High-Light versus Low-Light: Effects on Paired Photosystem II Supercomplex Structural Rearrangement in Pea Plants
Abstract
1. Introduction
2. Results and Discussion
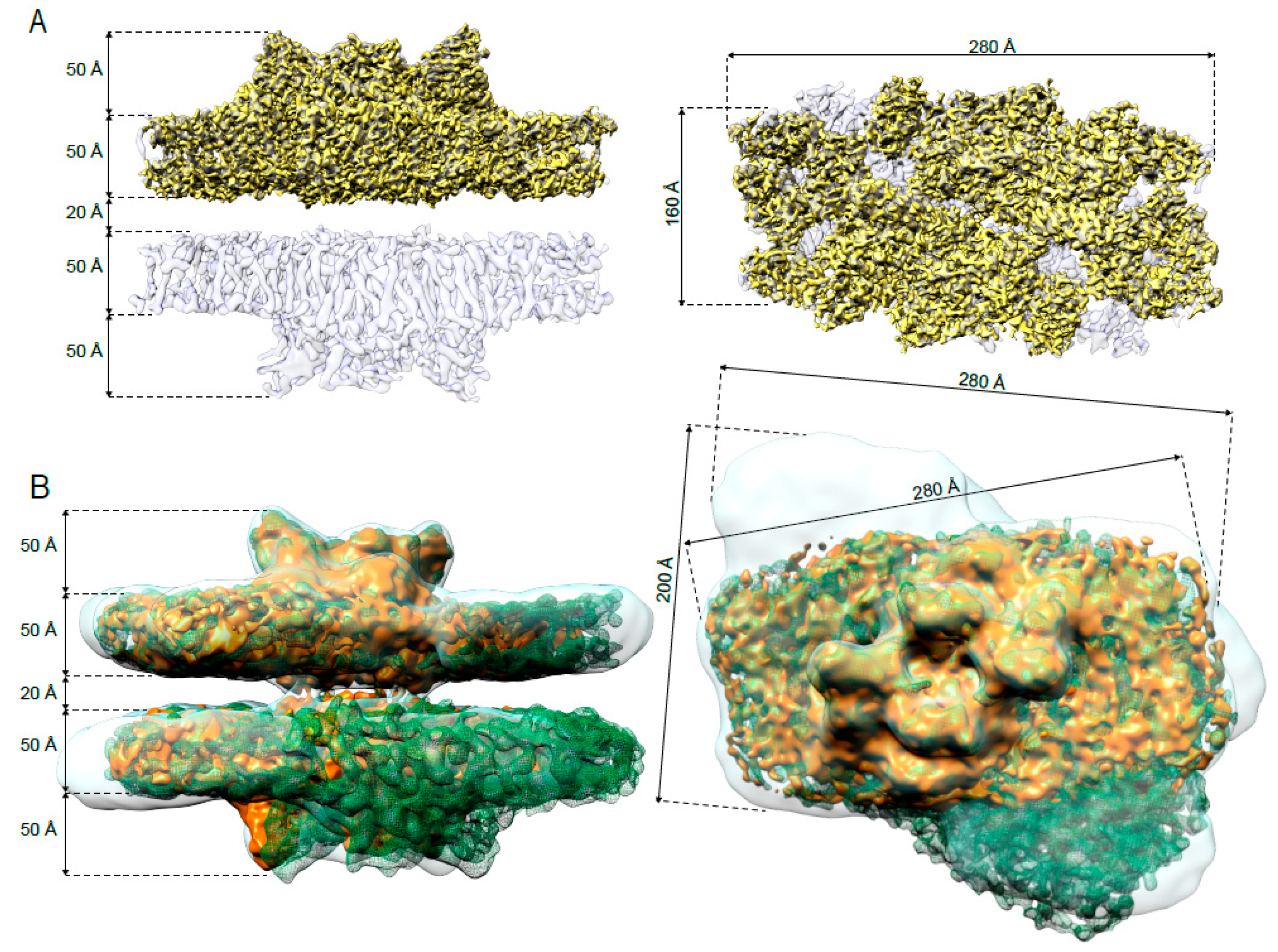
2.1. Cryo-EM Maps of Paired PSII-LHCII Supercomplexes Isolated from Plants Grown under High-Light and Low-Light
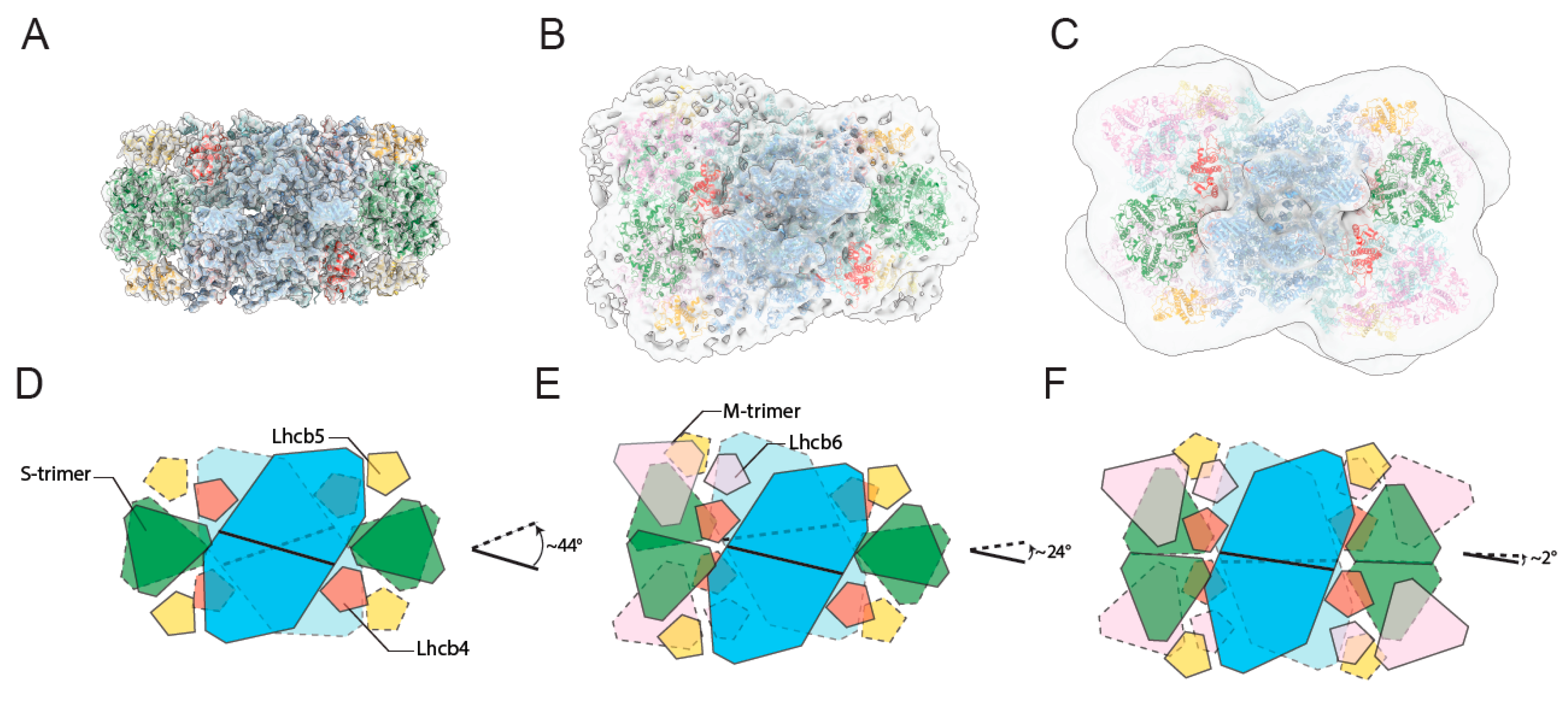
2.2. In Situ Arrangement of Paired PSII-LHCII Supercomplexes in Isolated Stacked Thylakoid Membranes
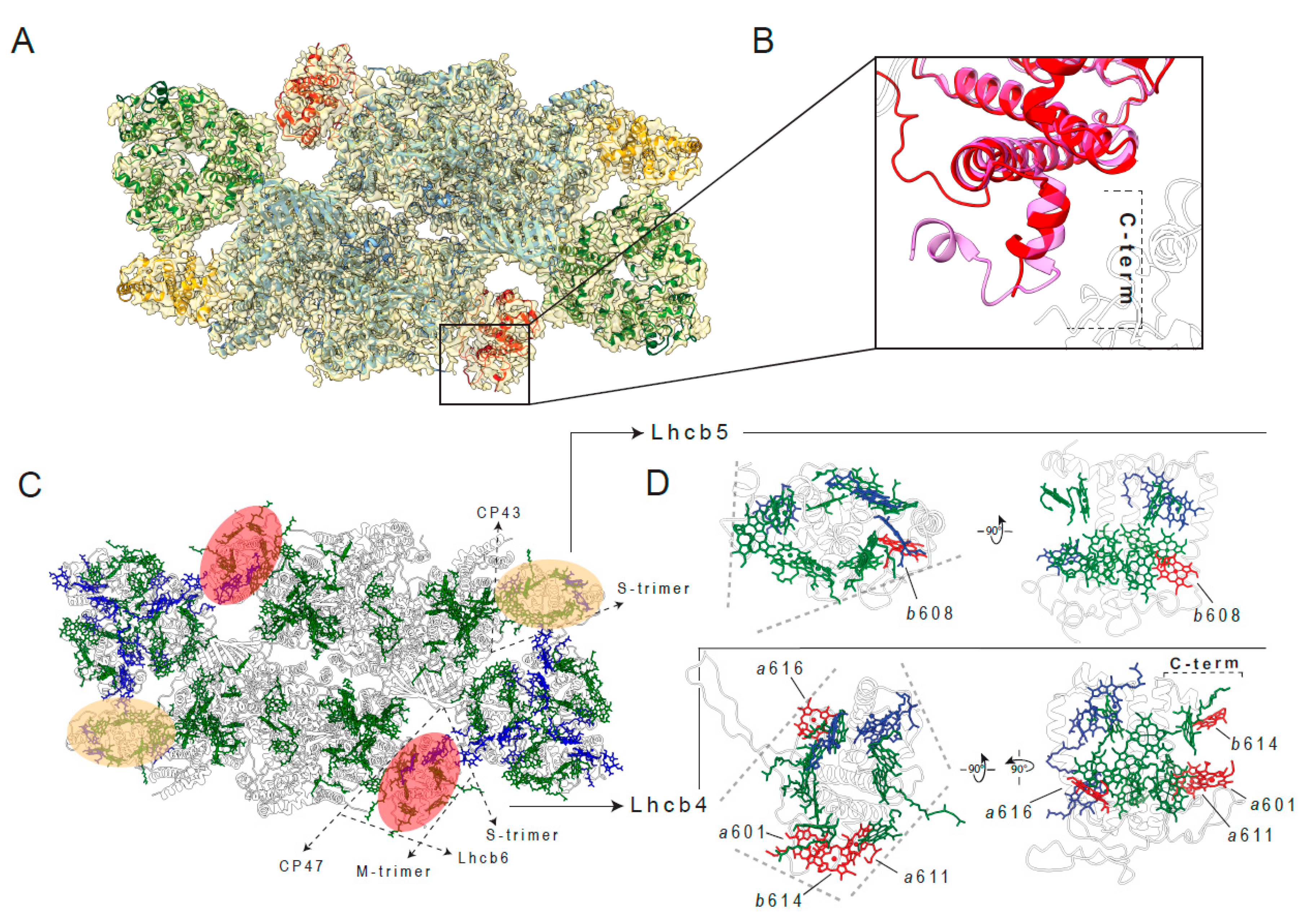
2.3. Peculiar Structural Features of the C2S2 Supercomplex in Plants Long-Term Acclimated to High-Light
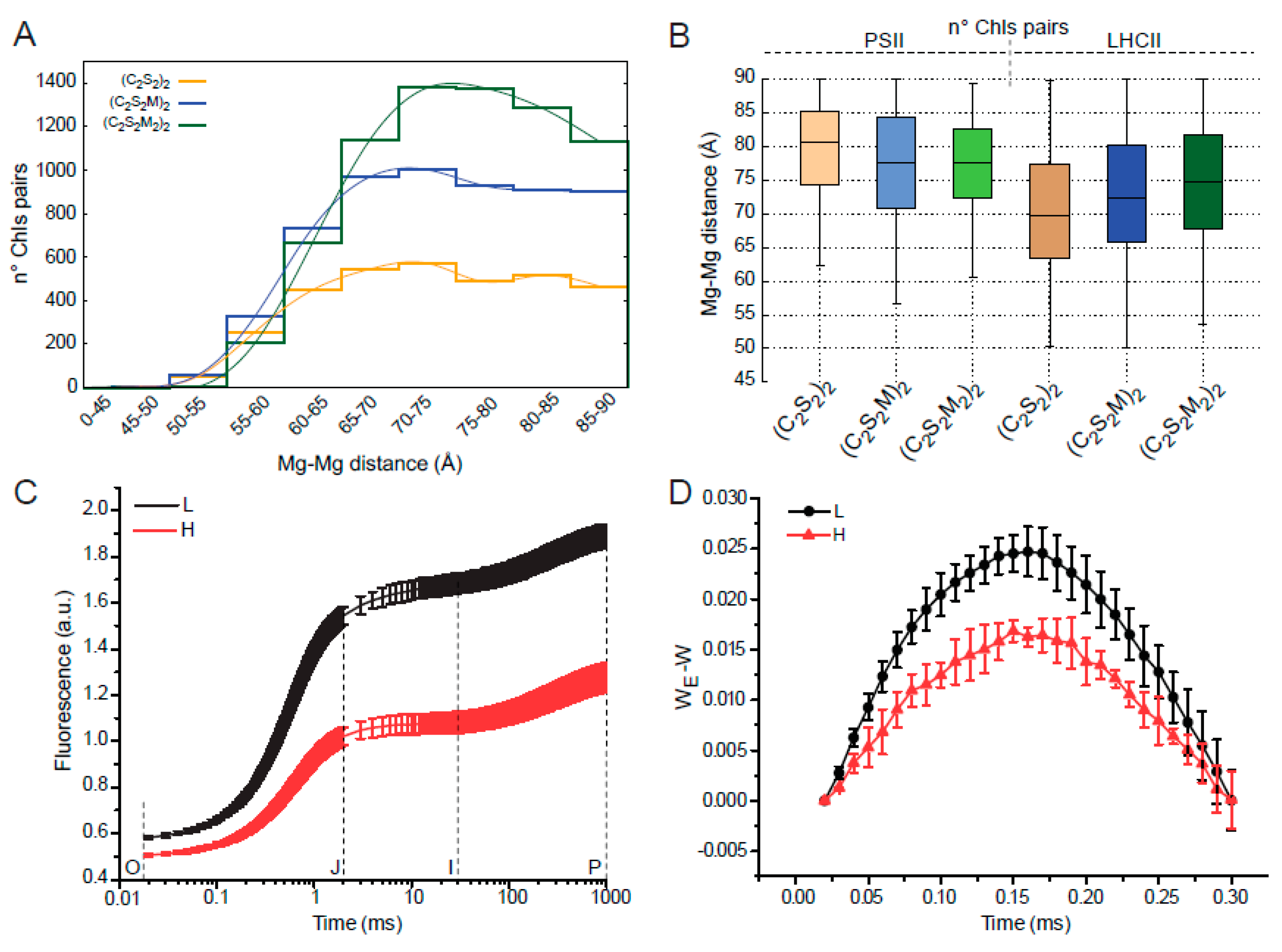
2.4. The Different Structural Arrangement of Paired PSII-LHCII Supercomplexes in High-Light and Low-Light Modifies Their Structural Interaction and Energetic Connectivity
3. Conclusions
4. Materials and Methods
4.1. Plant Growth and Isolation of Thylakoid Membranes
4.2. Cryo-ET Sample Preparation, Data Collection and Tomogram Reconstruction
4.3. Isolation of PSII-LHCII Supercomplexes
4.4. Cryo-EM Sample Preparation and Data Collection
4.5. Cryo-EM Data Processing and 3D Reconstruction
4.6. Modelling and Bioinformatics Tools
4.7. Chlorophyll Distance Measurements
4.8. Chlorophyll a Fluorescence Induction Measurements
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Lichtenthaler, H.K.; Buschmann, C.; Fietz, H.-J.; Bach, T.; Kozel, U.; Meier, D.; Rahmsdorf, U. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 1981, 2, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Mc Millen, G.G.; Mc Clendon, J.H. Leaf Angle: An Adaptive Feature of Sun and Shade Leaves. Int. J. Plant Sci. 1979, 140, 437–442. [Google Scholar] [CrossRef]
- Kasahara, M.; Kagawa, T.; Oikawa, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast avoidance movement reduces photodamage in plants. Nat. Cell Biol. 2002, 420, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Chow, W.; Goodchild, D. Thylakoid Membrane Organisation in Sun/Shade Acclimation. Funct. Plant Biol. 1988, 15, 11–26. [Google Scholar] [CrossRef]
- Barber, J. Photosystem II: The engine of life. Q. Rev. Biophys. 2003, 36, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J. Photoregulation of the Composition, Function, and Structure of Thylakoid Membranes. Annu. Rev. Plant Biol. 1986, 37, 93–136. [Google Scholar] [CrossRef]
- Daum, B.; Nicastro, D.; Austin, J.; McIntosh, J.R.; Kühlbrandt, W. Arrangement of Photosystem II and ATP Synthase in Chloroplast Membranes of Spinach and Pea. Plant Cell 2010, 22, 1299–1312. [Google Scholar] [CrossRef]
- Dekker, J.P.; Boekema, E.J. Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 12–39. [Google Scholar] [CrossRef]
- Caffarri, S.; Kouril, R.; Kereïche, S.; Boekema, E.J.; Croce, R. Functional architecture of higher plant photosystem II supercomplexes. EMBO J. 2009, 28, 3052–3063. [Google Scholar] [CrossRef]
- Kouril, R.; Wientjes, E.; Bultema, J.B.; Croce, R.; Boekema, E.J. High-light vs. low-light: Effect of light acclimation on photosystem II composition and organization in Arabidopsis thaliana. Biochim. Biophys. Acta Gen. Subj. 2013, 1827, 411–419. [Google Scholar] [CrossRef]
- Albanese, P.; Manfredi, M.; Meneghesso, A.; Marengo, E.; Saracco, G.; Barber, J.; Morosinotto, T.; Pagliano, C. Dynamic reorganization of photosystem II supercomplexes in response to variations in light intensities. Biochim. Biophys. Acta Gen. Subj. 2016, 1857, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Bielczynski, L.W.; Schansker, G.; Croce, R. Effect of Light Acclimation on the Organization of Photosystem II Super- and Sub-Complexes in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Klimmek, F.; Sjödin, A.; Noutsos, C.; Leister, D.; Jansson, S. Abundantly and Rarely Expressed Lhc Protein Genes Exhibit Distinct Regulation Patterns in Plants. Plant Physiol. 2006, 140, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Grebe, S.; Trotta, A.; A Bajwa, A.; Suorsa, M.; Gollan, P.J.; Jansson, S.; Tikkanen, M.; Aro, E.-M. The unique photosynthetic apparatus of Pinaceae: Analysis of photosynthetic complexes in Picea abies. J. Exp. Bot. 2019, 70, 3211–3225. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.E.; O’Cualain, R.; Selley, J.; Knight, D.; Karim, M.F.; Hubbard, S.J.; Johnson, G.N. Dynamic Acclimation to High Light in Arabidopsis thaliana Involves Widespread Reengineering of the Leaf Proteome. Front. Plant Sci. 2017, 8, 1239. [Google Scholar] [CrossRef] [PubMed]
- Albanese, P.; Manfredi, M.; Re, A.; Marengo, E.; Saracco, G.; Pagliano, C. Thylakoid proteome modulation in pea plants grown at different irradiances: Quantitative proteomic profiling in a non-model organism aided by transcriptomic data integration. Plant J. 2018, 96, 786–800. [Google Scholar] [CrossRef]
- Albanese, P.; Tamara, S.; Saracco, G.; Scheltema, R.A.; Pagliano, C. How paired PSII-LHCII supercomplexes mediate the stacking of plant thylakoid membranes unveiled by structural mass-spectrometry. Nat. Commun. 2020, 11, 1361. [Google Scholar] [CrossRef]
- Kouřil, R.; Dekker, J.P.; Boekema, E.J. Supramolecular organization of photosystem II in green plants. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 2–12. [Google Scholar] [CrossRef]
- Kirchhoff, H.; Haase, W.; Haferkamp, S.; Schott, T.; Borinski, M.; Kubitscheck, U.; Rögner, M. Structural and functional self-organization of Photosystem II in grana thylakoids. Biochim. Biophys. Gen. Subj. 2007, 1767, 1180–1188. [Google Scholar] [CrossRef]
- Wei, X.; Su, X.; Cao, P.; Liu, X.; Chang, W.; Li, M.; Zhang, X.; Liu, Z. Structure of spinach photosystem II–LHCII supercomplex at 3.2 Å resolution. Nat. Cell Biol. 2016, 534, 69–74. [Google Scholar] [CrossRef]
- Van Bezouwen, L.S.; Caffarri, S.; Kale, R.S.; Kouřil, R.; Thunnissen, A.-M.W.H.; Oostergetel, G.T.; Boekema, E.J. Subunit and chlorophyll organization of the plant photosystem II supercomplex. Nat. Plants 2017, 3, 17080. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Ma, J.; Wei, X.; Cao, P.; Zhu, D.; Chang, W.; Liu, Z.; Zhang, X.; Li, M. Structure and assembly mechanism of plant C2S2M2-type PSII-LHCII supercomplex. Science 2017, 357, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.; van Amerongen, H. Light harvesting in oxygenic photosynthesis: Structural biology meets spectroscopy. Science 2020, 369, eaay2058. [Google Scholar] [CrossRef]
- Albanese, P.; Melero, R.; Engel, B.D.; Grinzato, A.; Roberto, M.; Manfredi, M.; Chiodoni, A.; Vargas, J.; Sorzano, C.; Óscar, S.; et al. Pea PSII-LHCII supercomplexes form pairs by making connections across the stromal gap. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nield, J.; Orlova, E.V.; Morris, E.P.; Gowen, B.; Van Heel, M.; Barber, J. 3D map of the plant photosystem II supercomplex obtained by cryoelectron microscopy and single particle analysis. Nat. Genet. 2000, 7, 44–47. [Google Scholar] [CrossRef]
- Izawa, S.; Good, N.E. Effect of Salts and Electron Transport on the Conformation of Isolated Chloroplasts. II. Electron Microscopy. Plant Physiol. 1966, 41, 544–552. [Google Scholar] [CrossRef]
- Standfuss, J.; Van Scheltinga, A.C.T.; Lamborghini, M.; Kühlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. EMBO J. 2005, 24, 919–928. [Google Scholar] [CrossRef]
- Wan, T.; Li, M.; Zhao, X.; Zhang, J.; Liu, Z.; Chang, W. Crystal Structure of a Multilayer Packed Major Light-Harvesting Complex: Implications for Grana Stacking in Higher Plants. Mol. Plant 2014, 7, 916–919. [Google Scholar] [CrossRef]
- Pribil, M.; Labs, M.; Leister, D. Structure and dynamics of thylakoids in land plants. J. Exp. Bot. 2014, 65, 1955–1972. [Google Scholar] [CrossRef]
- Austin, J.R.; Staehelin, L.A. Three-Dimensional Architecture of Grana and Stroma Thylakoids of Higher Plants as Determined by Electron Tomography. Plant Physiol. 2011, 155, 1601–1611. [Google Scholar] [CrossRef]
- Anderson, J.M.; Horton, P.; Kim, E.-H.; Chow, W.S. Towards elucidation of dynamic structural changes of plant thylakoid architecture. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Schröppel-Meier, G.; Kaiser, W.M. Ion homeostasis in chloroplasts under salinity and mineral deficiency. II. Solute distribution between chloroplasts and extrachloroplastic space under excess or deficiency of sulfate, phosphate, or magnesium. Plant Physiol. 1988, 87, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Barera, S.; Pagliano, C.; Pape, T.; Saracco, G.; Barber, J. Characterization of PSII–LHCII supercomplexes isolated from pea thylakoid membrane by one-step treatment with α- and β-dodecyl-d-maltoside. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, H.; Hall, C.; Wood, M.; Herbstová, M.; Tsabari, O.; Nevo, R.; Charuvi, D.; Shimoni, E.; Reich, Z. Dynamic control of protein diffusion within the granal thylakoid lumen. Proc. Natl. Acad. Sci. USA 2011, 108, 20248–20253. [Google Scholar] [CrossRef]
- Wietrzynski, W.; Schaffer, M.; Tegunov, D.; Albert, S.; Kanazawa, A.; Plitzko, J.M.; Baumeister, W.; Engel, B.D. Charting the native architecture of Chlamydomonas thylakoid membranes with single-molecule precision. eLife 2020, 9. [Google Scholar] [CrossRef]
- Anderson, J.M. Lateral heterogeneity of plant thylakoid protein complexes: Early reminiscences. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3384–3388. [Google Scholar] [CrossRef]
- Wood, W.H.; Barnett, S.F.; Flannery, S.; Hunter, C.N.; Johnson, M.P. Dynamic Thylakoid Stacking Is Regulated by LHCII Phosphorylation but Not Its interaction with PSI. Plant Physiol. 2019, 180, 2152–2166. [Google Scholar] [CrossRef]
- Jansson, S. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 1999, 4, 236–240. [Google Scholar] [CrossRef]
- Sawchuk, M.G.; Donner, T.J.; Head, P.; Scarpella, E. Unique and Overlapping Expression Patterns among Members of Photosynthesis-Associated Nuclear Gene Families in Arabidopsis. Plant Physiol. 2008, 148, 1908–1924. [Google Scholar] [CrossRef]
- Alboresi, A.; Dall’Osto, L.; Aprile, A.; Carillo, P.; Roncaglia, E.; Cattivelli, L.; Bassi, R. Reactive oxygen species and transcript analysis upon excess light treatment in wild-type Arabidopsis thaliana vs. a photosensitive mutant lacking zeaxanthin and lutein. BMC Plant Biol. 2011, 11, 62. [Google Scholar] [CrossRef]
- Floris, M.; Bassi, R.; Robaglia, C.; Alboresi, A.; Lanet, E. Post-transcriptional control of light-harvesting genes expression under light stress. Plant Mol. Biol. 2013, 82, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Albanese, P.; Manfredi, M.; Marengo, E.; Saracco, G.; Pagliano, C. Structural and functional differentiation of the light-harvesting protein Lhcb4 during land plant diversification. Physiol. Plant. 2019, 166, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Li, M.; Wan, T.; Wang, L.; Jia, C.; Hou, Z.; Zhao, X.; Zhang, J.; Chang, W. Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat. Struct. Mol. Biol. 2011, 18, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Koochak, H.; Puthiyaveetil, S.; Mullendore, D.L.; Li, M.; Kirchhoff, H. The structural and functional domains of plant thylakoid membranes. Plant J. 2018, 97, 412–429. [Google Scholar] [CrossRef] [PubMed]
- Şener, M.; Strümpfer, J.; Hsin, J.; Chandler, D.; Scheuring, S.; Hunter, C.N.; Schulten, K. Förster Energy Transfer Theory as Reflected in the Structures of Photosynthetic Light-Harvesting Systems. ChemPhysChem 2011, 12, 518–531. [Google Scholar] [CrossRef]
- Strasser, R.J.; Stirbet, A.D. Estimation of the energetic connectivity of PS II centres in plants using the fluorescence rise O–J–I–P: Fitting of experimental data to three different PS II models. Math. Comput. Simul. 2001, 56, 451–462. [Google Scholar] [CrossRef]
- Stirbet, A. Excitonic connectivity between photosystem II units: What is it, and how to measure it? Photosynth. Res. 2013, 116, 189–214. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Palovcak, E.; Armache, J.-P.; Verba, K.A.; Cheng, Y.; Agard, S. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 2017, 14, 331–332. [Google Scholar] [CrossRef]
- Kremer, J.R.; Mastronarde, D.N.; Mc Intosh, J.R. Computer Visualization of Three-Dimensional Image Data Using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef]
- Li, X.; Mooney, P.; Zheng, S.; Booth, C.R.; Braunfeld, M.B.; Gubbens, S.; Agard, D.A.; Cheng, Y. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 2013, 10, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 2016, 193, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scheres, S.H. RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 2012, 180, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Peng, L.; Baldwin, P.R.; Mann, D.S.; Jiang, W.; Rees, I.; Ludtke, S.J. EMAN2: An extensible image processing suite for electron microscopy. J. Struct. Biol. 2007, 157, 38–46. [Google Scholar] [CrossRef]
- Punjani, A.; Rubinstein, J.L.; Fleet, D.J.; Brubaker, M.A. cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 2017, 14, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Scheres, S.H.; Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 2012, 9, 853–854. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Afonine, P.V.; Grosse-Kunstleve, R.W.; Echols, N.; Headd, J.J.; Moriarty, N.W.; Mustyakimov, M.; Terwilliger, T.C.; Urzhumtsev, A.; Zwart, P.H.; Adams, P.D. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 352–367. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development ofCoot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Joliot, P.; Joliot, A. Excitation transfer between photosynthetic units: The 1964 experiment. Photosynth. Res. 2003, 76, 241–245. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grinzato, A.; Albanese, P.; Marotta, R.; Swuec, P.; Saracco, G.; Bolognesi, M.; Zanotti, G.; Pagliano, C. High-Light versus Low-Light: Effects on Paired Photosystem II Supercomplex Structural Rearrangement in Pea Plants. Int. J. Mol. Sci. 2020, 21, 8643. https://doi.org/10.3390/ijms21228643
Grinzato A, Albanese P, Marotta R, Swuec P, Saracco G, Bolognesi M, Zanotti G, Pagliano C. High-Light versus Low-Light: Effects on Paired Photosystem II Supercomplex Structural Rearrangement in Pea Plants. International Journal of Molecular Sciences. 2020; 21(22):8643. https://doi.org/10.3390/ijms21228643
Chicago/Turabian StyleGrinzato, Alessandro, Pascal Albanese, Roberto Marotta, Paolo Swuec, Guido Saracco, Martino Bolognesi, Giuseppe Zanotti, and Cristina Pagliano. 2020. "High-Light versus Low-Light: Effects on Paired Photosystem II Supercomplex Structural Rearrangement in Pea Plants" International Journal of Molecular Sciences 21, no. 22: 8643. https://doi.org/10.3390/ijms21228643
APA StyleGrinzato, A., Albanese, P., Marotta, R., Swuec, P., Saracco, G., Bolognesi, M., Zanotti, G., & Pagliano, C. (2020). High-Light versus Low-Light: Effects on Paired Photosystem II Supercomplex Structural Rearrangement in Pea Plants. International Journal of Molecular Sciences, 21(22), 8643. https://doi.org/10.3390/ijms21228643








