Detailed Insight into the Interaction of Bicyclic Somatostatin Analogue with Cu(II) Ions
Abstract
1. Introduction
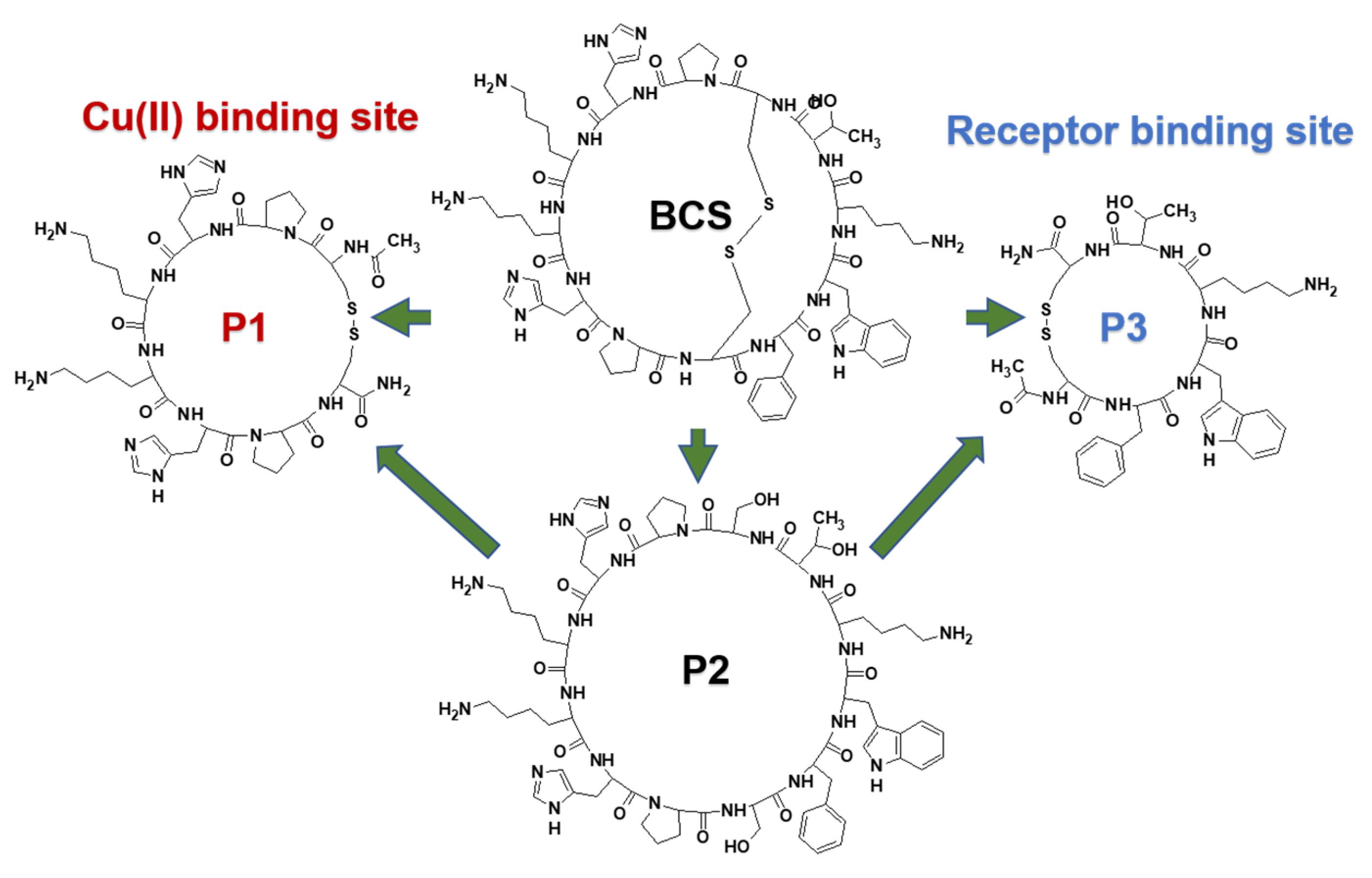
- P1. Ac-c(-S-Cys-Pro-Hisa-Lys-Lys-Hisb-Pro-Cys-S-)-NH2
- P2. c(Ser-Pro-Hisa-Lys-Lys-Hisb-Pro-Ser-Phe-Trp-Lys-Thr) (Figure 1).
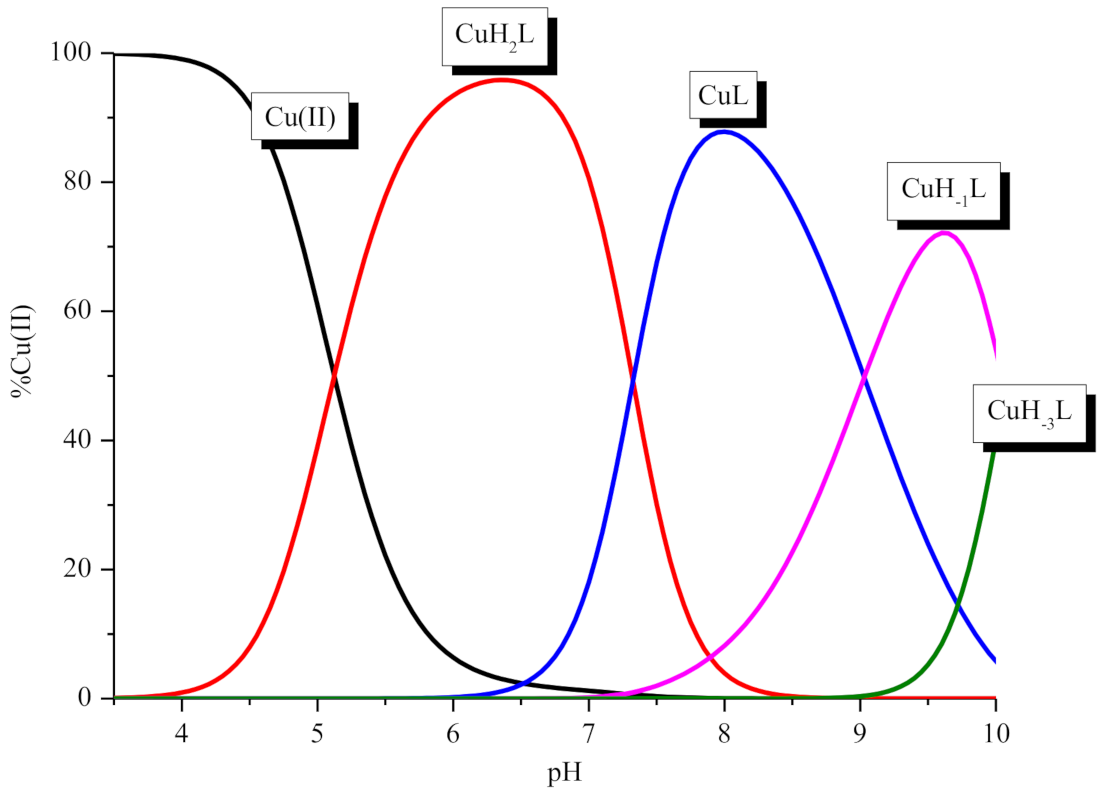
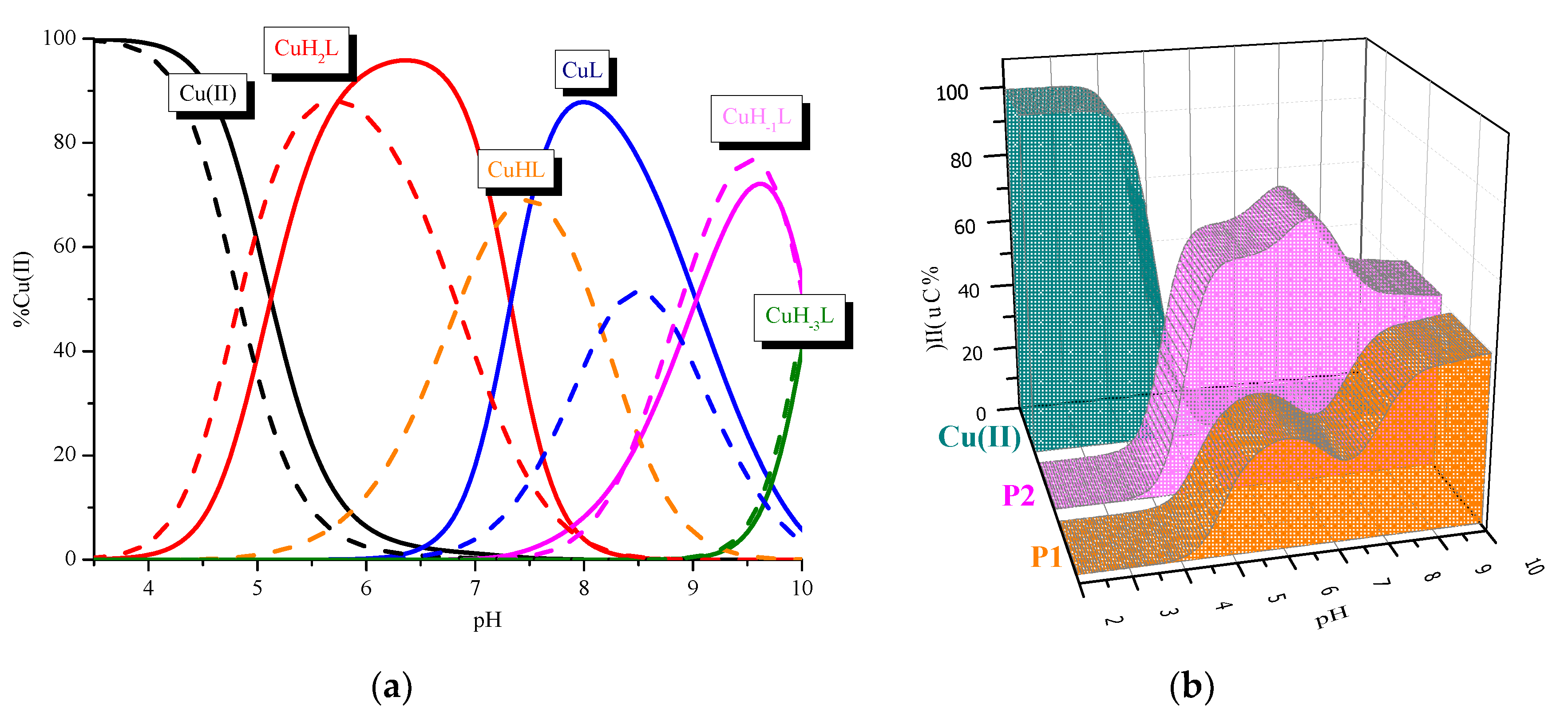
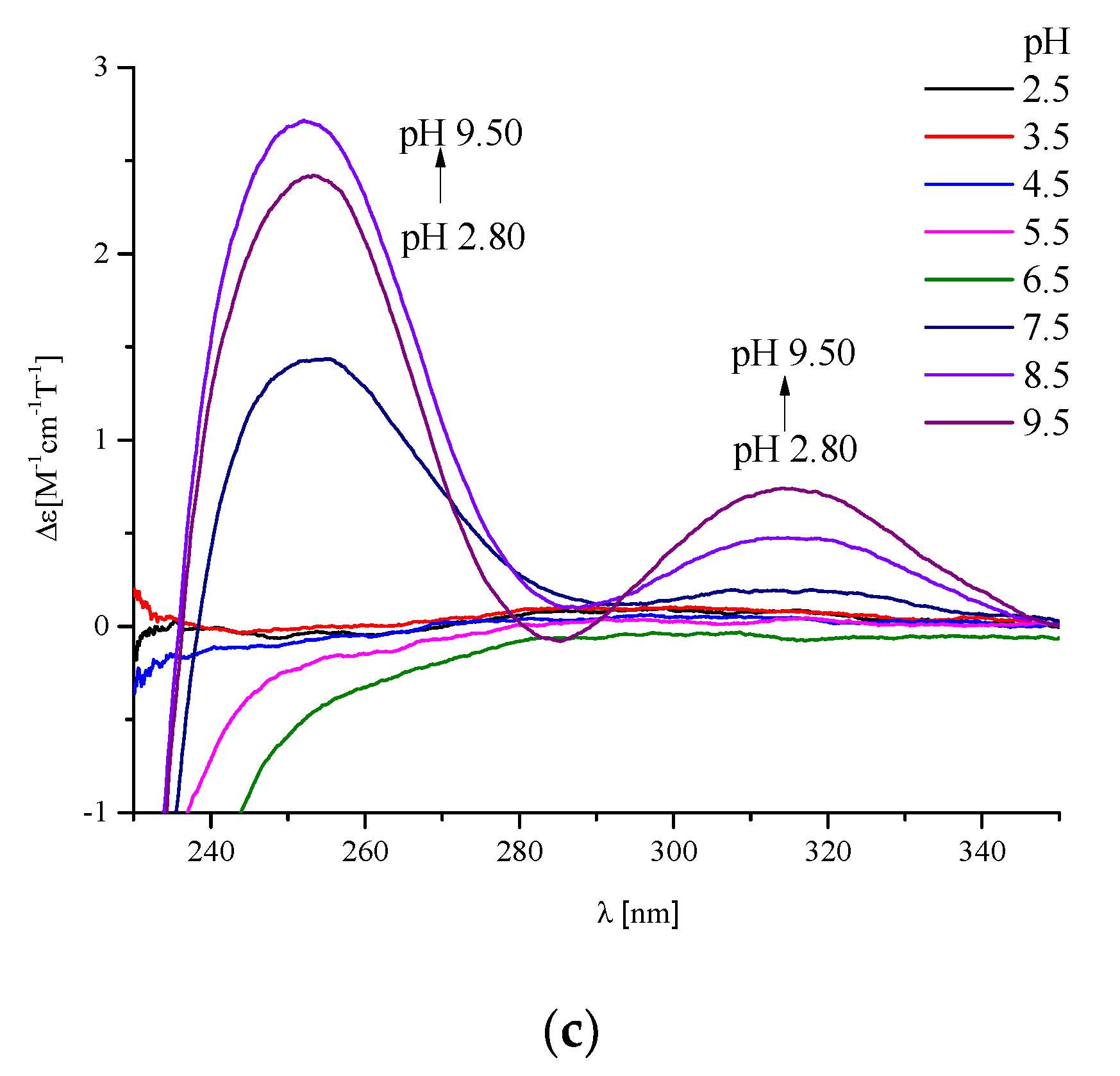
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of the Cyclopeptide C(Ser-Pro-His-Lys-Lys-His-Pro-Ser-Phe-Trp-Lys-Thr) (P2)
3.2. Potentiometric Measurements
3.3. Spectroscopic Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCS | Bicyclic Somatostatin analogue |
| CD | Circular Dichroism spectroscopy |
| CT | Charge Transfer |
| Dde | N-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl group |
| DIC | N,N′-Diisopropylcarbodiimide |
| DIPEA | N,N-Diisopropylethylamine |
| DMF | Dimethylformamide |
| MCD | Magnetic Circular Dichroism Spectroscopy |
| MW | Microwave |
| SST | Somatostatin |
| TFA | Trifluoroacetic acid |
| TIS | Triisopropyl silane |
References
- Eder, M.; Pavan, S.; Bauder-Wüst, U.; van Rietschoten, K.; Baranski, A.-C.; Harrison, H.; Campbell, S.; Stace, C.L.; Walker, E.H.; Chen, L.; et al. Bicyclic peptides as a new modality for imaging and targeting of proteins overexpressed by tumors. Cancer Res. 2019, 79, 841–852. [Google Scholar] [CrossRef]
- Veber, D.F.; Holly, F.W.; Nutt, R.F.; Bergstrand, S.J.; Brady, S.F.; Hisrschmann, R.; Glitzer, M.S.; Saperstein, R. Highly active cyclic and bicyclic somatostatin analogues of reduced ring size. Nature 1979, 280, 512–514. [Google Scholar] [CrossRef]
- Veber, D.F.; Holly, F.W.; Paleveda, W.J.; Nutt, R.F.; Bergstrand, S.J.; Torchiana, M.; Glitzer, M.S.; Saperstein, R.; Hirschmann, R. Conformationally restricted bicyclic analogs of somatostatin. Proc. Natl. Acad. Sci. USA 1978, 75, 2636–2640. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Freidinger, R.M.; Perlow, D.S.; Paleveda, W.J.; Holly, F.W.; Strachan, R.G.; Nutt, R.F.; Arison, B.H.; Homnick, C.; Randall, W.C.; et al. A potent cyclic hexapeptide analogue of somatostatin. Nature 1981, 292, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Falb, E.; Salitra, Y.; Yechezkel, T.; Bracha, M.; Litman, P.; Olender, R.; Rosenfeld, R.; Senderowitz, H.; Jiang, S.; Goodman, M. A bicyclic and hsst2 selective somatostatin analogue: Design, synthesis, conformational analysis and binding. Bioorg. Med. Chem. 2001, 9, 3255–3264. [Google Scholar] [CrossRef]
- Rivier, J.E.; Kirby, D.A.; Erchegyi, J.; Waser, B.; Eltschinger, V.; Cescato, R.; Reubi, J.C. Somatostatin receptor 1 selective analogues: 3. Dicyclic peptides. J. Med. Chem. 2005, 48, 515–522. [Google Scholar] [CrossRef]
- Fani, M.; Mueller, A.; Tamma, M.-L.; Nicolas, G.; Rink, H.R.; Cescato, R.; Reubi, J.C.; Maecke, H.R. Radiolabeled Bicyclic Somatostatin-Based Analogs: A Novel Class of Potential Radiotracers for SPECT/PET of Neuroendocrine Tumors. J. Nucl. Med. 2010, 51, 1771–1779. [Google Scholar] [CrossRef][Green Version]
- Kwekkeboom, D.J.; Teunissen, J.J.; Bakker, W.H.; Kooij, P.P.; De Herder, W.W.; Feelders, R.A.; Van Eijck, C.H.; Esser, J.P.; Kam, B.L.; Krenning, E.P. Radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J. Clin. Oncol. 2005, 23, 2754–2762. [Google Scholar] [CrossRef]
- Van Essen, M.; Krenning, E.P.; de Jong, M.; Valkema, R.; Kwekkeboom, D.J. Peptide Receptor Radionuclide Therapy with radiolabelled somatostatin analogues in patients with somatostatin receptor positive tumours. Acta Oncol. 2007, 46, 723–734. [Google Scholar] [CrossRef]
- Olsen, J.O.; Pozderac, R.V.; Hinkle, G.; Hill, T.; O’Dorisio, T.M.; Schirmer, W.J.; Ellison, E.C.; O’Dorisio, M.S. Somatostatin receptor imaging of neuroendocrine tumors with indium-111 pentetreotide (Octreoscan). Semin. Nucl. Med. 1995, 25, 251–261. [Google Scholar] [CrossRef]
- Pool, S.E.; Krenning, E.P.; Koning, G.A.; van Eijck, C.H.J.; Teunissen, J.J.M.; Kam, B.; Valkema, R.; Kwekkeboom, D.J.; de Jong, M. Preclinical and Clinical Studies of Peptide Receptor Radionuclide Therapy. Semin. Nucl. Med. 2010, 40, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Öksüz, M.; Winter, L.; Pfannenberg, C.; Reischl, G.; Müssig, K.; Bares, R.; Dittmann, H. Peptide receptor radionuclide therapy of neuroendocrine tumors with (90)Y-DOTATOC: Is treatment response predictable by pre-therapeutic uptake of (68)Ga-DOTATOC? Diagn. Interv. Imaging 2014, 95, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Witak, W.; Pieniężna, A.; Brasuń, J. The binding ability of a bicyclic somatostatin analogue towards Cu(II) ions. Chem. Biodivers. 2020, 17, e2000307. [Google Scholar] [CrossRef] [PubMed]
- Bataille, M.; Formicka-Kozlowska, G.; Kozlowski, H.; Pettit, L.D.; Steel, I. The L-proline residue as a ‘break-point’ in the co-ordination of metal–peptide systems. J. Chem. Soc. Chem. Commun. 1984, 231–232. [Google Scholar] [CrossRef]
- Marciniak, A.; Cebrat, M.; Brasuń, J. The Coordination Abilities of New Cyclic Analogs of Somatostatin. Int. J. Pept. Res. Ther. 2017, 23, 135–143. [Google Scholar] [CrossRef][Green Version]
- Prenesti, E.; Daniele, P.G.; Prencipe, M.; Ostacoli, G. Spectrum-structure correlation for visible absorption spectra of copper(II) complexes in aqueous solution. Polyhedron 1999, 18, 3233–3241. [Google Scholar] [CrossRef]
- Barth, G.; Voelter, W.; Bunnenberg, E.; Djerassi, C. Magnetic Circular Dichroism Studies. XVII. Magnetic Circular Dichroism Spectra of Proteins. New Method for the Quantitative Determination of Tryptophan. J. Am. Chem. Soc. 1972, 94, 1293–1298. [Google Scholar] [CrossRef]
- Barth, G.; Records, R.; Bunnenberg, E.; Djerassi, C.; Voelter, W. Magnetic Circular Dichroism Studies. XII. The Determination of Tryptophan in Proteins. J. Am. Chem. Soc. 1971, 93, 2545–2547. [Google Scholar] [CrossRef]
- Barrett, G. Chemistry and Biochemistry of the Amino Acids; Springer: Dordrecht, The Netherlands, 2012; ISBN 9789400948327. [Google Scholar]
- Irving, H.M.; Miles, M.G.; Pettit, L.D. A study of some problems in determining the stoicheiometric proton dissociation constants of complexes by potentiometric titrations using a glass electrode. Anal. Chim. Acta 1967, 38, 475–488. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. SUPERQUAD: An improved general program for computation of formation constants from potentiometric data. J. Chem. Soc. Dalton Trans. 1985, 1195–1200. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]




| Species | P1 | P2 | BCS [13] | |||||
|---|---|---|---|---|---|---|---|---|
| logβ a | logK b | logβ* c | logβ a | logK b | logβ* c | logβ | logK | |
| Ligands | ||||||||
| HL | 10.26 ± 0.02 | 10.26 | 10.75 ± 0.05 | 10.25 | 10.96 | 10.96 | ||
| H2L | 20.03 ± 0.01 | 9.78 | 19.81 ± 0.03 | 9.62 | 20.77 | 9.81 | ||
| H3L | 26.61 ± 0.02 | 6.58 | 26.38 ± 0.04 | 6.62 | 28.02 | 7.25 | ||
| H4L | 32.32 ± 0.02 | 5.71 | 31.85 ± 0.04 | 5.53 | 33.24 | 5.22 | ||
| Copper(II) Complexes | ||||||||
| CuH3L | - | - | 33.14 | 5.70 | ||||
| CuH2L | 25.00 ± 0.08 | 4.97 | 25.10 ± 0.04 | 5.29 | 27.44 | 7.10 | ||
| CuHL | - | 18.26 ± 0.06 | 6.79 | 7.51 | 20.34 | 8.82 | ||
| CuL | 10.35 ± 0.11 | 14.65 | 10.10 ± 0.07 | 8.13 | 11.52 | 9.54 | ||
| CuH−1L | 1.31 ± 0.14 | 9.04 | 1.28 ± 0.07 | 8.84 | 1.98 | 20.90 | ||
| CuH−3L | −18.81 ± 0.14 | 20.12 | −18.78 ± 0.07 | 19.74 | −18.92 | |||
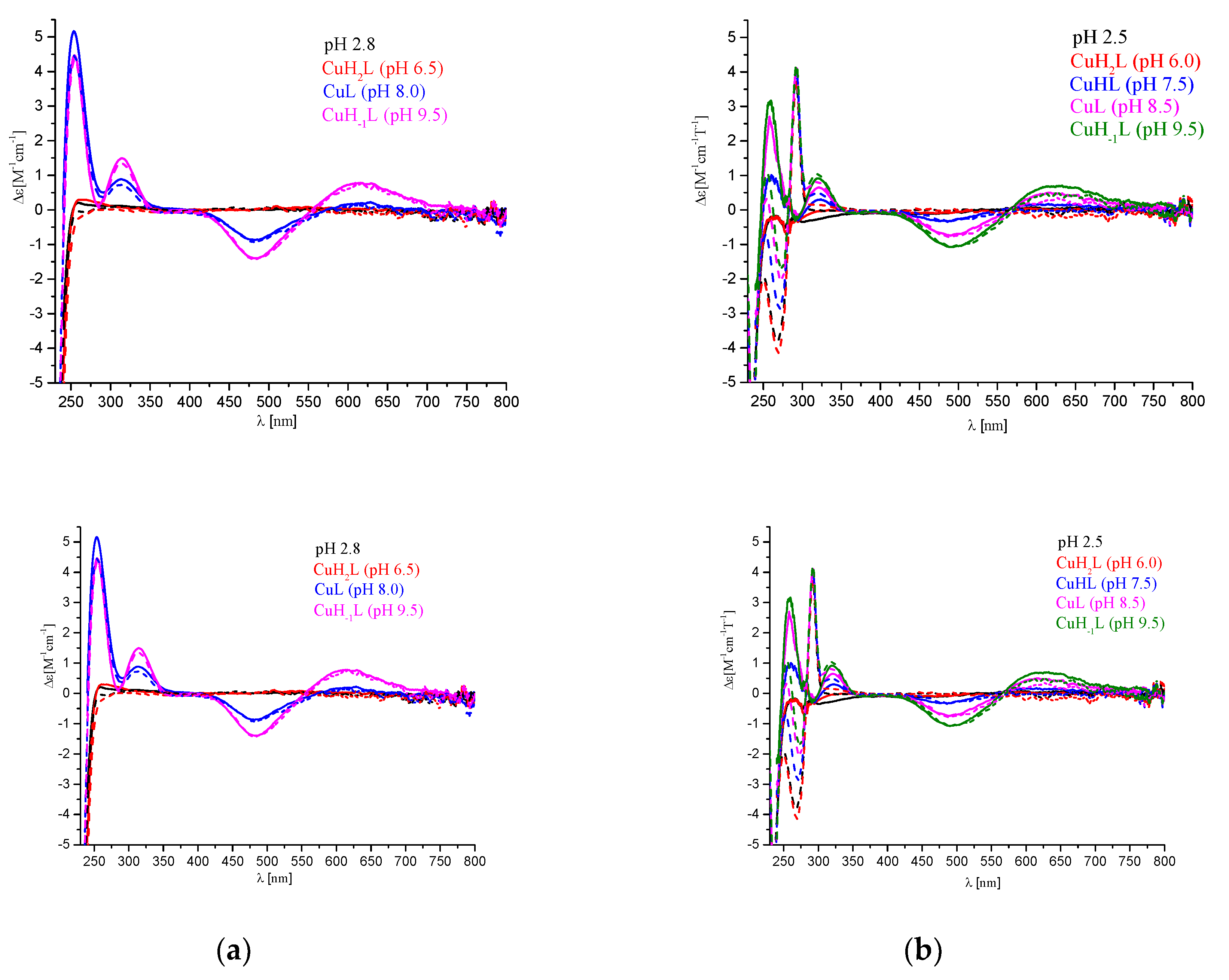
| UV-Vis | CD | ||
|---|---|---|---|
| Dominating Complex | λ [nm] | λ [nm] | Δε [M−1 cm−1] |
| P1. (Ac-c(-S-Cys-Pro-Hisa-Lys-Lys-Hisb-Pro-Cys-S-)-NH2) | |||
| CuH2L | 665 | - | - |
| CuL | 592 510 | 623 a 482 a 314 b 254 c | 0.211 −0.873 0.861 5.14 |
| CuH−1L | 580 sh 506 | 617 a 482 a 315 b 254 c | 0.782 −1.43 1.45 4.43 |
| CuH−3L | 580 sh 506 | 617 a 482 a 315 b 254 c | 0.899 −1.43 1.45 4.19 |
| P2. (c(Ser-Pro-Hisa-Lys-Lys-Hisb-Pro-Ser-Phe-Trp-Lys-Thr)) | |||
| CuH2L | 658 | - | - |
| CuHL | 609 | 624 a 486 a 323 b 260 c | 0.133 −0.325 0.289 0.935 |
| CuL | 606 sh 499 | 619 a 493 a 320 b 259 c | 0.487 −0.721 0.633 2.56 |
| CuH−1L | 606 sh 497 | 626 a 492 a 320 b 258 c | 0.689 −1.07 0.904 3.133 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marciniak, A.; Witak, W.; Sabatino, G.; Papini, A.M.; Brasuń, J. Detailed Insight into the Interaction of Bicyclic Somatostatin Analogue with Cu(II) Ions. Int. J. Mol. Sci. 2020, 21, 8794. https://doi.org/10.3390/ijms21228794
Marciniak A, Witak W, Sabatino G, Papini AM, Brasuń J. Detailed Insight into the Interaction of Bicyclic Somatostatin Analogue with Cu(II) Ions. International Journal of Molecular Sciences. 2020; 21(22):8794. https://doi.org/10.3390/ijms21228794
Chicago/Turabian StyleMarciniak, Aleksandra, Weronika Witak, Giuseppina Sabatino, Anna Maria Papini, and Justyna Brasuń. 2020. "Detailed Insight into the Interaction of Bicyclic Somatostatin Analogue with Cu(II) Ions" International Journal of Molecular Sciences 21, no. 22: 8794. https://doi.org/10.3390/ijms21228794
APA StyleMarciniak, A., Witak, W., Sabatino, G., Papini, A. M., & Brasuń, J. (2020). Detailed Insight into the Interaction of Bicyclic Somatostatin Analogue with Cu(II) Ions. International Journal of Molecular Sciences, 21(22), 8794. https://doi.org/10.3390/ijms21228794






