Human Endometrial Carcinogenesis Is Associated with Significant Reduction in Long Non-Coding RNA, TERRA
Abstract
1. Introduction
2. Results
2.1. TERRA Transcription Is Observed in Normal Human Endometrium and the Levels Are Significantly Reduced in Endometrial Cancer
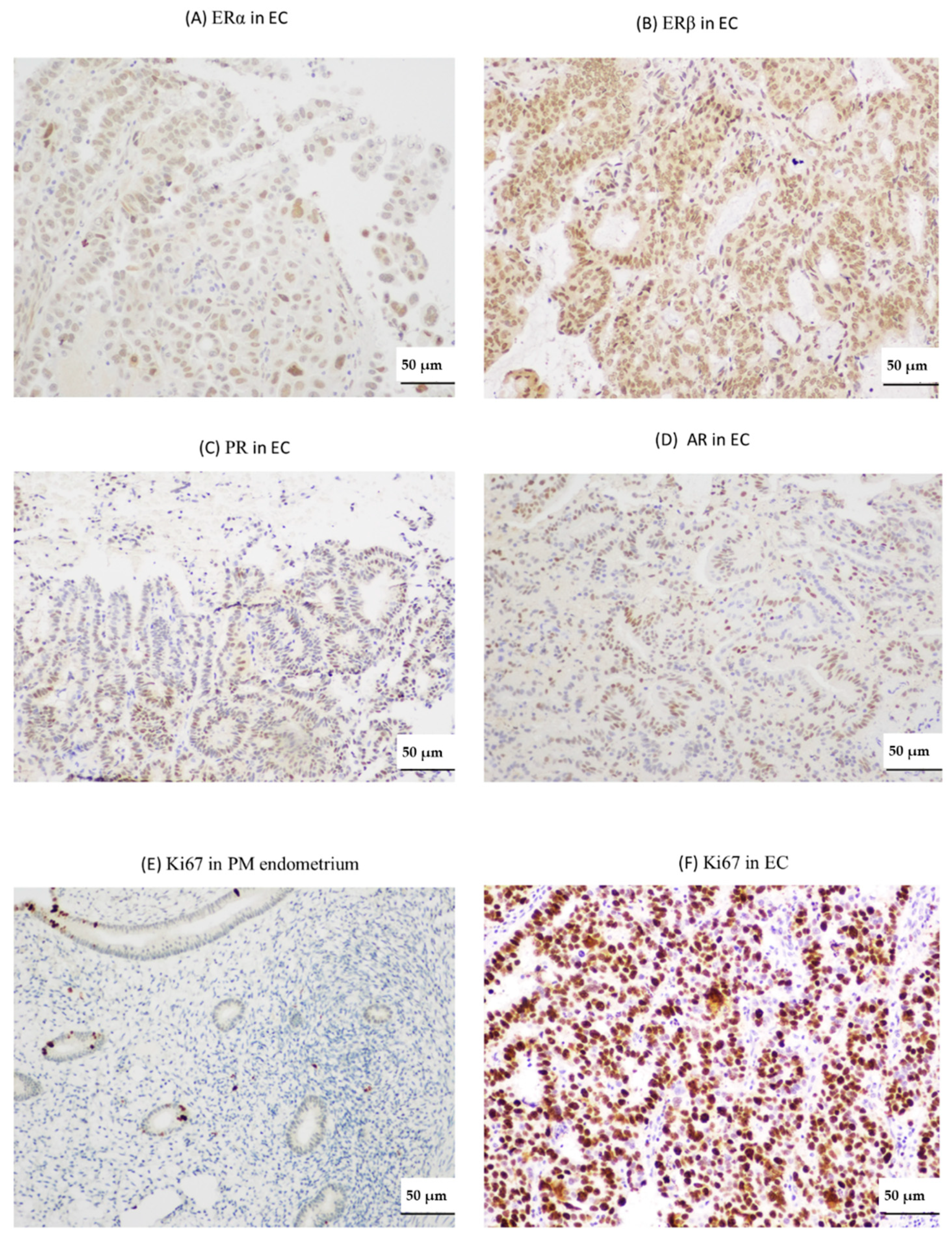
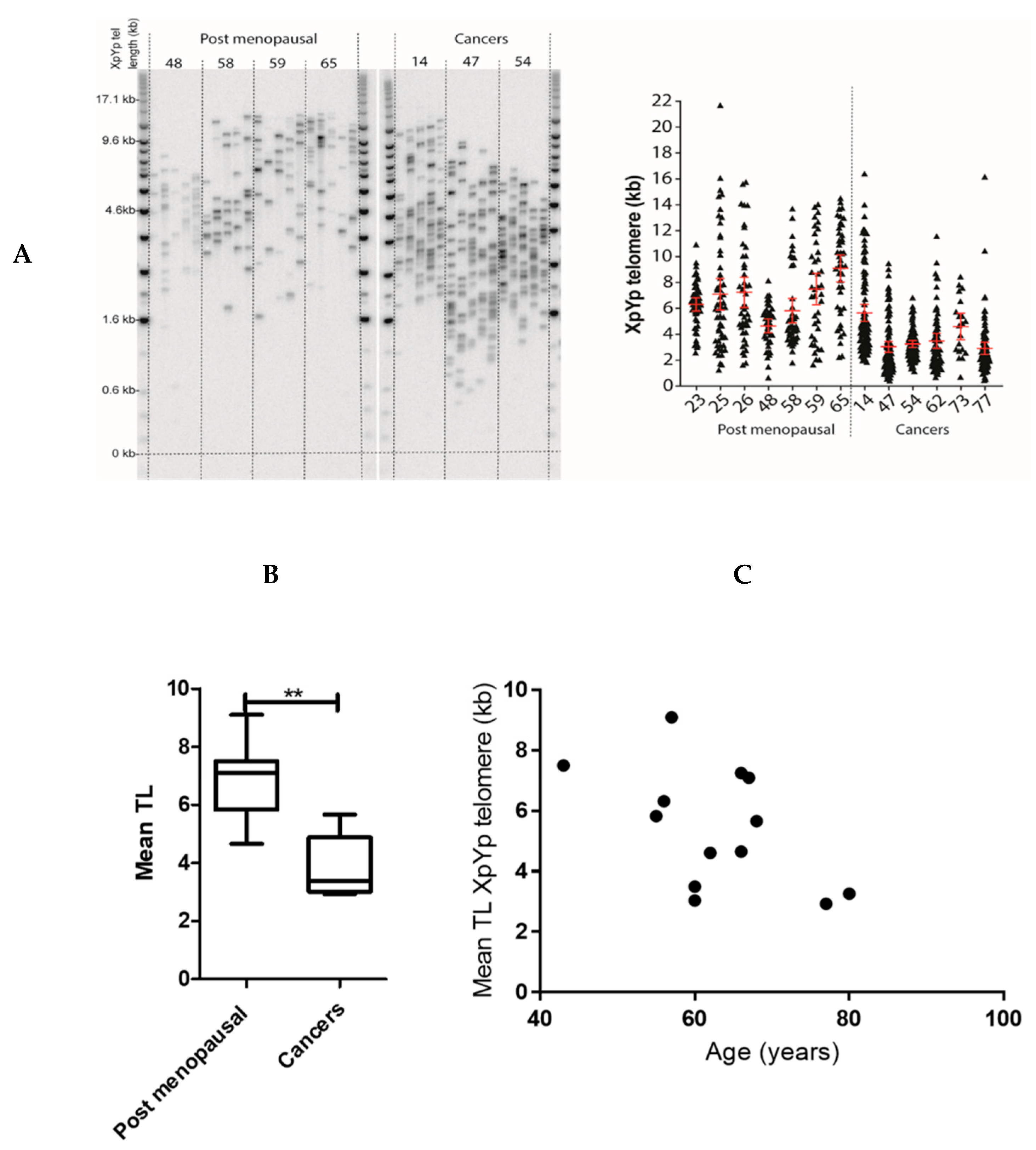
2.2. Endometrial TERRA Levels Correlated with Each Other, the Proliferative Marker Ki67, Steroid Receptor PR and Shelterin Protein TRF1 but Did Not Correlate with TA or with TLs at the XpYp Chromosomes
3. Discussion
4. Materials and Methods
4.1. Endometrial Tissue Samples
4.2. RNA Extraction and Real Time-qPCR
4.3. Telomerase Repeat Amplification Protocol (TRAP) Assay
4.4. Single Telomere Length Analysis (STELA)
4.5. Immunohistochemistry
4.6. Analysis of TCGA Dataset
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindemann, K.; Eskild, A.; Vatten, L.J.; Bray, F. Endometrial cancer incidence trends in Norway during 1953-2007 and predictions for 2008–2027. Int. J. Cancer 2010, 127, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- CRUK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer/mortality (accessed on 3 February 2020).
- Barcellini, A.; Roccio, M.; Laliscia, C.; Zanellini, F.; Pettinato, D.; Valvo, F.; Mirandola, A.; Orlandi, E.; Gadducci, A. Endometrial Cancer: When Upfront Surgery Is Not an Option. Oncology 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hapangama, D.K.; Kamal, A.; Saretzki, G. Implications of telomeres and telomerase in endometrial pathology. Hum. Reprod. Update 2017, 23, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Alnafakh, R.A.A.; Adishesh, M.; Button, L.; Saretzki, G.; Hapangama, D.K. Telomerase and Telomeres in Endometrial Cancer. Front. Oncol. 2019, 9, 344. [Google Scholar] [CrossRef]
- Smogorzewska, A.; de Lange, T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 2004, 73, 177–208. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Nergadze, S.G.; Farnung, B.O.; Wischnewski, H.; Khoriauli, L.; Vitelli, V.; Chawla, R.; Giulotto, E.; Azzalin, C.M. CpG-island promoters drive transcription of human telomeres. RNA 2009, 15, 2186–2194. [Google Scholar] [CrossRef]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef]
- Luke, B.; Panza, A.; Redon, S.; Iglesias, N.; Li, Z.; Lingner, J. The Rat1p 5′ to 3′ exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol. Cell 2008, 32, 465–477. [Google Scholar] [CrossRef]
- Vrbsky, J.; Akimcheva, S.; Watson, J.M.; Turner, T.L.; Daxinger, L.; Vyskot, B.; Aufsatz, W.; Riha, K. siRNA-mediated methylation of Arabidopsis telomeres. PLoS Genet. 2010, 6, e1000986. [Google Scholar] [CrossRef]
- Beishline, K.; Vladimirova, O.; Tutton, S.; Wang, Z.; Deng, Z.; Lieberman, P.M. CTCF driven TERRA transcription facilitates completion of telomere DNA replication. Nat. Commun. 2017, 8, 2114. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.J.; López de Silanes, I.; Graña, O.; Blasco, M.A. Telomeric RNAs are essential to maintain telomeres. Nat. Commun. 2016, 7, 12534. [Google Scholar] [CrossRef] [PubMed]
- Arnoult, N.; Van Beneden, A.; Decottignies, A. Telomere length regulates TERRA levels through increased trimethylation of telomeric H3K9 and HP1α. Nat. Struct. Mol. Biol. 2012, 19, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Smekalova, E.; Baumann, P. TERRA -a calling card for telomerase. Mol. Cell 2013, 51, 703–704. [Google Scholar] [CrossRef]
- Hirashima, K.; Seimiya, H. Telomeric repeat-containing RNA/G-quadruplex-forming sequences cause genome-wide alteration of gene expression in human cancer cells in vivo. Nucleic Acids Res. 2015, 43, 2022–2032. [Google Scholar] [CrossRef]
- Graf, M.; Bonetti, D.; Lockhart, A.; Serhal, K.; Kellner, V.; Maicher, A.; Jolivet, P.; Teixeira, M.T.; Luke, B. Telomere Length Determines TERRA and R-Loop Regulation through the Cell Cycle. Cell 2017, 170, 72–85.e14. [Google Scholar] [CrossRef]
- Redon, S.; Reichenbach, P.; Lingner, J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 2010, 38, 5797–5806. [Google Scholar] [CrossRef]
- Porro, A.; Feuerhahn, S.; Reichenbach, P.; Lingner, J. Molecular dissection of telomeric repeat-containing RNA biogenesis unveils the presence of distinct and multiple regulatory pathways. Mol. Cell. Biol. 2010, 30, 4808–4817. [Google Scholar] [CrossRef]
- Marion, R.M.; Montero, J.J.; Lopez de Silanes, I.; Grana-Castro, O.; Martinez, P.; Schoeftner, S.; Palacios-Fabrega, J.A.; Blasco, M.A. TERRA regulate the transcriptional landscape of pluripotent cells through TRF1-dependent recruitment of PRC2. eLife 2019, 8. [Google Scholar] [CrossRef]
- Hapangama, D.K.; Bulmer, J.N. Pathophysiology of heavy menstrual bleeding. Womens Health (Lond.) 2016, 12, 3–13. [Google Scholar] [CrossRef]
- Valentijn, A.J.; Saretzki, G.; Tempest, N.; Critchley, H.O.; Hapangama, D.K. Human endometrial epithelial telomerase is important for epithelial proliferation and glandular formation with potential implications in endometriosis. Hum. Reprod. 2015, 30, 2816–2828. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.M.; Simha, M.R. Telomerase and telomere length in normal and malignant human endometrium as prognostic markers. Indian J. Pathol. Microbiol. 2003, 46, 394–398. [Google Scholar] [PubMed]
- Saygan-Karamursel, B.; Dikmen, G.; Dogan, P.; Aksu, T.; Guven, S.; Ayhan, A. Quantitative telomerase activity in malignant, benign and normal gynecological tissues. Eur. J. Gynaecol. Oncol. 2005, 26, 83–86. [Google Scholar] [PubMed]
- Bradfield, A.; Button, L.; Drury, J.; Green, D.C.; Hill, C.J.; Hapangama, D.K. Investigating the Role of Telomere and Telomerase Associated Genes and Proteins in Endometrial Cancer. Methods Protoc. 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Boggess, J.F.; LaMarque, L.R.; Meyer, W.R.; Murray, M.J.; Fritz, M.A.; Lessey, B.A. A prospective, randomized study of endometrial telomerase during the menstrual cycle. J. Clin. Endocrinol. Metab. 2001, 86, 3912–3917. [Google Scholar] [CrossRef]
- Tanaka, M.; Kyo, S.; Takakura, M.; Kanaya, T.; Sagawa, T.; Yamashita, K.; Okada, Y.; Hiyama, E.; Inoue, M. Expression of telomerase activity in human endometrium is localized to epithelial glandular cells and regulated in a menstrual phase-dependent manner correlated with cell proliferation. Am. J. Pathol. 1998, 153, 1985–1991. [Google Scholar] [CrossRef]
- Wang, S.-J.; Sakamoto, T.; Yasuda, S.-i.; Fukasawa, I.; Ota, Y.; Hayashi, M.; Okura, T.; Zheng, J.-H.; Inaba, N. The Relationship between Telomere Length and Telomerase Activity in Gynecologic Cancers. Gynecol. Oncol. 2002, 84, 81–84. [Google Scholar] [CrossRef]
- Kyo, S.; Kanaya, T.; Ishikawa, H.; Ueno, H.; Inoue, M. Telomerase activity in gynecological tumors. Clin. Cancer Res. 1996, 2, 2023–2028. [Google Scholar]
- Kyo, S.; Takakura, M.; Kohama, T.; Inoue, M. Telomerase activity in human endometrium. Cancer Res. 1997, 57, 610–614. [Google Scholar]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef]
- Mathew, D.; Drury, J.A.; Valentijn, A.J.; Vasieva, O.; Hapangama, D.K. In silico, in vitro and in vivo analysis identifies a potential role for steroid hormone regulation of FOXD3 in endometriosis-associated genes. Hum. Reprod. 2016, 31, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, L.; Audry, J.; Matmati, S.; Arcangioli, B.; Geli, V.; Coulon, S. Eroded telomeres are rearranged in quiescent fission yeast cells through duplications of subtelomeric sequences. Nat. Commun. 2017, 8, 1684. [Google Scholar] [CrossRef] [PubMed]
- van Steensel, B.; de Lange, T. Control of telomere length by the human telomeric protein TRF1. Nature 1997, 385, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Schoeftner, S.; Blasco, M.A. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat. Cell Biol. 2008, 10, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Norseen, J.; Wiedmer, A.; Riethman, H.; Lieberman, P.M. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol. Cell 2009, 35, 403–413. [Google Scholar] [CrossRef]
- Hu, Y.; Bennett, H.W.; Liu, N.; Moravec, M.; Williams, J.F.; Azzalin, C.M.; King, M.C. RNA–DNA Hybrids Support Recombination-Based Telomere Maintenance in Fission Yeast. Genetics 2019, 213, 431–447. [Google Scholar] [CrossRef]
- Barthel, F.P.; Wei, W.; Tang, M.; Martinez-Ledesma, E.; Hu, X.; Amin, S.B.; Akdemir, K.C.; Seth, S.; Song, X.; Wang, Q.; et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat. Genet. 2017, 49, 349–357. [Google Scholar] [CrossRef]
- Baird, D.M.; Rowson, J.; Wynford-Thomas, D.; Kipling, D. Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat. Genet. 2003, 33, 203–207. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, L.; Lu, S. Role of TERRA in the regulation of telomere length. Int. J. Biol. Sci. 2015, 11, 316–323. [Google Scholar] [CrossRef]
- Cusanelli, E.; Romero, C.A.; Chartrand, P. Telomeric noncoding RNA TERRA is induced by telomere shortening to nucleate telomerase molecules at short telomeres. Mol. Cell 2013, 51, 780–791. [Google Scholar] [CrossRef]
- Takahama, K.; Takada, A.; Tada, S.; Shimizu, M.; Sayama, K.; Kurokawa, R.; Oyoshi, T. Regulation of telomere length by G-quadruplex telomere DNA- and TERRA-binding protein TLS/FUS. Chem. Biol. 2013, 20, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Porro, A.; Feuerhahn, S.; Lingner, J. TERRA-reinforced association of LSD1 with MRE11 promotes processing of uncapped telomeres. Cell Rep. 2014, 6, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.T.; Letsolo, B.T.; Jones, R.E.; Rowson, J.; Pratt, G.; Hewamana, S.; Fegan, C.; Pepper, C.; Baird, D.M. Telomere dysfunction and fusion during the progression of chronic lymphocytic leukemia: Evidence for a telomere crisis. Blood 2010, 116, 1899–1907. [Google Scholar] [CrossRef]
- Kamal, A.M.; Bulmer, J.N.; DeCruze, S.B.; Stringfellow, H.F.; Martin-Hirsch, P.; Hapangama, D.K. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br. J. Cancer 2016, 114, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Kupershmidt, I.; Su, Q.J.; Grewal, A.; Sundaresh, S.; Halperin, I.; Flynn, J.; Shekar, M.; Wang, H.; Park, J.; Cui, W.; et al. Ontology-based meta-analysis of global collections of high-throughput public data. PLoS ONE 2010, 5, e13066. [Google Scholar] [CrossRef]

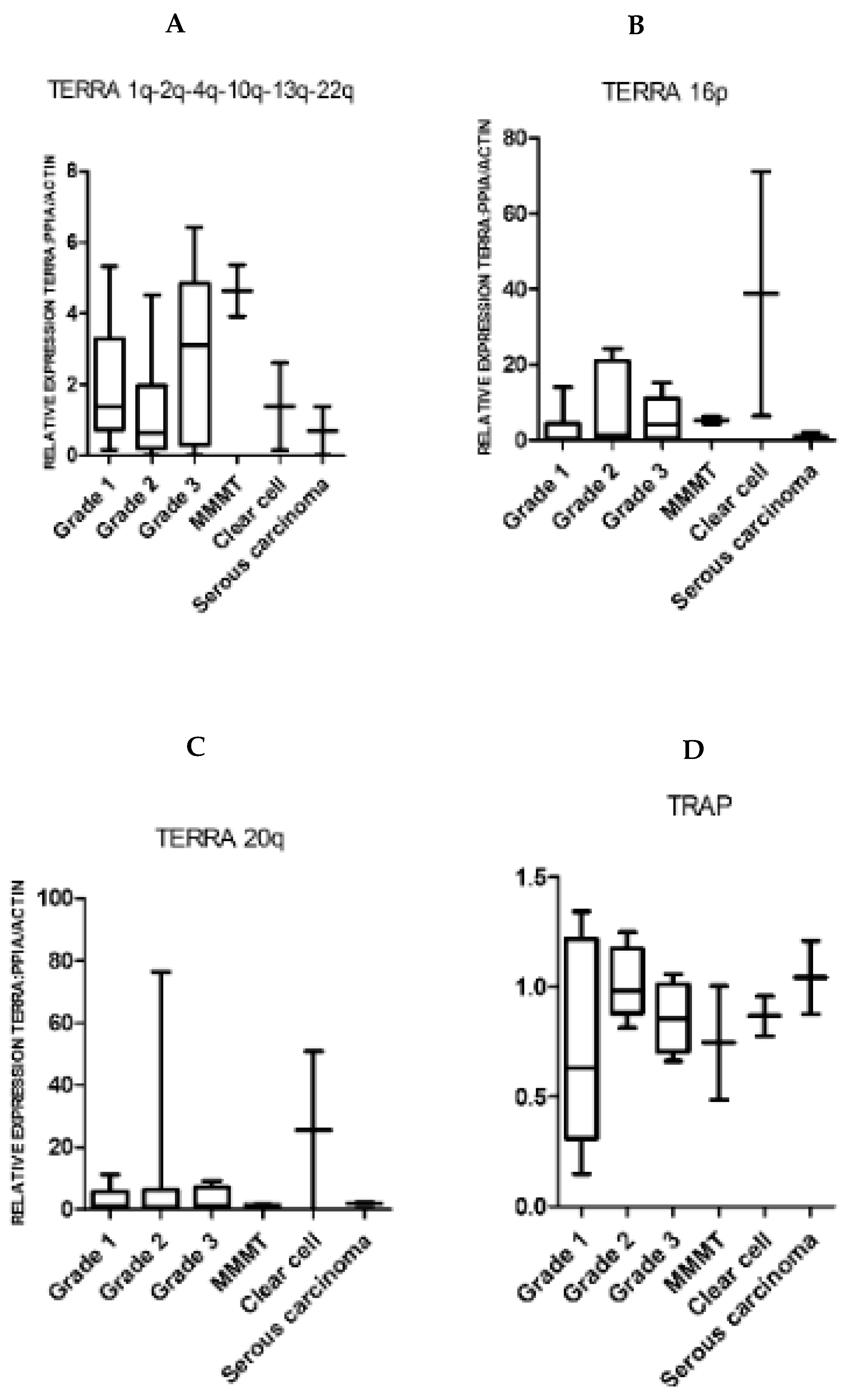
| TERRA1 | TERRA16 | TERRA20 | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| TERRA1 | 0.4885 | 0.0008 | 0.4841 | 0.0009 | ||
| TERRA16 | 0.4885 | 0.0008 | 0.7792 | <0.0001 | ||
| TERRA20 | 0.4841 | 0.0009 | 0.7792 | <0.0001 | ||
| AR | −0.08156 | 0.6465 | −0.2828 | 0.1051 | −0.1727 | 0.3288 |
| PR | −0.1068 | 0.5543 | −0.3997 | 0.0212 | −0.2094 | 0.2423 |
| ERα | −0.0579 | 0.7411 | 0.1462 | 0.4019 | 0.198 | 0.2543 |
| ERβ | 0.01555 | 0.9293 | 0.1325 | 0.4479 | 0.08311 | 0.635 |
| Ki67 | −0.147 | 0.3854 | −0.3487 | 0.0319 | −0.4156 | 0.0095 |
| TRF1 | 0.4547 | 0.0505 | 0.4414 | 0.0585 | 0.7083 | 0.0007 |
| TRF2 | 0.08588 | 0.8432 | 0.4269 | 0.2499 | 0.4809 | 0.1938 |
| TRAP | −0.1997 | 0.2292 | −0.251 | 0.1285 | −0.09328 | 0.5775 |
| Study Groups (n) | * Age (years) | * BMI (kg /m2) |
|---|---|---|
| Proliferative phase (7) | 43 (32–57) | 27.8 (22–40.5) |
| Secretory phase (9) | 41 (21–47) | 22.6 (18.9–31.6) |
| Postmenopausal (7) | 62 (52–85) | 24.3 (20–39.6) |
| Total Endometrial cancer pts (24) | 67 (37–80) | 30 (23.9–54.4) |
| Endometrioid Grade 1 (6/24) | 61 (46–73) | 37.8 (28.3–46.1) |
| Endometrioid Grade 2 (7/24) | 60 (37–77) | 28.9 (25.8–54.4) |
| Endometrioid Grade 3 (5/24) | 68 (60–80) | 29.8 (23.9–42.7) |
| Malignant Mixed Mullerian Tumor (2/24) | 72.5 (65–80) | 28.6 (24.2–32.9) |
| Clear Cell Carcinoma (2/24) | 71.5 (61–82) | 28.4 (26.6–30.1) |
| Serous Carcinoma (2/24) | 73 (68–78) | 32.7 (NK **-32.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adishesh, M.; Alnafakh, R.; Baird, D.M.; Jones, R.E.; Simon, S.; Button, L.; Kamal, A.M.; Kirwan, J.; DeCruze, S.B.; Drury, J.; et al. Human Endometrial Carcinogenesis Is Associated with Significant Reduction in Long Non-Coding RNA, TERRA. Int. J. Mol. Sci. 2020, 21, 8686. https://doi.org/10.3390/ijms21228686
Adishesh M, Alnafakh R, Baird DM, Jones RE, Simon S, Button L, Kamal AM, Kirwan J, DeCruze SB, Drury J, et al. Human Endometrial Carcinogenesis Is Associated with Significant Reduction in Long Non-Coding RNA, TERRA. International Journal of Molecular Sciences. 2020; 21(22):8686. https://doi.org/10.3390/ijms21228686
Chicago/Turabian StyleAdishesh, Meera, Rafah Alnafakh, Duncan M. Baird, Rhiannon E. Jones, Shannon Simon, Lucy Button, Areege M. Kamal, John Kirwan, S. Bridget DeCruze, Josephine Drury, and et al. 2020. "Human Endometrial Carcinogenesis Is Associated with Significant Reduction in Long Non-Coding RNA, TERRA" International Journal of Molecular Sciences 21, no. 22: 8686. https://doi.org/10.3390/ijms21228686
APA StyleAdishesh, M., Alnafakh, R., Baird, D. M., Jones, R. E., Simon, S., Button, L., Kamal, A. M., Kirwan, J., DeCruze, S. B., Drury, J., Saretzki, G., & Hapangama, D. K. (2020). Human Endometrial Carcinogenesis Is Associated with Significant Reduction in Long Non-Coding RNA, TERRA. International Journal of Molecular Sciences, 21(22), 8686. https://doi.org/10.3390/ijms21228686





