TRPV1 Channel: A Noxious Signal Transducer That Affects Mitochondrial Function
Abstract
1. Introduction
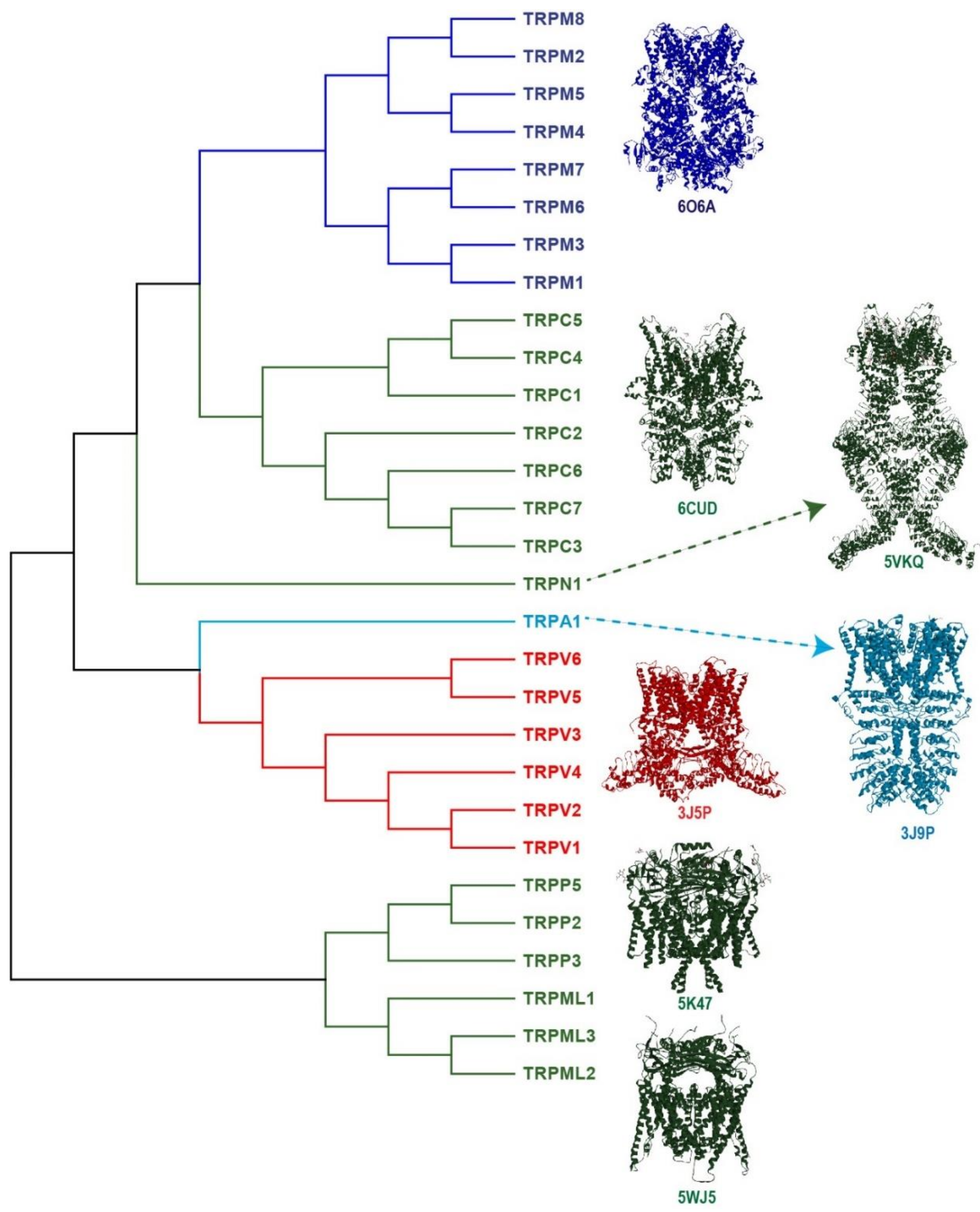
2. The TRPV1 Channel: A Key Transducer of Nociception
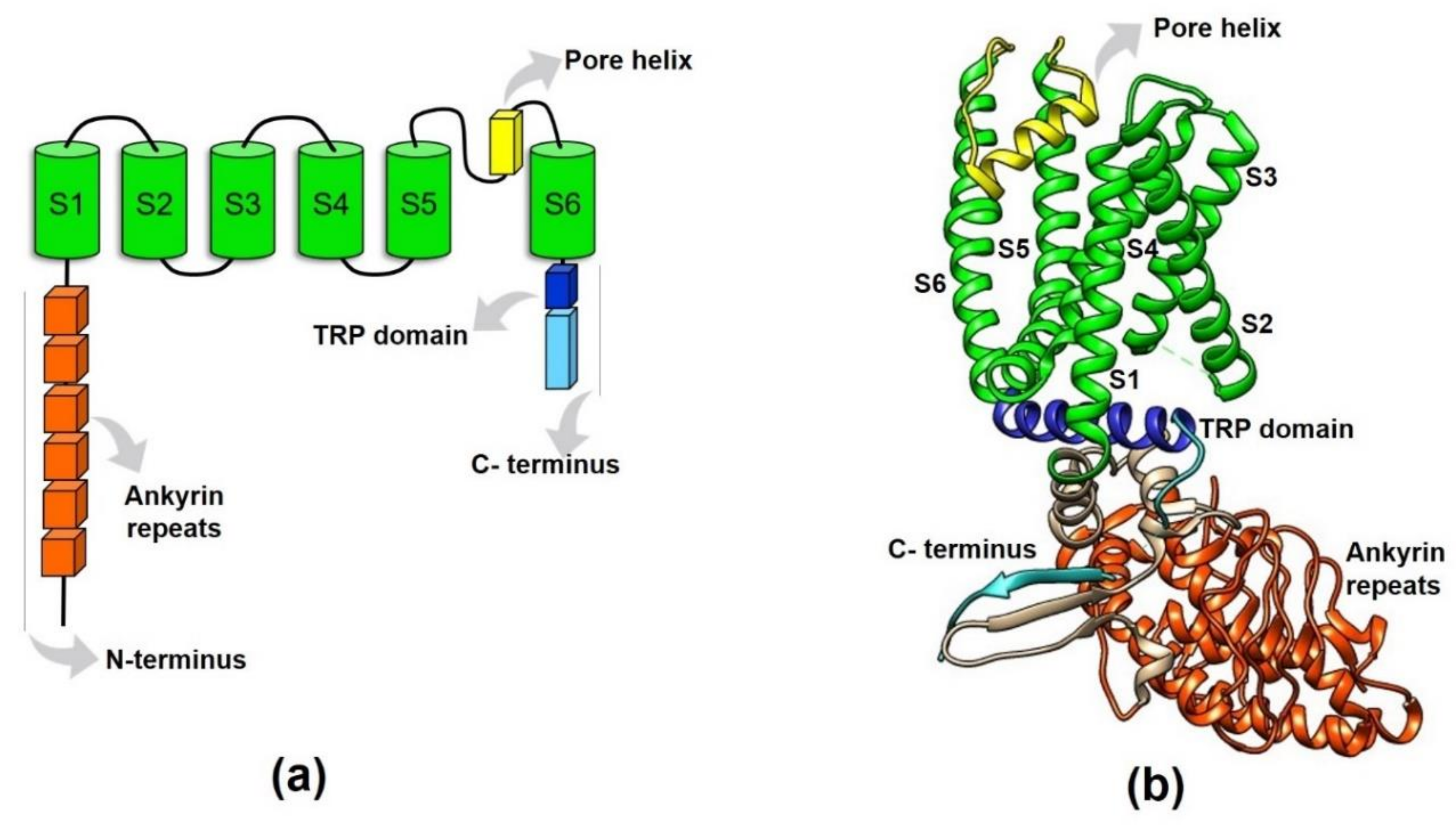
2.1. TRPV1 Structural Features
2.2. Intracellular TRPV1 Localization
2.3. TRPV1 Channels: Their Mitochondrial Actions in Non-Neuronal Cells
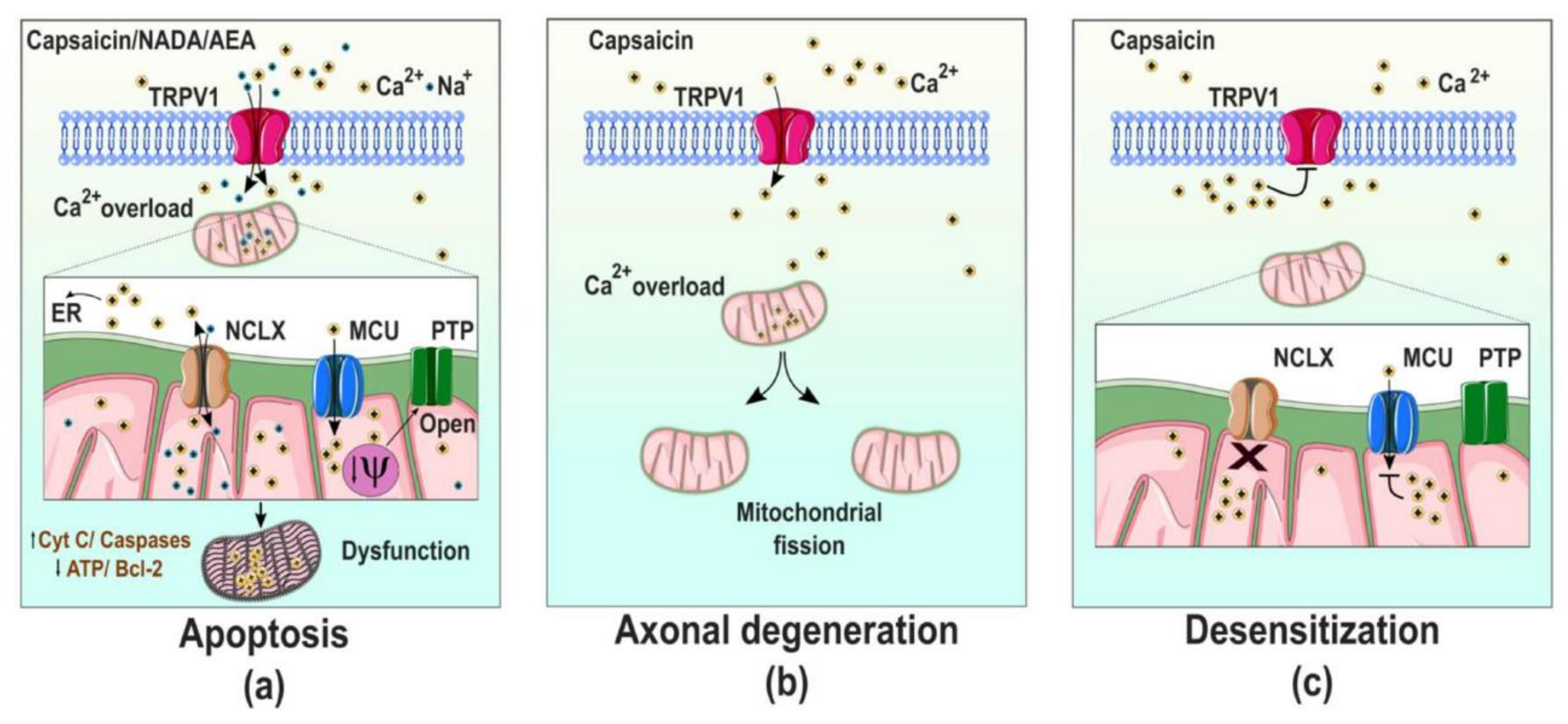
2.4. TRPV1 Channels and Mitochondrial Dysfunction in Neuronal Cells
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Apoe−/− mice | Atherosclerosis-prone apolipoprotein E-deficient mice |
| ARD | Ankyrin Repeats Domain |
| ASM | Airway Smooth Muscle |
| ATP | Adenosine Triphosphate |
| CaM | Calmodulin |
| CB1 | Cannabinoid Receptor 1 |
| cDNAs | Complementary DNA |
| COS7 cell | Fibroblast-like cell line derived from monkey kidney tissue |
| cryo-EM | Single-Particle Electron Cryo-Microscopy |
| C-terminus | Carboxyl-terminus |
| DRG | Dorsal Root Ganglion |
| ER | Endoplasmic Reticulum |
| H/R | Hypoxia/Reoxygenation |
| H9C2 cell line | Rat heart tissue-derived cardiac myoblast cell line |
| HEK293 cells | Human embryonic kidney 293 cells line |
| HeLa cells | Human Cervix Epithelial Carcinoma cell line |
| Kv channels | Voltage-gated K+ channels |
| LPA | Lysophosphatidic Acid |
| MAPK pathway | Mitogen-Activated Protein Kinase pathway |
| MCU | Mitochondrial Ca2+ Uniporter |
| mitROS | Mitochondrial reactive oxygen species |
| NADA | N-arachidonoyl-dopamine |
| NCLX | Na+/Ca2+/Li2+ exchanger |
| N-terminus | Amino-terminus |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| SERCA | Sarco/Endoplasmic Reticulum Ca2+-ATPase |
| SH-SY5Y cell | Human neuroblastoma cell line |
| S1-S6 | Transmembrane segments 1-6 |
| TG | Trigeminal Ganglion |
| TRP | Transient Receptor Potentials |
| TRPA | Transient Receptor Potentials Ankyrin |
| TRPA1 | Transient Receptor Potential Ankyrin 1 |
| TRPC | Transient Receptor Potentials Canonical |
| TRPC1 | Transient Receptor Potential Channel-related 1 |
| TRPC3 | Transient Receptor Potential Canonical type 3 |
| trpl | Transient Receptor Potential-Like |
| trp | Transient Receptor Potential Mutant |
| TRPM | Transient Receptor Potentials Melastatin |
| TRPM8 | Transient Receptor Potential Melastatin Member 8 |
| TRPML | Transient Receptor Potentials Mucolipin |
| TRPML1 | Transient Receptor Potential Mucolipin 1 |
| TRPN | Transient Receptor Potentials No Mechanoreceptor |
| TRPP | Transient Receptor Potentials Polycystin |
| TRPP1 | Transient Receptor Potential Polycystin 1 |
| TRPV | Transient Receptor Potentials Vanilloid |
| TRPV1 | The Transient Receptor Vanilloid 1 |
| Trpv1-/- | Transient Receptor Potential Vanilloid 1 Knockout |
| TRPV1-KO | Transient Receptor Potential Vanilloid 1 Knockout |
| TRPY | Transient Receptor Potentials Yeast |
| WT | Wild-Type |
| 3D | Three-dimensional |
| 12-(S)-HETE | 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid |
| 13-(S)-HODE | 13(S)-Hydroxy-9Z,11E-octadecadienoic acid |
References
- Cosens, D.J.; Manning, A. Abnormal electroretinogram from a Drosophila mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Cosens, D. Blindness in a Drosophila mutant. J. Insect Physiol. 1971, 17, 285–302. [Google Scholar] [CrossRef]
- Pak, W.L. Why Drosophila to study phototransduction? J. Neurogenet 2010, 24, 55–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Minke, B.; Wu, C.; Pak, W.L. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature 1975, 258, 84–87. [Google Scholar] [CrossRef]
- Barash, S.; Suss, E.; Stavenga, D.G.; Rubinstein, C.T.; Selinger, Z.; Minke, B. Light reduces the excitation efficiency in the nss mutant of the sheep blowfly Lucilia. J. Gen. Physiol. 1988, 92, 307–330. [Google Scholar] [CrossRef]
- Minke, B. Light-induced reduction in excitation efficiency in the trp mutant of Drosophila. J. Gen. Physiol. 1982, 79, 361–385. [Google Scholar] [CrossRef]
- Montell, C.; Jones, K.; Hafen, E.; Rubin, G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 1985, 230, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Hokanson, K.M.; Chang, L.T. Molecular basis of an inherited retinal defect in Drosophila. Investig. Ophthalmol. Vis. Sci. 1985, 26, 243–246. [Google Scholar]
- Montell, C.; Rubin, G.M. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- Wong, F.; Schaefer, E.L.; Roop, B.C.; LaMendola, J.N.; Johnson-Seaton, D.; Shao, D. Proper function of the Drosophila trp gene product during pupal development is important for normal visual transduction in the adult. Neuron 1989, 3, 81–94. [Google Scholar] [CrossRef]
- Minke, B. Drosophila mutant with a transducer defect. Eur. Biophys. J. 1977, 3, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Suss-Toby, E.; Selinger, Z.; Minke, B. Lanthanum reduces the excitation efficiency in fly photoreceptors. J. Gen. Physiol. 1991, 98, 849–868. [Google Scholar] [CrossRef] [PubMed]
- Minke, B.; Selinger, Z. The inositol-lipid pathway is necessary for light excitation in fly photoreceptors. Soc. Gen. Physiol. Ser. 1992, 47, 201–217. [Google Scholar]
- Hardie, R.C.; Minke, B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron 1992, 8, 643–651. [Google Scholar] [CrossRef]
- Phillips, A.M.; Bull, A.; Kelly, L.E. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron 1992, 8, 631–642. [Google Scholar] [CrossRef]
- Wes, P.D.; Chevesich, J.; Jeromin, A.; Rosenberg, C.; Stetten, G.; Montell, C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. USA 1995, 92, 9652–9656. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Palmer, C.P.; Zhou, X.L.; Lin, J.; Loukin, S.H.; Kung, C.; Saimi, Y. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA 2001, 98, 7801–7805. [Google Scholar] [CrossRef]
- Dong, X.P.; Wang, X.; Xu, H. TRP channels of intracellular membranes. J. Neurochem. 2010, 113, 313–328. [Google Scholar] [CrossRef]
- Zhao, R.; Tsang, S.Y. Versatile Roles of Intracellularly Located TRPV1 Channel. J. Cell. Physiol. 2017, 232, 1957–1965. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Guo, A.; Vulchanova, L.; Wang, J.; Li, X.; Elde, R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neuropeptides, the P2×3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 1999, 11, 946–958. [Google Scholar] [CrossRef]
- Menigoz, A.; Boudes, M. The expression pattern of TRPV1 in brain. J. Neurosci. 2011, 31, 13025–13027. [Google Scholar] [CrossRef]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Sand, C.A.; Grant, A.D.; Nandi, M. Vascular Expression of Transient Receptor Potential Vanilloid 1 (TRPV1). J. Histochem. Cytochem. 2015, 63, 449–453. [Google Scholar] [CrossRef]
- Salazar, H.; Llorente, I.; Jara-Oseguera, A.; Garcia-Villegas, R.; Munari, M.; Gordon, S.E.; Islas, L.D.; Rosenbaum, T. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat. Neurosci. 2008, 11, 255–261. [Google Scholar] [CrossRef]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.; Bandell, M.; Patapoutian, A. TRPV1 is activated by both acidic and basic pH. J. Neurosci. 2009, 29, 153–158. [Google Scholar] [CrossRef]
- Hwang, S.W.; Cho, H.; Kwak, J.; Lee, S.Y.; Kang, C.J.; Jung, J.; Cho, S.; Min, K.H.; Suh, Y.G.; Kim, D.; et al. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. USA 2000, 97, 6155–6160. [Google Scholar] [CrossRef]
- Nieto-Posadas, A.; Picazo-Juarez, G.; Llorente, I.; Jara-Oseguera, A.; Morales-Lazaro, S.; Escalante-Alcalde, D.; Islas, L.D.; Rosenbaum, T. Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat. Chem. Biol. 2011, 8, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.M.; Scotland, P.E.; Akopian, A.N.; Hargreaves, K.M. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc. Natl. Acad. Sci. USA 2009, 106, 18820–18824. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sorgard, M.; Di Marzo, V.; Julius, D.; Hogestatt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef]
- Bhave, G.; Hu, H.J.; Glauner, K.S.; Zhu, W.; Wang, H.; Brasier, D.J.; Oxford, G.S.; Gereau, R.W.T. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc. Natl. Acad. Sci. USA 2003, 100, 12480–12485. [Google Scholar] [CrossRef]
- Mohapatra, D.P.; Nau, C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 2005, 280, 13424–13432. [Google Scholar] [CrossRef]
- Sanz-Salvador, L.; Andres-Borderia, A.; Ferrer-Montiel, A.; Planells-Cases, R. Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J. Biol. Chem. 2012, 287, 19462–19471. [Google Scholar] [CrossRef]
- Fischer, M.J.; Btesh, J.; McNaughton, P.A. Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. J. Neurosci. 2013, 33, 7407–7414. [Google Scholar] [CrossRef] [PubMed]
- Lishko, P.V.; Procko, E.; Jin, X.; Phelps, C.B.; Gaudet, R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 2007, 54, 905–918. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Gordon-Shaag, A.; Munari, M.; Gordon, S.E. Ca2+/calmodulin modulates TRPV1 activation by capsaicin. J. Gen. Physiol. 2004, 123, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Numazaki, M.; Tominaga, T.; Takeuchi, K.; Murayama, N.; Toyooka, H.; Tominaga, M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc. Natl. Acad. Sci. USA 2003, 100, 8002–8006. [Google Scholar] [CrossRef] [PubMed]
- Moiseenkova-Bell, V.Y.; Stanciu, L.A.; Serysheva, I.I.; Tobe, B.J.; Wensel, T.G. Structure of TRPV1 channel revealed by electron cryomicroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 7451–7455. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef]
- Strang, C.; Cushman, S.J.; DeRubeis, D.; Peterson, D.; Pfaffinger, P.J. A central role for the T1 domain in voltage-gated potassium channel formation and function. J. Biol. Chem. 2001, 276, 28493–28502. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.Y.; Jung, S.J.; Park, Y.K.; Kwak, J.; Kim, S.J.; Kim, J. Effects of capsaicin on Ca(2+) release from the intracellular Ca(2+) stores in the dorsal root ganglion cells of adult rats. Biochem. Biophys. Res. Commun. 2001, 285, 1114–1120. [Google Scholar] [CrossRef]
- Liu, M.; Liu, M.C.; Magoulas, C.; Priestley, J.V.; Willmott, N.J. Versatile regulation of cytosolic Ca2+ by vanilloid receptor I in rat dorsal root ganglion neurons. J. Biol. Chem. 2003, 278, 5462–5472. [Google Scholar] [CrossRef]
- Olah, Z.; Szabo, T.; Karai, L.; Hough, C.; Fields, R.D.; Caudle, R.M.; Blumberg, P.M.; Iadarola, M.J. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J. Biol. Chem. 2001, 276, 11021–11030. [Google Scholar] [CrossRef]
- Gallego-Sandín, S.; Rodríguez-García, A.; Alonso, M.T.; García-Sancho, J. The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels. J. Biol. Chem. 2009, 284, 32591–32601. [Google Scholar] [CrossRef] [PubMed]
- Karai, L.J.; Russell, J.T.; Iadarola, M.J.; Olah, Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J. Biol. Chem. 2004, 279, 16377–16387. [Google Scholar] [CrossRef]
- Miyake, T.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Activation of mitochondrial transient receptor potential vanilloid 1 channel contributes to microglial migration. Glia 2015, 63, 1870–1882. [Google Scholar] [CrossRef]
- Yocum, G.T.; Chen, J.; Choi, C.H.; Townsend, E.A.; Zhang, Y.; Xu, D.; Fu, X.W.; Sanderson, M.J.; Emala, C.W. Role of transient receptor potential vanilloid 1 in the modulation of airway smooth muscle tone and calcium handling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 312, L812–L821. [Google Scholar] [CrossRef]
- Hurt, C.M.; Lu, Y.; Stary, C.M.; Piplani, H.; Small, B.A.; Urban, T.J.; Qvit, N.; Gross, G.J.; Mochly-Rosen, D.; Gross, E.R. Transient Receptor Potential Vanilloid 1 Regulates Mitochondrial Membrane Potential and Myocardial Reperfusion Injury. J. Am. Heart Assoc. 2016, 5, e003774. [Google Scholar] [CrossRef]
- Olah, Z.; Karai, L.; Iadarola, M.J. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J. Biol. Chem. 2001, 276, 31163–31170. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, F.; Takanishi, C.L.; Zheng, J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J. Gen. Physiol. 2007, 129, 191–207. [Google Scholar] [CrossRef]
- Sun, Z.; Han, J.; Zhao, W.; Zhang, Y.; Wang, S.; Ye, L.; Liu, T.; Zheng, L. TRPV1 activation exacerbates hypoxia/reoxygenation-induced apoptosis in H9C2 cells via calcium overload and mitochondrial dysfunction. Int. J. Mol. Sci. 2014, 15, 18362–18380. [Google Scholar] [CrossRef]
- Faussone-Pellegrini, M.S.; Taddei, A.; Bizzoco, E.; Lazzeri, M.; Vannucchi, M.G.; Bechi, P. Distribution of the vanilloid (capsaicin) receptor type 1 in the human stomach. Histochem. Cell Biol. 2005, 124, 61–68. [Google Scholar] [CrossRef]
- Wen, W.; Que, K.; Zang, C.; Wen, J.; Sun, G.; Zhao, Z.; Li, Y. Expression and distribution of three transient receptor potential vanilloid(TRPV) channel proteins in human odontoblast-like cells. J. Mol. Histol. 2017, 48, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.; Bucher, C.; Liu, W.; Müller, M.; Schmidt, T.; Kardell, M.; Driessen, M.N.; Rossaint, J.; Gross, E.R.; Wagner, N.M. 12(S)-HETE mediates diabetes-induced endothelial dysfunction by activating intracellular endothelial cell TRPV1. J. Clin. Investig. 2020, 130, 4999–5010. [Google Scholar] [CrossRef] [PubMed]
- Wisnoskey, B.J.; Sinkins, W.G.; Schilling, W.P. Activation of vanilloid receptor type I in the endoplasmic reticulum fails to activate store-operated Ca2+ entry. Biochem. J. 2003, 372, 517–528. [Google Scholar] [CrossRef]
- Lotteau, S.; Ducreux, S.; Romestaing, C.; Legrand, C.; Van Coppenolle, F. Characterization of functional TRPV1 channels in the sarcoplasmic reticulum of mouse skeletal muscle. PLoS ONE 2013, 8, e58673. [Google Scholar] [CrossRef]
- Xin, H.; Tanaka, H.; Yamaguchi, M.; Takemori, S.; Nakamura, A.; Kohama, K. Vanilloid receptor expressed in the sarcoplasmic reticulum of rat skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 332, 756–762. [Google Scholar] [CrossRef]
- Lozano, C.; Córdova, C.; Marchant, I.; Zúñiga, R.; Ochova, P.; Ramírez-Barrantes, R.; González-Arriagada, W.A.; Rodriguez, B.; Olivero, P. Intracellular aggregated TRPV1 is associated with lower survival in breast cancer patients. Breast Cancer (Dove Med. Press) 2018, 10, 161–168. [Google Scholar] [CrossRef]
- Lang, H.; Li, Q.; Yu, H.; Li, P.; Lu, Z.; Xiong, S.; Yang, T.; Zhao, Y.; Huang, X.; Gao, P.; et al. Activation of TRPV1 attenuates high salt-induced cardiac hypertrophy through improvement of mitochondrial function. Br. J. Pharmacol. 2015, 172, 5548–5558. [Google Scholar] [CrossRef]
- Jung, J.; Shin, J.S.; Lee, S.Y.; Hwang, S.W.; Koo, J.; Cho, H.; Oh, U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J. Biol. Chem. 2004, 279, 7048–7054. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, P.; Ma, L.; Gao, P.; Gong, L.; Li, L.; Li, Q.; Sun, F.; Zhou, X.; He, H.; et al. Ameliorating Endothelial Mitochondrial Dysfunction Restores Coronary Function via Transient Receptor Potential Vanilloid 1-Mediated Protein Kinase A/Uncoupling Protein 2 Pathway. Hypertension 2016, 67, 451–460. [Google Scholar] [CrossRef]
- Tessier, N.; Chouabe, C.; Crola Da Silva, C.; Bonvallet, R.; Ovize, M.; Ducreux, S.; Van Coppenolle, F. Cardioprotective Role of Transient Receptor Potential Vanilloid 1 (TRPV1) Channels in H9C2 Cell Line. Arch. Cardiovasc. Dis. Suppl 2018, 10, 186. [Google Scholar]
- Ristoiu, V.; Shibasaki, K.; Uchida, K.; Zhou, Y.; Ton, B.H.; Flonta, M.L.; Tominaga, M. Hypoxia-induced sensitization of transient receptor potential vanilloid 1 involves activation of hypoxia-inducible factor-1 alpha and PKC. Pain 2011, 152, 936–945. [Google Scholar] [CrossRef]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Singh, S.; Leishangthem, G.D.; Aich, J.; Kumar, M.; Khanna, K.; Singh, V.P.; et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci. Rep. 2013, 3, 1349. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.Y.; Shin, J.; Kim, B.M.; Wang, M.H.; Jang, J.H.; Surh, Y.J.; Oh, U. Essential role of mitochondrial permeability transition in vanilloid receptor 1-dependent cell death of sensory neurons. Mol. Cell. Neurosci. 2003, 24, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Stueber, T.; Eberhardt, M.J.; Caspi, Y.; Lev, S.; Binshtok, A.; Leffler, A. Differential cytotoxicity and intracellular calcium-signalling following activation of the calcium-permeable ion channels TRPV1 and TRPA1. Cell Calcium 2017, 68, 34–44. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef]
- Flatters, S.J. The contribution of mitochondria to sensory processing and pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 119–146. [Google Scholar] [CrossRef]
- Özdemir, Ü.S.; Nazıroğlu, M.; Şenol, N.; Ghazizadeh, V. Hypericum perforatum Attenuates Spinal Cord Injury-Induced Oxidative Stress and Apoptosis in the Dorsal Root Ganglion of Rats: Involvement of TRPM2 and TRPV1 Channels. Mol. Neurobiol. 2016, 53, 3540–3551. [Google Scholar] [CrossRef]
- Ramírez-Barrantes, R.; Córdova, C.; Gatica, S.; Rodriguez, B.; Lozano, C.; Marchant, I.; Echeverria, C.; Simon, F.; Olivero, P. Transient Receptor Potential Vanilloid 1 Expression Mediates Capsaicin-Induced Cell Death. Front. Physiol. 2018, 9, 682. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, D.Y.; Chung, E.S.; Oh, U.T.; Kim, S.U.; Jin, B.K. Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro. J. Neurosci. 2005, 25, 662–671. [Google Scholar] [CrossRef]
- Davies, J.W.; Hainsworth, A.H.; Guerin, C.J.; Lambert, D.G. Pharmacology of capsaicin-, anandamide-, and N-arachidonoyl-dopamine-evoked cell death in a homogeneous transient receptor potential vanilloid subtype 1 receptor population. Br. J. Anaesth. 2010, 104, 596–602. [Google Scholar] [CrossRef]
- Chiang, H.; Ohno, N.; Hsieh, Y.L.; Mahad, D.J.; Kikuchi, S.; Komuro, H.; Hsieh, S.T.; Trapp, B.D. Mitochondrial fission augments capsaicin-induced axonal degeneration. Acta Neuropathol. 2015, 129, 81–96. [Google Scholar] [CrossRef]
- Dedov, V.N.; Roufogalis, B.D. Mitochondrial calcium accumulation following activation of vanilloid (VR1) receptors by capsaicin in dorsal root ganglion neurons. Neuroscience 2000, 95, 183–188. [Google Scholar] [CrossRef]
- Nita, I.I.; Caspi, Y.; Gudes, S.; Fishman, D.; Lev, S.; Hersfinkel, M.; Sekler, I.; Binshtok, A.M. Privileged crosstalk between TRPV1 channels and mitochondrial calcium shuttling machinery controls nociception. Biochim. Et Biophys. Acta 2016, 1863, 2868–2880. [Google Scholar] [CrossRef]
- Medvedeva, Y.V.; Kim, M.S.; Usachev, Y.M. Mechanisms of prolonged presynaptic Ca2+ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons. J. Neurosci. 2008, 28, 5295–5311. [Google Scholar] [CrossRef]
- Shutov, L.P.; Kim, M.S.; Houlihan, P.R.; Medvedeva, Y.V.; Usachev, Y.M. Mitochondria and plasma membrane Ca2+-ATPase control presynaptic Ca2+ clearance in capsaicin-sensitive rat sensory neurons. J. Physiol. 2013, 591, 2443–2462. [Google Scholar] [CrossRef]
- Ortiz-Renteria, M.; Juarez-Contreras, R.; Gonzalez-Ramirez, R.; Islas, L.D.; Sierra-Ramirez, F.; Llorente, I.; Simon, S.A.; Hiriart, M.; Rosenbaum, T.; Morales-Lazaro, S.L. TRPV1 channels and the progesterone receptor Sig-1R interact to regulate pain. Proc. Natl. Acad. Sci. USA 2018, 115, E1657–E1666. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
| Intracellular Localization | Cell Type | Reference |
|---|---|---|
| Mitochondria | Murine cardiomyocytes | [51] |
| Microglial cells | [49] | |
| Heart-derived H9C2 cells | [51,54] | |
| Human parietal cells | [55] | |
| Human odontoblast-like cells | [56] | |
| Human endothelial cells | [57] | |
| Endoplasmic Reticulum | DRG neurons | [44,45,46,47,48] |
| Airway smooth muscle cells | [50] | |
| TRPV1-expressing cells: HEK293, COS7, Sf9, HeLa | [44,45,46,47,52,58] | |
| Sarcoplasmic Reticulum | Skeletal muscle cells | [59,60] |
| Golgi Complex | DRG neurons | [24] |
| Microglia cells | [49] | |
| Breast cancer cell lines | [61] | |
| Lysosome | Microglia cells | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juárez-Contreras, R.; Méndez-Reséndiz, K.A.; Rosenbaum, T.; González-Ramírez, R.; Morales-Lázaro, S.L. TRPV1 Channel: A Noxious Signal Transducer That Affects Mitochondrial Function. Int. J. Mol. Sci. 2020, 21, 8882. https://doi.org/10.3390/ijms21238882
Juárez-Contreras R, Méndez-Reséndiz KA, Rosenbaum T, González-Ramírez R, Morales-Lázaro SL. TRPV1 Channel: A Noxious Signal Transducer That Affects Mitochondrial Function. International Journal of Molecular Sciences. 2020; 21(23):8882. https://doi.org/10.3390/ijms21238882
Chicago/Turabian StyleJuárez-Contreras, Rebeca, Karina Angélica Méndez-Reséndiz, Tamara Rosenbaum, Ricardo González-Ramírez, and Sara Luz Morales-Lázaro. 2020. "TRPV1 Channel: A Noxious Signal Transducer That Affects Mitochondrial Function" International Journal of Molecular Sciences 21, no. 23: 8882. https://doi.org/10.3390/ijms21238882
APA StyleJuárez-Contreras, R., Méndez-Reséndiz, K. A., Rosenbaum, T., González-Ramírez, R., & Morales-Lázaro, S. L. (2020). TRPV1 Channel: A Noxious Signal Transducer That Affects Mitochondrial Function. International Journal of Molecular Sciences, 21(23), 8882. https://doi.org/10.3390/ijms21238882





