Efficient Suppression of Abdominal Aortic Aneurysm Expansion in Rats through Systemic Administration of Statin-Loaded Nanomedicine
Abstract
1. Introduction
2. Results and Discussion
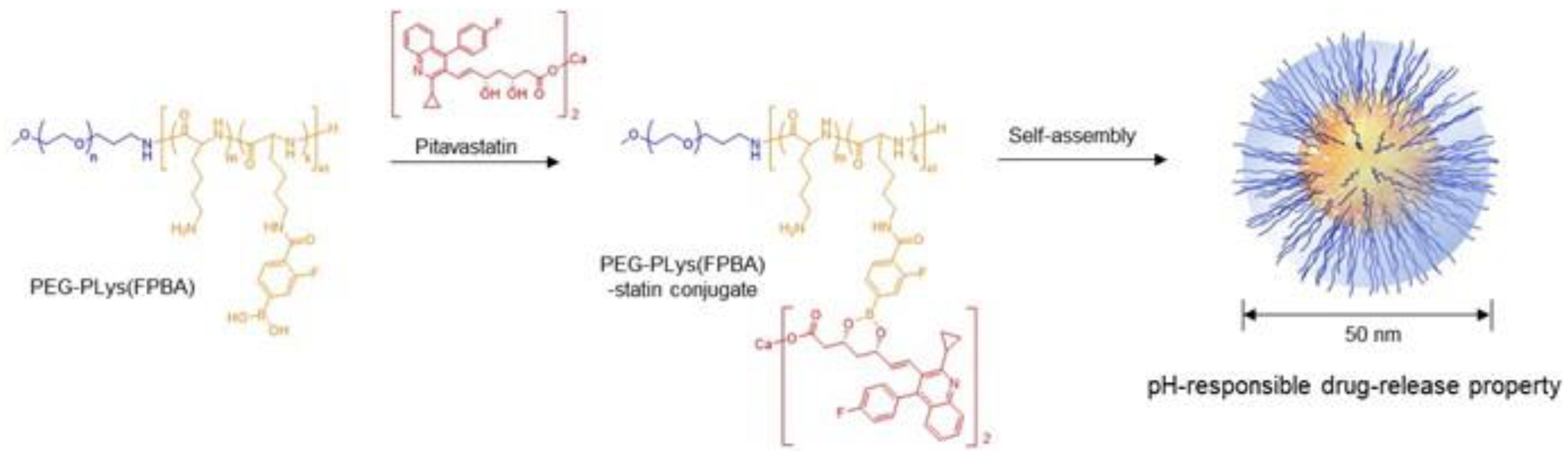
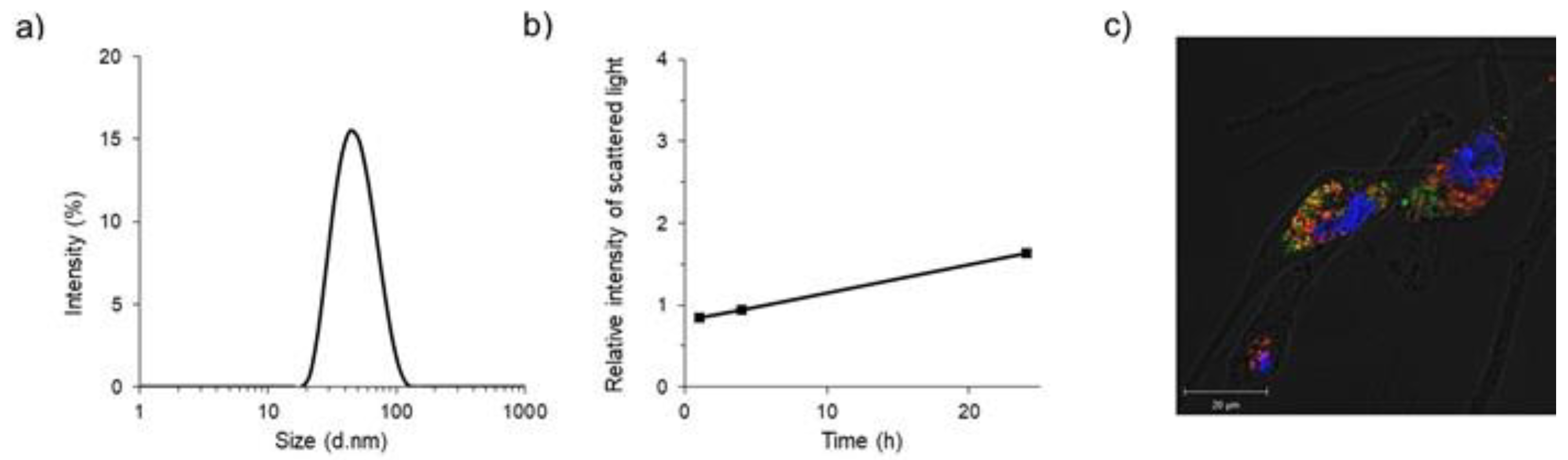
2.1. Preparation and In Vitro Characteristics of Polymeric Micelles
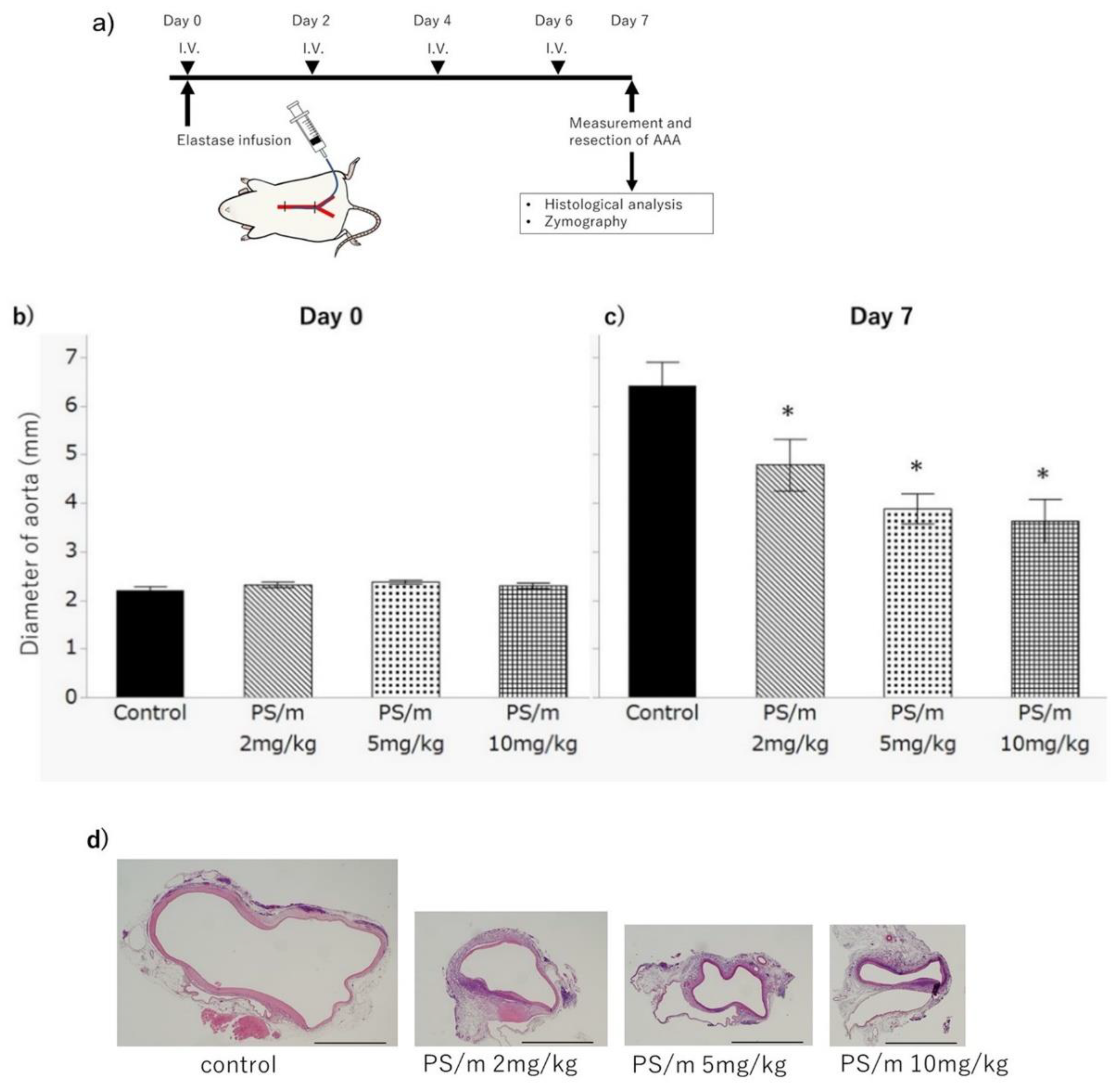
2.2. Therapeutic Efficacy of Pitavastatin-Loaded Polymeric Micelles for AAA
2.3. Histology after Treatment with Pitavastatin-Loaded Polymeric Micelle
2.4. Gelatinatic Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of PEG-Poly(l-lysine) Block Copolymer (PEG-PLys)
3.3. Synthesis of Phenylboronic Acid Modified Polymers [PEG-PLys(FPBA)]
3.4. Alexa647 Conjugation to PEG-PLys(FPBA)
3.5. Preparation of Pitavastatin-Loaded Polymeric Micelle
3.6. Cell Culture
3.7. Cytotoxicity
3.8. Confocal Laser Scanning Microscopic Observation
3.9. Elastase Infusion Model of AAA in Rat
3.10. Therapeutic Effect of Pitavastatin-Loaded Polymeric Micelle
3.11. Histological Analysis of the AAA
3.12. Gelatinase Activity
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; Bakal, C.W.; Creager, M.A.; Halperin, J.L.; Hiratzka, L.F.; Murphy, W.R.; Olin, J.W.; Puschett, J.B.; et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006, 113, e463–e654. [Google Scholar] [PubMed]
- Fujiwara, Y.; Shiraya, S.; Miyake, T.; Yamakawa, S.; Aoki, M.; Makino, H.; Nishimura, M.; Morishita, R. Inhibition of experimental abdominal aortic aneurysm in a rat model by the angiotensin receptor blocker valsartan. Int. J. Mol. Med. 2009, 22, 703–708. [Google Scholar]
- Petrinec, D.; Liao, S.; Holmes, D.R.; Reilly, J.M.; Parks, W.C.; Thompson, R.W. Doxycycline inhibition of aneurysmal degeneration in an elastase-induced rat model of abdominal aortic aneurysm: Prevention of aortic elastin associated with suppressed production of 92 kD gelatinase. J. Vasc. Surg. 1996, 23, 336–346. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.H.; Xia, R.P.; Duan, X.H.; Jiang, Y.B.; Jiang, Q.; Sun, W.J. Suppression of experimental abdominal aortic aneurysm in a rat model by the phosphodiesterase 3 inhibitor cilostazol. J. Surg. Res. 2011, 167, e385–e393. [Google Scholar] [CrossRef] [PubMed]
- Baxter, B.T.; Pearce, W.H.; Waltke, E.A.; Littooy, F.N.; Hallett, J.W., Jr.; Kent, K.C.; Upchurch, G.R., Jr.; Chaikof, E.L.; Mills, J.L.; Fleckten, B.; et al. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: Report of a prospective (Phase II) multicenter study. J. Vasc. Surg. 2002, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- The Propranolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: Results of a randomized trial. J. Vasc. Surg. 2002, 35, 72–79. [Google Scholar] [CrossRef]
- Shanks, N.; Greek, R.; Greek, J. Are animal models predictive for humans? Philos. Ethics. Humanit. Med. 2009, 4, 1–20. [Google Scholar] [CrossRef]
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892. [Google Scholar] [CrossRef]
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv. Drug Deliv. Rev. 2001, 47, 113–131. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 2006, 112, 630–648. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kataoka, K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009, 100, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y. Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Wilson, R.H.; Calvert, H.; Boddy, A.V.; Griffin, M.; Sludden, J.; Tilby, M.J.; Eatock, M.; Pearson, D.G.; Ottley, C.J.; et al. A phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Br. J. Cancer 2011, 104, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Shirasu, T.; Koyama, H.; Miura, Y.; Hoshina, K.; Kataoka, K.; Watanabe, T. Nanoparticles effectively target rapamycin delivery to sites of experimental aortic aneurysm in rats. PLoS ONE 2016, 23, e0157813. [Google Scholar] [CrossRef]
- Ferguson, C.D.; Clancy, P.; Bourke, B.; Walker, P.J.; Dear, A.; Buckenham, T.; Norman, P.; Golledge, J. Association of statin prescription with small abdominal aortic aneurysm progression. Am. Heart J. 2010, 159, 307–313. [Google Scholar] [CrossRef]
- Hurks, R.; Hoefer, I.E.; Vink, A.; Pasterkamp, G.; Schoneveld, A.; Kerver, M.; de Vries, J.P.; Tangelder, M.J.; Moll, F.L. Different effects of commonly prescribed statins on abdominal aortic aneurysm wall biology. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 569–576. [Google Scholar] [CrossRef]
- Salata, K.; Syed, M.; Hussain, M.A.; de Mestral, C.; Greco, E.; Mamdani, M.; Tu, J.V.; Forbes, T.L.; Bhatt, D.L.; Verma, S.; et al. Statins reduced abdominal aortic aneurysm growth, rupture, and perioperative mortality: A systematic review and meta-analysis. J. Am. Heart Assoc. 2018, 7, e008657. [Google Scholar] [CrossRef]
- Itoga, N.K.; Rothenberg, K.A.; Suarez, P.; Ho, T.V.; Mell, M.W.; Xu, B.; Curtin, C.M.; Dalman, R.L. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J. Vasc. Surg. 2018, 69, 710–716. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nakagami, H.; Nozaki, K.; Morishita, R.; Hashimoto, N. Pitavastatin suppresses formation and progression of cerebral aneurysm through inhibition of the nuclear factor κB pathway. Neurosurgery 2009, 64, 557–566. [Google Scholar] [CrossRef]
- Honda, Y.; Nomoto, T.; Matsui, M.; Takemoto, H.; Kaihara, Y.; Miura, Y.; Nishiyama, N. Sequential Self-Assembly Using Tannic Acid and Phenylboronic Acid-Modified Copolymers for Potential Protein Delivery. Biomacromolecules 2020, 21, 3826–3835. [Google Scholar] [CrossRef]
- Naito, M.; Yoshinaga, N.; Ishii, T.; Matsumoto, A.; Miyahara, Y.; Miyata, K.; Kataoka, K. Enhanced Intracellular Delivery of siRNA by Controlling ATP-Responsivity of Phenylboronic Acid-Function- alized Polyion Complex Micelles. Macromol. Biosci. 2018, 18, 1700357. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Stephenson-Brown, A.J.; Khan, T.; Miyazawa, T.; Cabral, H.; Kataoka, K.; Miyahara, Y. Heterocyclic boronic acids display sialic acid selective binding in a hypoxic tumor relevant acidic environment. Chem. Sci. 2017, 8, 6165–6170. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Thompson, P.D.; Panza, G.; Zalenski, A.; Taylor, B. Statin-associated side effects. J. Am. Coll. Cardiol. 2016, 67, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Shiraya, S.; Miyake, T.; Aoki, M.; Fujiwara, Y.; Ohgi, S.; Nishimura, M.; Ogihara, T.; Morishita, R. Inhibition of development of experimental aortic abdominal aneurysm in rat model by atorvastatin through inhibition of macrophage migration. Atherosclerosis 2009, 202, 34–40. [Google Scholar] [CrossRef]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, R.; Oo, A.Y.; Xiao, Q. Matrix Metalloproteinase in Abdominal Aortic Aneurysm and Aortic Dissection. Pharmaceuticals 2019, 12, 118. [Google Scholar] [CrossRef]
- Longo, G.M.; Xiong, W.; Greiner, T.C.; Zhao, Y.; Fiotti, N.; Baxter, B.T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J. Clin. Investig. 2002, 110, 625–632. [Google Scholar] [CrossRef]
- Steinmetz, E.F.; Burkley, C.; Shames, M.L.; Ennis, T.L.; Vanvickle-Chavez, S.J.; Mao, D.; Goeddel, L.A.; Hawkins, C.J.; Thompson, R.W. Treatment With Simvastatin Suppresses the Development of Experimental Abdominal Aortic Aneurysms in Normal and Hypercholesterolemic Mice. Ann. Surg. 2005, 241, 92–101. [Google Scholar] [CrossRef]
- Ramella, M.; Boccafoschi, F.; Bellofatto, K.; Follenzi, A.; Fusaro, L.; Boldorini, R.; Casella, F.; Porta, C.; Settembrini, P.; Cannas, M. Endothelial MMP-9 drives the inflammatory response in abdominal aortic aneurysm (AAA). Am. J. Transl. Res. 2017, 9, 5485–5495. [Google Scholar]
- Yao, F.; Yao, Z.; Zhong, T.; Zhang, J.; Wang, T.; Zhang, B.; He, Q.; Ding, L.; Yang, B. Imatinib prevents elastase-induced abdominal aortic aneurysm progression by regulating macrophage-derived MMP9. Eur. J. Pharmacol. 2019, 860, 172559. [Google Scholar] [CrossRef] [PubMed]
- Anidjar, S.; Salzmann, J.-L.; Gentric, D.; Lagneau, P.; Camilleri, J.-P.; Michel, J.-B. Elastase-Induced Experimental Aneurysms in Rats. Circulation 1990, 82, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Anidjar, S.; Dobrin, P.B.; Eichorst, M.; Graham, G.P.; Chejfec, G. Correlation of inflammatory infiltrate with the enlargement of experimental aortic aneurysms. J. Vasc. Surg. 1992, 16, 139–147. [Google Scholar] [CrossRef][Green Version]


| Free Pitavastatin | PS/m | |
|---|---|---|
| IC50 (μM) | 8.71 | 0.26 |
| Control | PS/m 2 mg/kg | PS/m 5 mg/kg | PS/m 10 mg/kg | |
|---|---|---|---|---|
| AST (IU/L) | 62 ± 5.0 | 55 ± 4.2 | 61 ± 3.6 | 51 ± 7.9 |
| ALT (IU/L) | 21 ± 1.5 | 21 ± 1.3 | 21 ± 1.1 | 20 ± 2.4 |
| BUN (mg/dL) | 16.1 ± 0.9 | 17.0 ± 0.7 | 17.0 ± 0.6 | 18.3 ± 1.4 |
| CRE (mg/dL) | 0.25 ± 0.01 | 0.26 ± 0.01 | 0.26 ± 0.01 | 0.25 ± 0.02 |
| CK (IU/L) | 183 ± 31 | 210 ± 26 | 190 ± 22 | 154 ± 50 |
| TG (mg/dL) | 137 ± 11 | 72 ± 9 * | 105 ± 8 | 56 ± 18 * |
| LDL-C (mg/dL) | 9 ± 1.2 | 12 ± 1.0 | 12 ± 0.8 | 14 ± 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuhara, N.; Honda, Y.; Ukita, N.; Matsui, M.; Miura, Y.; Hoshina, K. Efficient Suppression of Abdominal Aortic Aneurysm Expansion in Rats through Systemic Administration of Statin-Loaded Nanomedicine. Int. J. Mol. Sci. 2020, 21, 8702. https://doi.org/10.3390/ijms21228702
Fukuhara N, Honda Y, Ukita N, Matsui M, Miura Y, Hoshina K. Efficient Suppression of Abdominal Aortic Aneurysm Expansion in Rats through Systemic Administration of Statin-Loaded Nanomedicine. International Journal of Molecular Sciences. 2020; 21(22):8702. https://doi.org/10.3390/ijms21228702
Chicago/Turabian StyleFukuhara, Natsumi, Yuto Honda, Nao Ukita, Makoto Matsui, Yutaka Miura, and Katsuyuki Hoshina. 2020. "Efficient Suppression of Abdominal Aortic Aneurysm Expansion in Rats through Systemic Administration of Statin-Loaded Nanomedicine" International Journal of Molecular Sciences 21, no. 22: 8702. https://doi.org/10.3390/ijms21228702
APA StyleFukuhara, N., Honda, Y., Ukita, N., Matsui, M., Miura, Y., & Hoshina, K. (2020). Efficient Suppression of Abdominal Aortic Aneurysm Expansion in Rats through Systemic Administration of Statin-Loaded Nanomedicine. International Journal of Molecular Sciences, 21(22), 8702. https://doi.org/10.3390/ijms21228702






