Impairment of Hypoxia-Induced CA IX by Beta-Blocker Propranolol—Impact on Progression and Metastatic Potential of Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
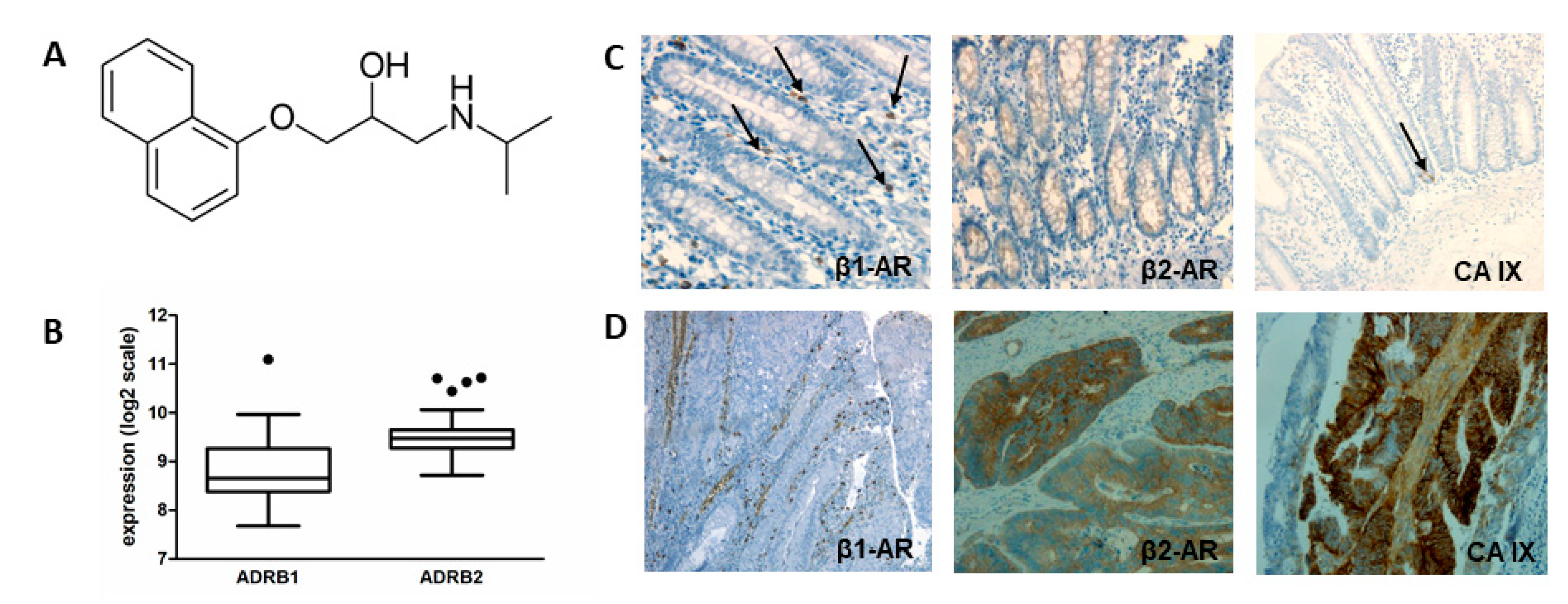
2.1. Expression of Beta Adrenoreceptors in Colorectal Carcinomas-In Silico Analysis
2.2. Expression of β-AR in Colorectal Carcinoma Tissues
2.3. Beta-Blockade by Propranolol Influences Extracellular pH of Colorectal Cancer Cells in Hypoxia
2.4. Beta-Blockade by Propranolol Decreases Level of Phosphorylated form of Catalytic Subunit of PKA under Hypoxic Conditions
2.5. Propranolol Decreases Expression of CA IX in Hypoxic Colorectal Cancer Cells Cultivated in Monolayer
2.6. Propranolol Influences Adaptation to Hypoxic Microenvironment by Decreased Levels of HIF1α and CA IX in 3D Spheroids
2.7. Propranolol Decreases Migration Properties of Colorectal Cancer Cells
2.8. Distribution of CA IX in 3D Spheroids Is Polarized
2.9. Propranolol Affects Cell Stress Proteome in Spheroids
2.10. Propranolol Reduces Number of Mitochondria and Mitochondrial ox-phos Enzymes
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Growth Conditions
4.2. Western Blotting
4.3. Proteome Profiler Array
4.4. Flow Cytometry Analysis of Cell Viability
4.5. Quantitative Real-Time PCR
4.6. Formation of Spheroids in Hanging Drops
4.7. Clonogenic Assay
4.8. Immunofluorescence of Spheroids
4.9. Immunohistochemistry
4.10. In Silico Analysis
4.11. Time-Lapse Microscopy
4.12. Fluorescent Labelling and Analysis of Mitochondrial Network
4.13. Ethic Approval and Patients Tissue Samples
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CA IX | Carbonic anhydrase IX |
| β-AR | Beta adrenergic receptor |
| HIF1 | Hypoxia inducible factor 1 |
| PKA | Protein kinase A |
References
- Dronkers, E.A.; Mes, S.W.; Wieringa, M.H.; van der Schroeff, M.P.; Baatenburg de Jong, R.J. Noncompliance to guidelines in head and neck cancer treatment; associated factors for both patient and physician. BMC Cancer 2015, 15, 515. [Google Scholar] [CrossRef]
- Boje, C.R. Impact of comorbidity on treatment outcome in head and neck squamous cell carcinoma—A systematic review. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 110, 81–90. [Google Scholar] [CrossRef]
- Pule, M.L.; Buckley, E.; Niyonsenga, T.; Roder, D. The effects of comorbidity on colorectal cancer mortality in an Australian cancer population. Sci. Rep. 2019, 9, 8580. [Google Scholar] [CrossRef]
- Sarfati, D.; Koczwara, B.; Jackson, C. The impact of comorbidity on cancer and its treatment. CA Cancer J. Clin. 2016, 66, 337–350. [Google Scholar] [CrossRef]
- Chen, R.; Ryan, P.; Natarajan, K.; Falconer, T.; Crew, K.D.; Reich, C.G.; Vashisht, R.; Randhawa, G.; Shah, N.H.; Hripcsak, G. Treatment Patterns for Chronic Comorbid Conditions in Patients With Cancer Using a Large-Scale Observational Data Network. JCO Clin. Cancer Inform. 2020, 4, 171–183. [Google Scholar] [CrossRef]
- Cohen, J.B.; Geara, A.S.; Hogan, J.J.; Townsend, R.R. Hypertension in Cancer Patients and Survivors: Epidemiology, Diagnosis, and Management. JACC CardioOncol. 2019, 1, 238–251. [Google Scholar] [CrossRef]
- Edwards, B.K.; Noone, A.M.; Mariotto, A.B.; Simard, E.P.; Boscoe, F.P.; Henley, S.J.; Jemal, A.; Cho, H.; Anderson, R.N.; Kohler, B.A.; et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014, 120, 1290–1314. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef]
- Ali, A.; Herndon, D.N.; Mamachen, A.; Hasan, S.; Andersen, C.R.; Grogans, R.J.; Brewer, J.L.; Lee, J.O.; Heffernan, J.; Suman, O.E.; et al. Propranolol attenuates hemorrhage and accelerates wound healing in severely burned adults. Crit. Care 2015, 19, 217. [Google Scholar] [CrossRef]
- Peskind, E.R.; Tsuang, D.W.; Bonner, L.T.; Pascualy, M.; Riekse, R.G.; Snowden, M.B.; Thomas, R.; Raskind, M.A. Propranolol for disruptive behaviors in nursing home residents with probable or possible Alzheimer disease: A placebo-controlled study. Alzheimer Dis. Assoc. Disord. 2005, 19, 23–28. [Google Scholar] [CrossRef]
- Greendyke, R.M.; Kanter, D.R.; Schuster, D.B.; Verstreate, S.; Wootton, J. Propranolol treatment of assaultive patients with organic brain disease. A double-blind crossover, placebo-controlled study. J. Nerv. Ment. Dis. 1986, 174, 290–294. [Google Scholar] [CrossRef]
- De Giorgi, V.; Grazzini, M.; Gandini, S.; Benemei, S.; Lotti, T.; Marchionni, N.; Geppetti, P. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch. Intern. Med. 2011, 171, 779–781. [Google Scholar] [CrossRef]
- Melhem-Bertrandt, A.; Chavez-Macgregor, M.; Lei, X.; Brown, E.N.; Lee, R.T.; Meric-Bernstam, F.; Sood, A.K.; Conzen, S.D.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2645–2652. [Google Scholar] [CrossRef]
- Diaz, E.S.; Karlan, B.Y.; Li, A.J. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol. Oncol. 2012, 127, 375–378. [Google Scholar] [CrossRef]
- Jansen, L.; Hoffmeister, M.; Arndt, V.; Chang-Claude, J.; Brenner, H. Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer 2014, 120, 1178–1186. [Google Scholar] [CrossRef]
- Wu, F.Q.; Fang, T.; Yu, L.X.; Lv, G.S.; Lv, H.W.; Liang, D.; Li, T.; Wang, C.Z.; Tan, Y.X.; Ding, J.; et al. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1alpha. J. Hepatol. 2016, 65, 314–324. [Google Scholar] [CrossRef]
- Antoni, M.H.; Lutgendorf, S.K.; Cole, S.W.; Dhabhar, F.S.; Sephton, S.E.; McDonald, P.G.; Stefanek, M.; Sood, A.K. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat. Rev. Cancer 2006, 6, 240–248. [Google Scholar] [CrossRef]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef]
- Grytli, H.H.; Fagerland, M.W.; Fossa, S.D.; Tasken, K.A. Association between use of beta-blockers and prostate cancer-specific survival: A cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur. Urol. 2014, 65, 635–641. [Google Scholar] [CrossRef]
- Kaapu, K.J.; Murtola, T.J.; Maattanen, L.; Talala, K.; Taari, K.; Tammela, T.L.; Auvinen, A. Prostate cancer risk among users of digoxin and other antiarrhythmic drugs in the Finnish Prostate Cancer Screening Trial. Cancer Causes Control 2016, 27, 157–164. [Google Scholar] [CrossRef]
- Kulik, G. ADRB2-Targeting Therapies for Prostate Cancer. Cancers 2019, 11, 358. [Google Scholar] [CrossRef]
- Botteri, E.; Munzone, E.; Rotmensz, N.; Cipolla, C.; De Giorgi, V.; Santillo, B.; Zanelotti, A.; Adamoli, L.; Colleoni, M.; Viale, G.; et al. Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res. Treat. 2013, 140, 567–575. [Google Scholar] [CrossRef]
- Powe, D.G.; Voss, M.J.; Zanker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef]
- Choy, C.; Raytis, J.L.; Smith, D.D.; Duenas, M.; Neman, J.; Jandial, R.; Lew, M.W. Inhibition of beta2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative beta-blockade. Oncol. Rep. 2016, 35, 3135–3142. [Google Scholar] [CrossRef]
- Montoya, A.; Amaya, C.N.; Belmont, A.; Diab, N.; Trevino, R.; Villanueva, G.; Rains, S.; Sanchez, L.A.; Badri, N.; Otoukesh, S.; et al. Use of non-selective beta-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget 2017, 8, 6446–6460. [Google Scholar] [CrossRef]
- Lemeshow, S.; Sorensen, H.T.; Phillips, G.; Yang, E.V.; Antonsen, S.; Riis, A.H.; Lesinski, G.B.; Jackson, R.; Glaser, R. beta-Blockers and survival among Danish patients with malignant melanoma: A population-based cohort study. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 2273–2279. [Google Scholar] [CrossRef]
- Choi, C.H.; Song, T.; Kim, T.H.; Choi, J.K.; Park, J.Y.; Yoon, A.; Lee, Y.Y.; Kim, T.J.; Bae, D.S.; Lee, J.W.; et al. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J. Cancer Res. Clin. Oncol. 2014, 140, 1179–1188. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Coleman, H.G.; Murray, L.J.; Entschladen, F.; Powe, D.G. Beta-blocker usage and breast cancer survival: A nested case-control study within a UK clinical practice research datalink cohort. Int. J. Epidemiol. 2013, 42, 1852–1861. [Google Scholar] [CrossRef]
- Livingstone, E.; Hollestein, L.M.; van Herk-Sukel, M.P.; van de Poll-Franse, L.; Nijsten, T.; Schadendorf, D.; de Vries, E. beta-Blocker use and all-cause mortality of melanoma patients: Results from a population-based Dutch cohort study. Eur. J. Cancer 2013, 49, 3863–3871. [Google Scholar] [CrossRef]
- McCourt, C.; Coleman, H.G.; Murray, L.J.; Cantwell, M.M.; Dolan, O.; Powe, D.G.; Cardwell, C.R. Beta-blocker usage after malignant melanoma diagnosis and survival: A population-based nested case-control study. Br. J. Dermatol. 2014, 170, 930–938. [Google Scholar] [CrossRef]
- Parker, W.P.; Lohse, C.M.; Zaid, H.B.; Cheville, J.C.; Boorjian, S.A.; Leibovich, B.C.; Thompson, R.H. Evaluation of beta-blockers and survival among hypertensive patients with renal cell carcinoma. Urol. Oncol. 2017, 35, 36.e1–36.e6. [Google Scholar] [CrossRef]
- Yap, A.; Lopez-Olivo, M.A.; Dubowitz, J.; Pratt, G.; Hiller, J.; Gottumukkala, V.; Sloan, E.; Riedel, B.; Schier, R. Effect of beta-blockers on cancer recurrence and survival: A meta-analysis of epidemiological and perioperative studies. Br. J. Anaesth. 2018, 121, 45–57. [Google Scholar] [CrossRef]
- Bristow, M.R.; Hershberger, R.E.; Port, J.D.; Minobe, W.; Rasmussen, R. Beta 1- and beta 2-adrenergic receptor-mediated adenylate cyclase stimulation in nonfailing and failing human ventricular myocardium. Mol. Pharmacol. 1989, 35, 295–303. [Google Scholar]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Pastorek, J.; Pastorekova, S.; Callebaut, I.; Mornon, J.P.; Zelnik, V.; Opavsky, R.; Zat’ovicova, M.; Liao, S.; Portetelle, D.; Stanbridge, E.J.; et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 1994, 9, 2877–2888. [Google Scholar]
- Pastorek, J.; Pastorekova, S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: From biology to clinical use. Semin. Cancer Biol. 2015, 31, 52–64. [Google Scholar] [CrossRef]
- Wykoff, C.C.; Beasley, N.J.; Watson, P.H.; Turner, K.J.; Pastorek, J.; Sibtain, A.; Wilson, G.D.; Turley, H.; Talks, K.L.; Maxwell, P.H.; et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000, 60, 7075–7083. [Google Scholar]
- Ditte, P.; Dequiedt, F.; Svastova, E.; Hulikova, A.; Ohradanova-Repic, A.; Zatovicova, M.; Csaderova, L.; Kopacek, J.; Supuran, C.T.; Pastorekova, S.; et al. Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res. 2011, 71, 7558–7567. [Google Scholar] [CrossRef]
- Dorai, T.; Sawczuk, I.S.; Pastorek, J.; Wiernik, P.H.; Dutcher, J.P. The role of carbonic anhydrase IX overexpression in kidney cancer. Eur. J. Cancer 2005, 41, 2935–2947. [Google Scholar] [CrossRef]
- Zatovicova, M.; Jelenska, L.; Hulikova, A.; Csaderova, L.; Ditte, Z.; Ditte, P.; Goliasova, T.; Pastorek, J.; Pastorekova, S. Carbonic anhydrase IX as an anticancer therapy target: Preclinical evaluation of internalizing monoclonal antibody directed to catalytic domain. Curr. Pharm. Des. 2010, 16, 3255–3263. [Google Scholar] [CrossRef]
- Bourseau-Guilmain, E.; Menard, J.A.; Lindqvist, E.; Indira Chandran, V.; Christianson, H.C.; Cerezo Magana, M.; Lidfeldt, J.; Marko-Varga, G.; Welinder, C.; Belting, M. Hypoxia regulates global membrane protein endocytosis through caveolin-1 in cancer cells. Nat. Commun. 2016, 7, 11371. [Google Scholar] [CrossRef] [PubMed]
- Zatovicova, M.; Sedlakova, O.; Svastova, E.; Ohradanova, A.; Ciampor, F.; Arribas, J.; Pastorek, J.; Pastorekova, S. Ectodomain shedding of the hypoxia-induced carbonic anhydrase IX is a metalloprotease-dependent process regulated by TACE/ADAM17. Br. J. Cancer 2005, 93, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Vidlickova, I.; Dequiedt, F.; Jelenska, L.; Sedlakova, O.; Pastorek, M.; Stuchlik, S.; Pastorek, J.; Zatovicova, M.; Pastorekova, S. Apoptosis-induced ectodomain shedding of hypoxia-regulated carbonic anhydrase IX from tumor cells: A double-edged response to chemotherapy. BMC Cancer 2016, 16, 239. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Parkkila, S.; Parkkila, A.K.; Opavsky, R.; Zelnik, V.; Saarnio, J.; Pastorek, J. Carbonic anhydrase IX, MN/CA IX: Analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology 1997, 112, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Pantziarka, P.; Bouche, G.; Sukhatme, V.; Meheus, L.; Rooman, I.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)-Propranolol as an anti-cancer agent. Ecancermedicalscience 2016, 10, 680. [Google Scholar] [CrossRef]
- Chim, H.; Armijo, B.S.; Miller, E.; Gliniak, C.; Serret, M.A.; Gosain, A.K. Propranolol induces regression of hemangioma cells through HIF-1alpha-mediated inhibition of VEGF-A. Ann. Surg. 2012, 256, 146–156. [Google Scholar] [CrossRef]
- Cheong, H.I.; Asosingh, K.; Stephens, O.R.; Queisser, K.A.; Xu, W.; Willard, B.; Hu, B.; Dermawan, J.K.T.; Stark, G.R.; Naga Prasad, S.V.; et al. Hypoxia sensing through beta-adrenergic receptors. JCI Insight 2016, 1, e90240. [Google Scholar] [CrossRef]
- Brohee, L.; Peulen, O.; Nusgens, B.; Castronovo, V.; Thiry, M.; Colige, A.C.; Deroanne, C.F. Propranolol sensitizes prostate cancer cells to glucose metabolism inhibition and prevents cancer progression. Sci. Rep. 2018, 8, 7050. [Google Scholar] [CrossRef]
- Lucido, C.T.; Callejas-Valera, J.L.; Colbert, P.L.; Vermeer, D.W.; Miskimins, W.K.; Spanos, W.C.; Vermeer, P.D. beta2-Adrenergic receptor modulates mitochondrial metabolism and disease progression in recurrent/metastatic HPV(+) HNSCC. Oncogenesis 2018, 7, 81. [Google Scholar] [CrossRef]
- Lucido, C.T.; Miskimins, W.K.; Vermeer, P.D. Propranolol Promotes Glucose Dependence and Synergizes with Dichloroacetate for Anti-Cancer Activity in HNSCC. Cancers 2018, 10, 476. [Google Scholar] [CrossRef]
- Wolter, J.K.; Wolter, N.E.; Blanch, A.; Partridge, T.; Cheng, L.; Morgenstern, D.A.; Podkowa, M.; Kaplan, D.R.; Irwin, M.S. Anti-tumor activity of the beta-adrenergic receptor antagonist propranolol in neuroblastoma. Oncotarget 2014, 5, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Zavadova, Z.; Kostal, M.; Babusikova, O.; Zavada, J. A novel quasi-viral agent, MaTu, is a two-component system. Virology 1992, 187, 620–626. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.F.; Janssen-Heijnen, M.L.; van der Heijden, L.H.; Coebergh, J.W. Comorbidity and colorectal cancer according to subsite and stage: A population-based study. Eur. J. Cancer 2000, 36, 95–99. [Google Scholar] [CrossRef]
- Chang, P.Y.; Huang, W.Y.; Lin, C.L.; Huang, T.C.; Wu, Y.Y.; Chen, J.H.; Kao, C.H. Propranolol Reduces Cancer Risk: A Population-Based Cohort Study. Medicine 2015, 94, e1097. [Google Scholar] [CrossRef]
- Hanns, P.; Paczulla, A.M.; Medinger, M.; Konantz, M.; Lengerke, C. Stress and catecholamines modulate the bone marrow microenvironment to promote tumorigenesis. Cell Stress 2019, 3, 221–235. [Google Scholar] [CrossRef]
- Uchea, C.; Owen, S.F.; Chipman, J.K. Functional xenobiotic metabolism and efflux transporters in trout hepatocyte spheroid cultures. Toxicol. Res. 2015, 4, 494–507. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control Rel. 2012, 164, 192–204. [Google Scholar] [CrossRef]
- Simko, V.; Iuliano, F.; Sevcikova, A.; Labudova, M.; Barathova, M.; Radvak, P.; Pastorekova, S.; Pastorek, J.; Csaderova, L. Hypoxia induces cancer-associated cAMP/PKA signalling through HIF-mediated transcriptional control of adenylyl cyclases VI and VII. Sci. Rep. 2017, 7, 10121. [Google Scholar] [CrossRef]
- Minchenko, A.; Leshchinsky, I.; Opentanova, I.; Sang, N.; Srinivas, V.; Armstead, V.; Caro, J. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene. Its possible role in the Warburg effect. J. Biol. Chem. 2002, 277, 6183–6187. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J. Bioenerg. Biomembr. 2007, 39, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bosch-Marce, M.; Shimoda, L.A.; Tan, Y.S.; Baek, J.H.; Wesley, J.B.; Gonzalez, F.J.; Semenza, G.L. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 2008, 283, 10892–10903. [Google Scholar] [CrossRef] [PubMed]
- Albinana, V.; Villar Gomez de Las Heras, K.; Serrano-Heras, G.; Segura, T.; Perona-Moratalla, A.B.; Mota-Perez, M.; de Campos, J.M.; Botella, L.M. Propranolol reduces viability and induces apoptosis in hemangioblastoma cells from von Hippel-Lindau patients. Orphanet. J. Rare Dis. 2015, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Moz, M.; Correia, G.; Teixeira, A.; Medeiros, R.; Ribeiro, L. Antiproliferative effects of beta-blockers on human colorectal cancer cells. Oncol. Rep. 2015, 33, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.C.; Li, J.M.; Lee, K.F.; Huang, Y.C.; Wang, K.C.; Lai, H.C.; Cheng, C.C.; Kuo, Y.H.; Shi, C.S. Selective beta2-AR Blockage Suppresses Colorectal Cancer Growth Through Regulation of EGFR-Akt/ERK1/2 Signaling, G1-Phase Arrest, and Apoptosis. J. Cell. Physiol. 2016, 231, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Wolter, N.E.; Wolter, J.K.; Enepekides, D.J.; Irwin, M.S. Propranolol as a novel adjunctive treatment for head and neck squamous cell carcinoma. J. Otolaryngol. Head Neck Surg. 2012, 41, 334–344. [Google Scholar]
- Zhang, D.; Ma, Q.Y.; Hu, H.T.; Zhang, M. beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol. Ther. 2010, 10, 19–29. [Google Scholar] [CrossRef]
- Palm, D.; Lang, K.; Niggemann, B.; Drell, T.L.T.; Masur, K.; Zaenker, K.S.; Entschladen, F. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int. J. Cancer 2006, 118, 2744–2749. [Google Scholar] [CrossRef]
- Liu, D.; Yang, Z.; Wang, T.; Yang, Z.; Chen, H.; Hu, Y.; Hu, C.; Guo, L.; Deng, Q.; Liu, Y.; et al. beta2-AR signaling controls trastuzumab resistance-dependent pathway. Oncogene 2016, 35, 47–58. [Google Scholar] [CrossRef]
- Kramer, N.; Walzl, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschlager, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef]
- Barbieri, A.; Bimonte, S.; Palma, G.; Luciano, A.; Rea, D.; Giudice, A.; Scognamiglio, G.; La Mantia, E.; Franco, R.; Perdona, S.; et al. The stress hormone norepinephrine increases migration of prostate cancer cells in vitro and in vivo. Int. J. Oncol. 2015, 47, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; He, X.; Tan, J.; Zhou, X.; Zou, L. beta-arrestin2 mediates beta-2 adrenergic receptor signaling inducing prostate cancer cell progression. Oncol. Rep. 2011, 26, 1471–1477. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Z.; Li, G.; Zhang, Z.; Gu, R.; Wang, W.; Lai, X.; Cui, Z.K.; Zeng, F.; Xu, S.; Deng, F. beta2AR-HIF-1alpha-CXCL12 signaling of osteoblasts activated by isoproterenol promotes migration and invasion of prostate cancer cells. BMC Cancer 2019, 19, 1142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Svastova, E.; Witarski, W.; Csaderova, L.; Kosik, I.; Skvarkova, L.; Hulikova, A.; Zatovicova, M.; Barathova, M.; Kopacek, J.; Pastorek, J.; et al. Carbonic anhydrase IX interacts with bicarbonate transporters in lamellipodia and increases cell migration via its catalytic domain. J. Biol. Chem. 2012, 287, 3392–3402. [Google Scholar] [CrossRef]
- Hruz, T.; Laule, O.; Szabo, G.; Wessendorp, F.; Bleuler, S.; Oertle, L.; Widmayer, P.; Gruissem, W.; Zimmermann, P. Genevestigator v3: A reference expression database for the meta-analysis of transcriptomes. Adv. Bioinform. 2008, 2008, 420747. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barathova, M.; Grossmannova, K.; Belvoncikova, P.; Kubasova, V.; Simko, V.; Skubla, R.; Csaderova, L.; Pastorek, J. Impairment of Hypoxia-Induced CA IX by Beta-Blocker Propranolol—Impact on Progression and Metastatic Potential of Colorectal Cancer Cells. Int. J. Mol. Sci. 2020, 21, 8760. https://doi.org/10.3390/ijms21228760
Barathova M, Grossmannova K, Belvoncikova P, Kubasova V, Simko V, Skubla R, Csaderova L, Pastorek J. Impairment of Hypoxia-Induced CA IX by Beta-Blocker Propranolol—Impact on Progression and Metastatic Potential of Colorectal Cancer Cells. International Journal of Molecular Sciences. 2020; 21(22):8760. https://doi.org/10.3390/ijms21228760
Chicago/Turabian StyleBarathova, Monika, Katarina Grossmannova, Petra Belvoncikova, Veronika Kubasova, Veronika Simko, Rudolf Skubla, Lucia Csaderova, and Jaromir Pastorek. 2020. "Impairment of Hypoxia-Induced CA IX by Beta-Blocker Propranolol—Impact on Progression and Metastatic Potential of Colorectal Cancer Cells" International Journal of Molecular Sciences 21, no. 22: 8760. https://doi.org/10.3390/ijms21228760
APA StyleBarathova, M., Grossmannova, K., Belvoncikova, P., Kubasova, V., Simko, V., Skubla, R., Csaderova, L., & Pastorek, J. (2020). Impairment of Hypoxia-Induced CA IX by Beta-Blocker Propranolol—Impact on Progression and Metastatic Potential of Colorectal Cancer Cells. International Journal of Molecular Sciences, 21(22), 8760. https://doi.org/10.3390/ijms21228760




