Interplay between Oxidative Stress and Metabolic Derangements in Non-Alcoholic Fatty Liver Disease: The Role of Selenoprotein P
Abstract
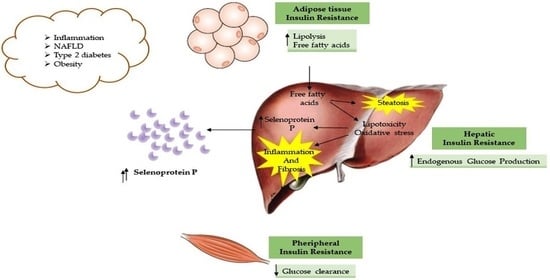
1. Introduction
2. Results
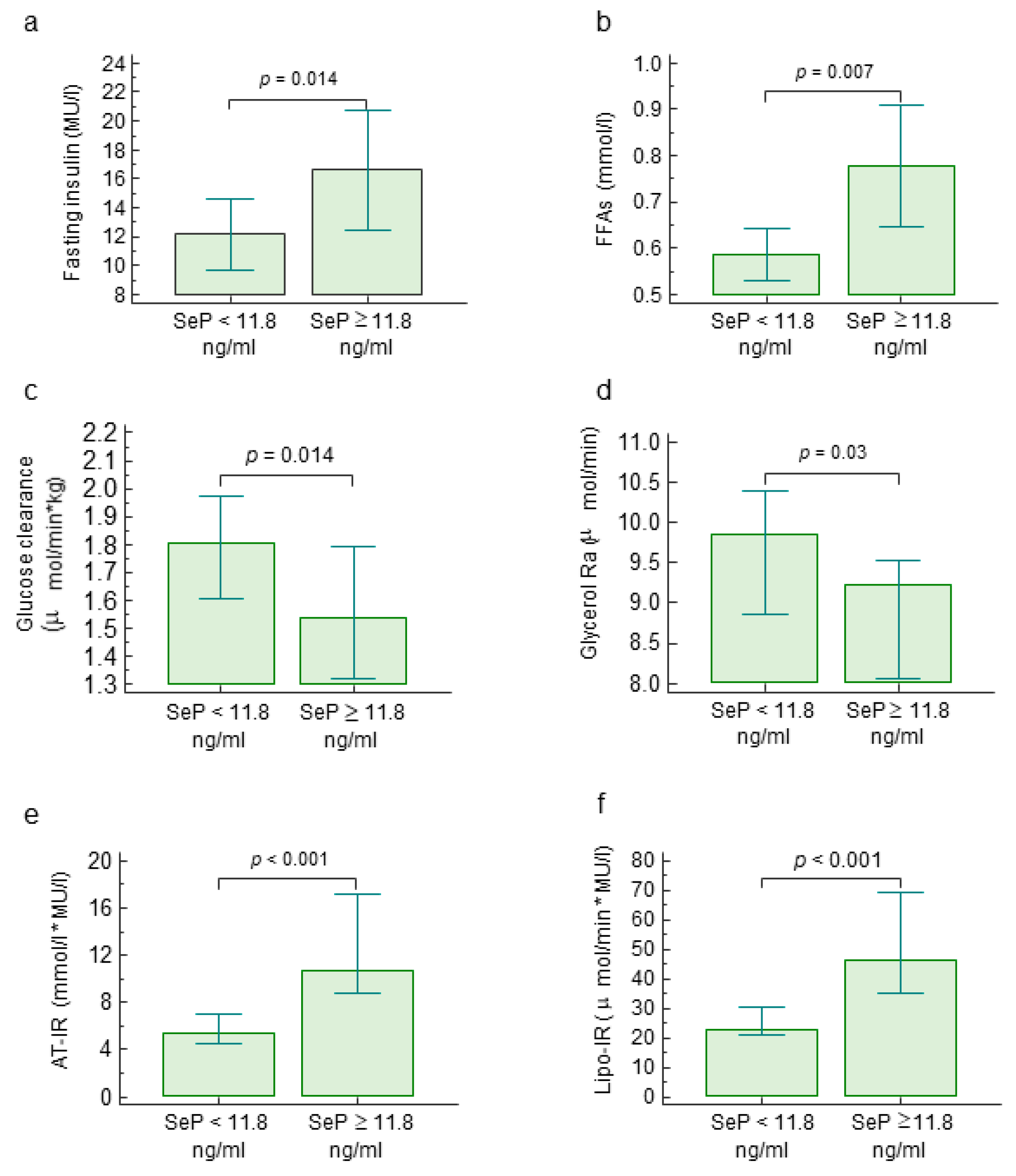
2.1. Circulating Selenoprotein P Levels, Metabolic Parameters and Insulin Resistance
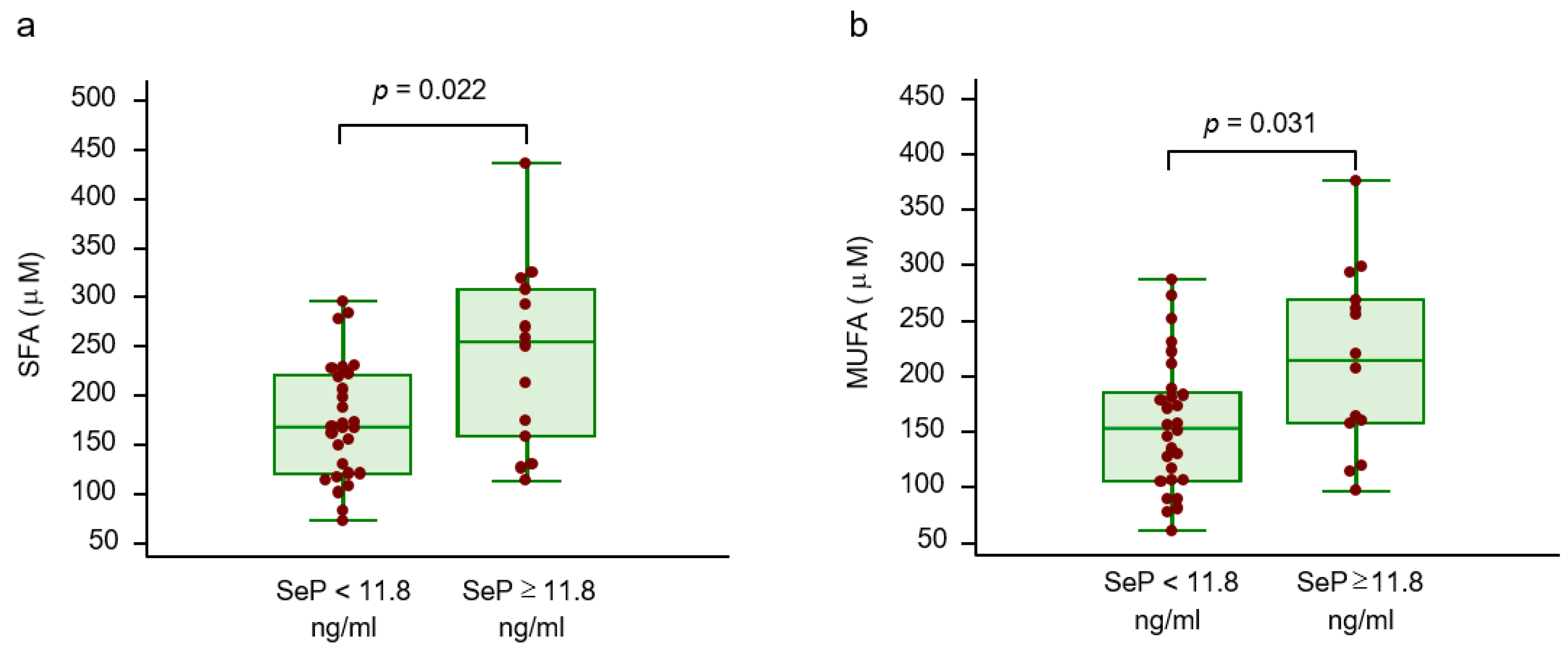
2.2. Circulating Selenoprotein P, Amino Acid Profile and Lipid Profile
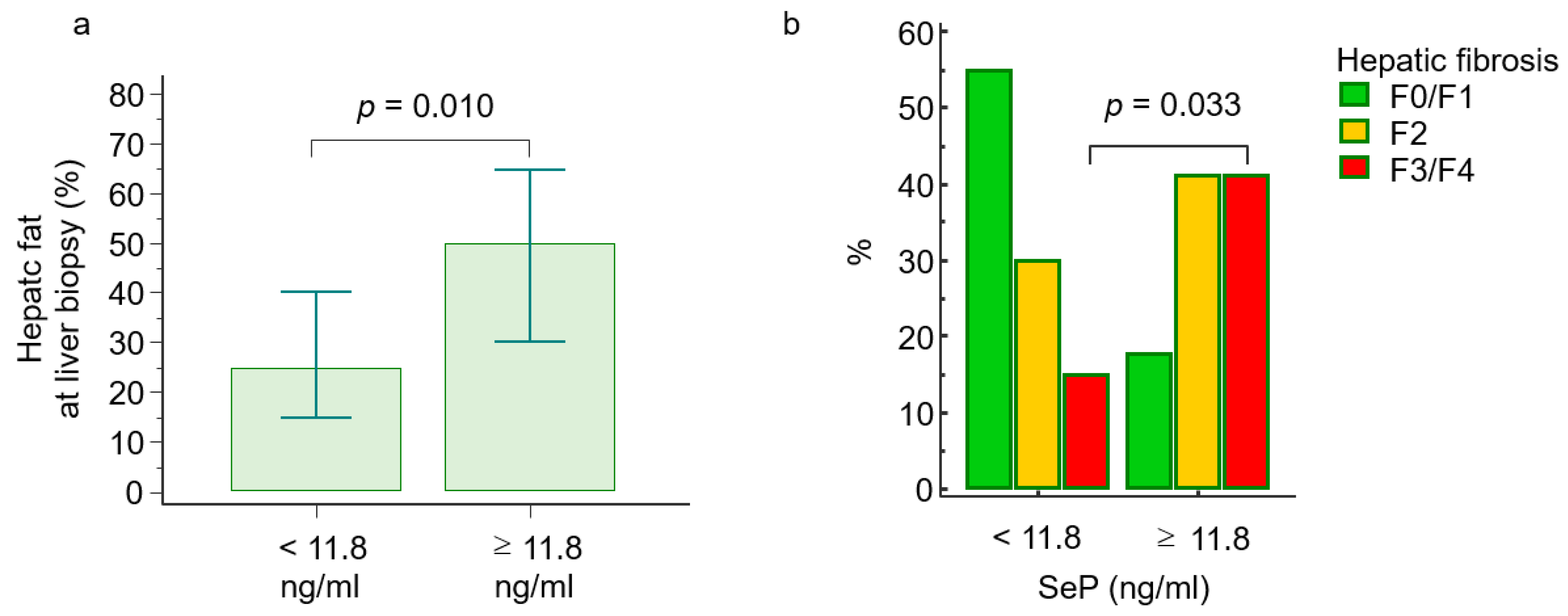
2.3. Selenoprotein P Levels and Histological Features
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Histological Features
4.3. Analytical Determinations
4.4. Study Design
4.5. “ In Vivo” Tracer Protocol
4.6. Circulating Selenoprotein P Measurement
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Enry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef]
- Marengo, A.; Jouness, R.I.; Bugianesi, E. Progression and natural history of non-alcoholic fatty liver disease in adults. Clin. Liver Dis. 2016, 20, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, A.; Gentilini, A.; Marra, F. Molecular pathogenesis of NASH. Int. J. Mol. Sci. 2016, 17, 1575. [Google Scholar] [CrossRef] [PubMed]
- Baffy, G.; Brunt, E.M.; Caldwell, S.H. Hepatocellular carcinoma in non-alcoholicfatty liver disease: An emerging menace. J. Hepatol. 2012, 56, 1384–1391. [Google Scholar] [CrossRef]
- Day, C.P.; James, O.F. Steatohepetitis: A tale of two “hits”? Gastroenterology 1998, 114, 842–845. [Google Scholar] [CrossRef]
- Rolo, A.P.; Teodoro, J.S.; Palmeira, C.M. Role of oxidative stress in the pathogenesis of non-alcoholic steatohepatitis. Free Radic. Biol. Med. 2012, 52, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Moscatiello, S.; Ciaravella, M.F.; Marchesini, G. Insulin resistance in non-alcoholic fatty liver disease. Curr. Pharm. Des. 2010, 16, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, R.; Ortiz-Lopez, C.; Orsak, B.; Webb, A.; Hardies, J.; Darland, C.; Finch, J.; Gastaldelli, A.; Harrison, S.; Tio, F.; et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in non obese patients with nonalcoholic fatty liver disease. Hepatology 2012, 5, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Carli, F.; Rosso, C.; Younes, R.; D’Aurizio, R.; Bugianesi, E.; Gastaldelli, A. Altered metabolic profile and adipocyte insulin resistance mark severe liver fibrosis in patients with chronic liver disease. Int. J. Mol. Sci. 2019, 48, 6333. [Google Scholar] [CrossRef]
- Rosso, C.; Kazankov, K.; Younes, R.; Esmaili, S.; Marietti, M.; Sacco, M.; Carli, F.; Gaggini, M.; Salomone, F.; Moller, H.J.; et al. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 1012–1021. [Google Scholar] [CrossRef]
- Oliveira, C.P.; da Costa Gayotto, L.C.; Tatai, C.; Della Bina, B.I.; Janiszewski, M.; Lima, E.S.; Abdalla, D.S.; Lopasso, F.P.; Laurindo, F.R.M.; Laudanna, A.A. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with choline-deficient diet. J. Cell. Mol. Med. 2002, 6, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Deeg, M.A.; Crabb, D.W. Systemic levels of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2005, 100, 850–855. [Google Scholar]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Misu, H. Identification of hepatokines involved in pathology of type 2 diabetes and obesity. Endocr. J. 2019, 66, 659–662. [Google Scholar] [CrossRef]
- Choi, H.Y.; Hwang, S.Y.; Lee, C.H.; Hong, H.C.; Yang, S.J.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Increased selenoprotein P levels in subjects with visceral obesity and nonalcoholic fatty liver disease. Diabetes Metab. J. 2013, 37, 63–71. [Google Scholar] [CrossRef]
- Chen, M.; Liu, B.; Wilkinson, D.; Hutchison, A.T.; Thompson, C.H.; Wittert, G.A.; Heilbronn, L.K. Selenoprotein P is elevated in individuals with obesity, but is not independently associated with insulin resistance. Obes. Res. Clin. Pract. 2017, 11, 227–232. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Mavrouli, M.; Katsinelos, P.; Doulberis, M.; Gavana, E.; Duntas, L. Selenoprotein P in patients with nonalcoholic fatty liver disease. Exp. Clin. Endocrinol. Diabetes 2019, 127, 598–602. [Google Scholar] [CrossRef]
- Yang, S.J.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Serum selenoprotein P levels in patients with type 2 diabetes and prediabetes: Implications for insulin resistance, inflammation, and atherosclerosis. J. Clin. Endocrinol. Metab. 2011, 96, 1325–1329. [Google Scholar] [CrossRef]
- di Giuseppe, R.; Koch, M.; Nothlings, U.; Kastenmuller, G.; Artati, A.; Adamski, J.; Jacobs, G.; Lieb, W. Metabolomics signature associated with circulating serum selenoprotein P levels. Endocrine 2019, 64, 486–495. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Mostert, V.; Dreher, I.; Kohrle, J.; Abel, J. Transforming growth factor-beta1 inhibits expression of Selenoprotein P in cultured human liver cells. FEBS Lett. 1999, 460, 23–26. [Google Scholar] [CrossRef]
- Chen, Y.; He, X.; Chen, X.; Li, Y.; Ke, Y. SeP is elevated in NAFLD and participates in NAFLD pathogenesis through AMPK/ACC pathway. J. Cell Physiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Selenoprotein P-expression, functions, and roles in mammals. Biochim. Biophys. Acta 2009, 1790, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.E.; Wu, S.; Motley, A.K.; Stevenson, T.D.; Winfrey, V.P.; Capecchi, M.R.; Atkins, J.F.; Burk, R.F. Production of Selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis. J. Biol. Chem. 2012, 48, 40414–40424. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H. Interference of selenium and selenoproteins with the insulin-regulated carbohydrate and lipid metabolism. Free Radic. Biol. Med. 2013, 65, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Misu, H.; Takamura, T.; Takayama, H.; Hayashi, H.; Matsuzawa-Nagata, N.; Kurita, S.; Ishikura, K.; Ando, H.; Takeshita, Y.; Ota, T.; et al. A liver-derived secretory protein, selenoprotein-P, cause insulin resistance. Cell Metab. 2010, 12, 483–495. [Google Scholar] [CrossRef]
- Gambino, R.; Bugianesi, E.; Rosso, C.; Mezzabotta, L.; Pinach, S.; Alemanno, N.; Saba, F.; Cassader, M. Different serum free fatty acids profile in NAFLD subjects and healthy controls after oral fat load. Int. J. Mol. Sci. 2016, 17, 479. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrel, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Casolaro, A.; Pettiti, M.; Nannipieri, M.; Ciociaro, D.; Frascerra, S.; Buzzigoli, E.; Baldi, S.; Mari, A.; Ferrannini, E. Effect of pioglitazone on the metabolic and hormonal response to a mixed meal in type II diabetes. Clin. Pharmacol. Ther. 2007, 81, 205–212. [Google Scholar] [CrossRef]
- Bugianesi, E.; Gastaldelli, A.; Vanni, E.; Gambino, R.; Cassader, M.; Baldi, S.; Ponti, V.; Pagano, G.; Ferrannini, E.; Rizzetto, M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia 2005, 48, 634–642. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total n = 57 | <11.7 ng/mL n = 40 | ≥11.8 ng/mL n = 17 | p Value |
|---|---|---|---|---|
| Age (years), median (95% CI) | 43 (38–48) | 42 (36–50) | 44 (35–51) | 0.794 |
| Male/Female gender, n (%) | 44/13 (77/23) | 36/4 (90/10) | 8/9 (47/53) | <0.001 |
| BMI (kg/m2), median (95% CI) | 26.8 (25.8–28.8) | 25.7 (24.8–27.8) | 30.1 (26.8–33) | 0.002 |
| Waist circumference (cm), median (95% CI) | 95 (91–99) | 94 (90–99) | 95 (89–103) | 0.407 |
| AST (IU/l), median (95% CI) | 33 (30–39) | 31 (26–36) | 56 (31–65) | 0.003 |
| ALT (IU/l), median (95% CI) | 62 (46–70) | 53 (41–70) | 63 (47–96) | 0.114 |
| Platelets (x 109/l), median (95% CI) | 225 (216–246) | 227 (215–264) | 224 (189–246) | 0.589 |
| Fasting glucose (mg/dl), median (95% CI) | 93 (90–98) | 93 (90–99) | 93 (87–108) | 0.868 |
| Total cholesterol (mg/dl), median (95% CI) | 190 (182–199) | 189 (181–200) | 194 (169–238) | 0.565 |
| HDL cholesterol (mg/dl), median (95% CI) | 46 (41–49) | 46 (41–49) | 48 (31–54) | 0.513 |
| Triglycerides (mg/dl), median (95% CI) | 100 (89–119) | 99 (86–120) | 107 (86–215) | 0.448 |
| Histological features | ||||
| Hepatic steatosis (%), median (range) | 32 (21–43) | 25 (15–40) | 50 (30–65) | 0.010 |
| Steatosis score (1/2/3), n (%) | 29/20/8 (51/35/14) | 24/12/4 (60/30/10) | 5/8/4 (29/47/24) | 0.033 |
| Ballooning (0/1/2), n (%) | 6/27/24 (11/47/42) | 5/21/14 (12/53/35) | 1/6/10 (6/35/59) | 0.107 |
| Lobular inflammation (0/1/2), n (%) | 11/43/3 (19/76/5) | 7/31/2 (17/78/5) | 4/12/1 (23/71/6) | 0.708 |
| Fibrosis (0/1/2/3/4), n (%) | 20/5/19/11/2 (36/7/30/25/2) | 17/5/12/6/0 (42/13/30/15/0) | 3/0/7/5/2 (18/0/41/29/12) | 0.005 |
| NASH, n (%) | 42 (74) | 30 (75) | 12 (71) | 0.732 |
| Variables | rS | 95% CI | p Value |
|---|---|---|---|
| Fasting insulin (MU/l) | 0.28 | 0.02–0.50 | 0.035 |
| Fasting glucose (mg/dl) | −0.05 | −0.31–0.21 | 0.691 |
| Fasting FFAs (mmol/l) | 0.42 | 0.17–0.61 | 0.001 |
| Glucose clearance (μmol/min × kg) | −0.33 | −0.56–−0.06 | 0.019 |
| Endogenous glucose production (μmol/min × kg) | −0.22 | −0.46–0.05 | 0.106 |
| Glycerol Ra (μmol/min) | −0.34 | −0.60–−0.02 | 0.039 |
| Insulin resistance components | |||
| HOMA-IR | 0.26 | −0.01–0.49 | 0.051 |
| Hep-IR (μmol/min × kg × MU/l) | 0.27 | 0.01–0.49 | 0.043 |
| AT-IR (mmol/l × MU/l) | 0.50 | 0.27–0.67 | <0.001 |
| Lipo-IR (μmol/min × MU/l) | 0.40 | 0.15–0.60 | 0.002 |
| Free Fatty Acids Profile | rS | p Value | |
|---|---|---|---|
| FFAs (mmol/l) | 0.42 | 0.01 | |
| Palmitic acid (μM) | 0.40 | 0.010 | |
| Stearic acid (μM) | 0.33 | 0.034 | SFA |
| Margaric acid (μM) | 0.44 | 0.005 | |
| Miristic acid (μM) | 0.28 | 0.086 | |
| Palmitoleic acid (μM) | 0.45 | 0.003 | |
| Oleic acid (μM) | 0.39 | 0.011 | MUFA |
| Linoleic acid (μM) | 0.25 | 0.104 | |
| Arachidonic acid (μM) | 0.17 | 0.293 | PUFA |
| SFA (μM) | 0.41 | 0.007 | |
| MUFA (μM) | 0.40 | 0.009 | |
| PUFA (μM) | 0.27 | 0.086 |
| Univariate Regression Analysis | Multivariable Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | t | rp | p Value | t | rp | p Value |
| Age (years) | 0.928 | 0.124 | 0.358 | 1.217 | 0.168 | 0.229 |
| Gender | 1.752 | 0.230 | 0.085 | 0.616 | 0.086 | 0.541 |
| BMI (kg/m2) | 3.707 | 0.447 | <0.001 | 2.763 | 0.361 | 0.008 |
| SeP (ng/mL) | 3.787 | 0.455 | <0.001 | 2.369 | 0.315 | 0.022 |
| AT-IR (mmol/l * MU/l) | 3.215 | 0.398 | 0.002 | 0.599 | 0.084 | 0.552 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caviglia, G.P.; Rosso, C.; Armandi, A.; Gaggini, M.; Carli, F.; Abate, M.L.; Olivero, A.; Ribaldone, D.G.; Saracco, G.M.; Gastaldelli, A.; et al. Interplay between Oxidative Stress and Metabolic Derangements in Non-Alcoholic Fatty Liver Disease: The Role of Selenoprotein P. Int. J. Mol. Sci. 2020, 21, 8838. https://doi.org/10.3390/ijms21228838
Caviglia GP, Rosso C, Armandi A, Gaggini M, Carli F, Abate ML, Olivero A, Ribaldone DG, Saracco GM, Gastaldelli A, et al. Interplay between Oxidative Stress and Metabolic Derangements in Non-Alcoholic Fatty Liver Disease: The Role of Selenoprotein P. International Journal of Molecular Sciences. 2020; 21(22):8838. https://doi.org/10.3390/ijms21228838
Chicago/Turabian StyleCaviglia, Gian Paolo, Chiara Rosso, Angelo Armandi, Melania Gaggini, Fabrizia Carli, Maria Lorena Abate, Antonella Olivero, Davide Giuseppe Ribaldone, Giorgio Maria Saracco, Amalia Gastaldelli, and et al. 2020. "Interplay between Oxidative Stress and Metabolic Derangements in Non-Alcoholic Fatty Liver Disease: The Role of Selenoprotein P" International Journal of Molecular Sciences 21, no. 22: 8838. https://doi.org/10.3390/ijms21228838
APA StyleCaviglia, G. P., Rosso, C., Armandi, A., Gaggini, M., Carli, F., Abate, M. L., Olivero, A., Ribaldone, D. G., Saracco, G. M., Gastaldelli, A., & Bugianesi, E. (2020). Interplay between Oxidative Stress and Metabolic Derangements in Non-Alcoholic Fatty Liver Disease: The Role of Selenoprotein P. International Journal of Molecular Sciences, 21(22), 8838. https://doi.org/10.3390/ijms21228838