.J.; Martin, N.M.B.; Orr, A.I.; Reaper, P.M.; Jackson, S.P.; Curtin, N.J.; Smith, G.C.M. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004, 64, 9152–9159. [Google Scholar] [CrossRef]
- Azami, S.H.; Nazarian, H.; Abdollahifar, M.A.; Eini, F.; Farsani, M.A.; Novin, M.G. The antioxidant curcumin postpones ovarian aging in young and middle-aged mice. Reprod. Fertil. Dev. 2020, 32, 292–303. [Google Scholar] [CrossRef]
- Salumets, A.; Suikkari, A.M.; Mols, T.; Soderstrom-Anttila, V.; Tuuri, T. Influence of oocytes and spermatozoa on early embryonic development. Fertil. Steril. 2002, 78, 1082–1087. [Google Scholar] [CrossRef]
- Kupka, M.S.; Ferraretti, A.P.; De Mouzon, J.; Erb, K.; D’Hooghe, T.; Castilla, J.A.; Calhaz-Jorge, C.; De Geyter, C.; Goossens, V.; Strohmer, H.; et al. Assisted reproductive technology in Europe, 2010: Results generated from European registers by ESHRE. Hum. Reprod. 2014, 29, 2099–2113. [Google Scholar] [CrossRef]
- Lydall, D.; Nikolsky, Y.; Bishop, D.K.; Weinert, T. A meiotic recombination checkpoint controlled by mitotic checkpoint genes. Nature 1996, 383, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Perez, E.; Colaiácovo, M.P. Distribution of meiotic recombination events: Talking to your neighbors. Curr. Opin. Genet. Dev. 2009, 19, 105–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roeder, G.S.; Bailis, J.M. The pachytene checkpoint. Trends Genet. 2000, 16, 395–403. [Google Scholar] [CrossRef]
- Kurahashi, H.; Kogo, H.; Tsutsumi, M.; Inagaki, H.; Ohye, T. Failure of homologous synapsis and sex-specific reproduction problems. Front. Genet. 2012, 3, 112. [Google Scholar] [CrossRef]
- Tease, C. X-ray-induced chromosome aberrations in dictyate oocytes of young and old female mice. Mutat. Res. Lett. 1983, 119, 191–194. [Google Scholar] [CrossRef]
- Jacquet, P.; Adriaens, I.; Buset, J.; Neefs, M.; Vankerkom, J. Cytogenetic studies in mouse oocytes irradiated in vitro at different stages of maturation, by use of an early preantral follicle culture system. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2005, 583, 168–177. [Google Scholar] [CrossRef]
- Bartek, J.; Lukas, J. DNA damage checkpoints: From initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007, 19, 238–245. [Google Scholar] [CrossRef]
- Ciccia, A.; Elledge, S.J. The DNA Damage Response: Making it safe to play with knives. Mol. Cell. 2010, 40, 179–204. [Google Scholar] [CrossRef]
- Tay, V.S.Y.; Devaraj, S.; Koh, T.; Ke, G.; Crasta, K.C.; Ali, Y. Increased double strand breaks in diabetic beta-cells with a p21 response that limits apoptosis. Sci. Rep. 2019, 9, 19341. [Google Scholar] [CrossRef]
- Kaushal, N.; Bansal, M.P. Inhibition of CDC2/Cyclin B1 in response to selenium-induced oxidative stress during spermatogenesis: Potential role of Cdc25c and p21. Mol. Cell. Biochem. 2007, 298, 139–150. [Google Scholar] [CrossRef]
- Zhao, G.-M.; Yang, W.-L.; Wu, S.-J.; Yun, Y.; Lei, A.-M. Detection for p21 Gene Expression in Bovine Oocytes Maturation and Construction of Eukaryotic Expression Vector pVenus-P21. Acta Vet. Zootech. Inica. 2011, 42, 1071–1080. [Google Scholar]
- Zhao, G.-M. Effect of P21 on Bovine Oocytes Meiotic Maturation; Northwest Agriculture and Forestry University: Yangling, China, 2011; pp. 1–90. [Google Scholar]
- Levasseur, M.; Carroll, M.; Jones, K.T.; McDougall, A. A novel mechanism controls the Ca2+ oscillations triggered by activation of ascidian eggs and has an absolute requirement for Cdk1 activity. J. Cell Sci. 2007, 120, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.D.; Ayabe, T.; Kopf, G.S.; Schultz, R.M. Temporal patterns of gene expression of G1-S cyclins and cdks during the first and second mitotic cell cycles in mouse embryos. Mol. Reprod. Dev. 1996, 45, 264–275. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.-Q. The ATM Inhibitor KU-55933 Suppresses Cell Proliferation and Induces Apoptosis by Blocking Akt In Cancer Cells with Overactivated Akt. Mol. Cancer Ther. 2010, 9, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Yuen, W.S.; Merriman, J.A.; O’Bryan, M.K.; Jones, K.T. DNA Double Strand Breaks but Not Interstrand Crosslinks Prevent Progress through Meiosis in Fully Grown Mouse Oocytes. PLoS ONE 2012, 7, e43875. [Google Scholar] [CrossRef] [PubMed]
- Tanghe, S.; Van Soom, A.; Nauwynck, H.; Coryn, M.; de Kruif, A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol. Reprod. Dev. 2002, 61, 414–424. [Google Scholar] [CrossRef]
- Sugiura, K.; Pendola, F.L.; Eppig, J.J. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: Energy metabolism. Dev. Biol. 2005, 279, 20–30. [Google Scholar] [CrossRef]
- Reinhardt, H.C.; Yaffe, M.B. Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr. Opin. Cell Biol. 2009, 21, 245–255. [Google Scholar] [CrossRef]
- Smith, J.; Tho, L.M.; Xu, N.; Gillespie, D.A. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv. Cancer Res. 2010, 108, 73–112. [Google Scholar]
- Paull, T.T.; Rogakou, E.P.; Yamazaki, V.; Kirchgessner, C.U.; Bonner, W.M. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000, 10, 886–895. [Google Scholar] [CrossRef]
- Ward, I.M.; Chen, J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 2001, 276, 47759–47762. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; O’Donnell, A.H.; Kim, S.T.; Kastan, M.B. Phosphorylation of Serine 1387 in Brca1 Is Specifically Required for the Atm-mediated S-Phase Checkpoint after Ionizing Irradiation. Cancer Res. 2002, 62, 4588–4591. [Google Scholar] [PubMed]
- Li, G.; Liu, D.; Zhang, X.; Quan, R.; Zhong, C.; Mo, J.; Huang, Y.; Wang, H.; Ruan, X.; Xu, Z. Suppressing Ku70/Ku80 expression elevates homology-directed repair efficiency in primary fibroblasts. Int. J. Biochem. Cell Biol. 2018, 99, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Pannunzio, N.R.; Watanabe, G.; Lieber, M.R. Nonhomologous DNA end-joining for repair of DNA double-strand breaks. J. Biol. Chem. 2018, 293, 10512–10523. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Susor, A.; Conti, M. Protein Tyrosine Kinase Wee1B Is Essential for Metaphase II Exit in Mouse Oocytes. Science 2011, 332, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.; Rego, M.A.; Boisvert, R.A.; Esashi, F.; Cavallo, F.; Jasin, M.; Howlett, N.G. p21 promotes error-free replication-coupled DNA double-strand break repair. Nucleic Acids Res. 2012, 40, 8348–8360. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, G.; Ricci, C.; Zannini, L.; Fontanella, E.; Plevani, P.; Delia, D. Bimodal regulation of p21(waf1) protein as function of DNA damage levels. Cell Cycle 2014, 13, 2901–2912. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, L.; Chao, S.B.; Wang, Z.B.; Qi, S.T.; Sun, Q.-Y. Checkpoint kinase 1 is essential for meiotic cell cycle regulation in mouse oocytes. Cell Cycle 2012, 11, 1948–1955. [Google Scholar] [CrossRef][Green Version]
- Nagai, T.; Ibata, K.; Park, E.S.; Kubota, M.; Mikoshiba, K.; Miyawaki, A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002, 20, 87–90. [Google Scholar] [CrossRef]
- Rekas, A. 6Crystal Structure of Venus, a Yellow Fluorescent Protein with Improved Maturation and Reduced Environmental Sensitivity. J. Biol. Chem. 2002, 277, 50573–50578. [Google Scholar] [CrossRef]
- Yang, W.L.; Li, J.; An, P.; Lei, A.M. Cdc20 downregulation impairs spindle morphology and causes reduced first polar body emission during bovine oocyte maturation. Theriogenology 2014, 81, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Eisa, A.A.; De, S.; Detwiler, A.; Gilker, E.; Ignatious, A.C.; Vijayaraghavan, S.; Kline, D. YWHA (14-3-3) protein isoforms and their interactions with CDC25B phosphatase in mouse oogenesis and oocyte maturation. BMC Dev. Biol. 2019, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Hu, Y.; Ren, C.; Cao, Q.; Huo, R. METTL3-mediated m 6 A is required for murine oocyte maturation and maternal-to-zygotic transition. Cell Cycle 2020, 19, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, J.; Zhao, G.; Yun, Y.; Wu, S.; Jones, K.T.; Lei, A. Differential regulation of cyclin b1 degradation between the first and second meiotic divisions of bovine oocytes. Theriogenology 2012, 78, 1171–1181. [Google Scholar] [CrossRef]
- Yun, Y.; An, P.; Ning, J.; Zhao, G.M.; Yang, W.L.; Lei, A.M. H1foo is essential for in vitro meiotic maturation of bovine oocytes. Zygote 2015, 23, 416–425. [Google Scholar] [CrossRef]

| Group | Samples Microinjected |
|---|---|
| Overexpression | p21-Venus group: 200 ng/μL p21-Venus cRNA |
| Venus group: 200 ng/μL Venus cRNA | |
| Interference | p21-Morpholino group: 2 mmol/L p21-Morpholino |
| Control Morpholino group: 2 mmol/L Control Morpholino | |
| Control | nuclease-free H2O group: nuclease-free H2O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xu, X.; Teng, M.; Zhao, G.; Lei, A. Coping with DNA Double-Strand Breaks via ATM Signaling Pathway in Bovine Oocytes. Int. J. Mol. Sci. 2020, 21, 8892. https://doi.org/10.3390/ijms21238892
Wang L, Xu X, Teng M, Zhao G, Lei A. Coping with DNA Double-Strand Breaks via ATM Signaling Pathway in Bovine Oocytes. International Journal of Molecular Sciences. 2020; 21(23):8892. https://doi.org/10.3390/ijms21238892
Chicago/Turabian StyleWang, Lili, Xiaolei Xu, Mingming Teng, Guimin Zhao, and Anmin Lei. 2020. "Coping with DNA Double-Strand Breaks via ATM Signaling Pathway in Bovine Oocytes" International Journal of Molecular Sciences 21, no. 23: 8892. https://doi.org/10.3390/ijms21238892
APA StyleWang, L., Xu, X., Teng, M., Zhao, G., & Lei, A. (2020). Coping with DNA Double-Strand Breaks via ATM Signaling Pathway in Bovine Oocytes. International Journal of Molecular Sciences, 21(23), 8892. https://doi.org/10.3390/ijms21238892





