Injectable Gel Form of a Decellularized Bladder Induces Adipose-Derived Stem Cell Differentiation into Smooth Muscle Cells In Vitro
Abstract
1. Introduction
2. Results and Discussion
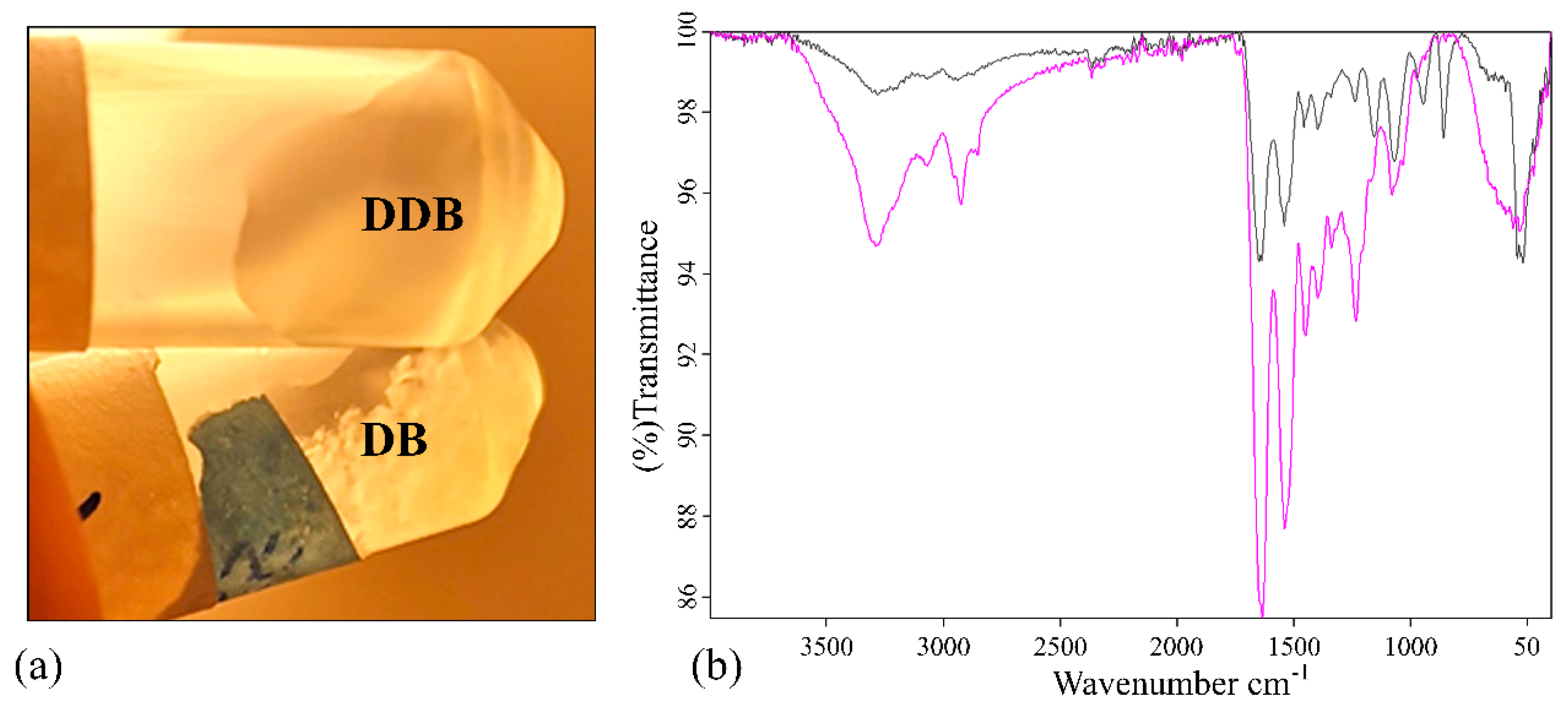
2.1. Material Characterization
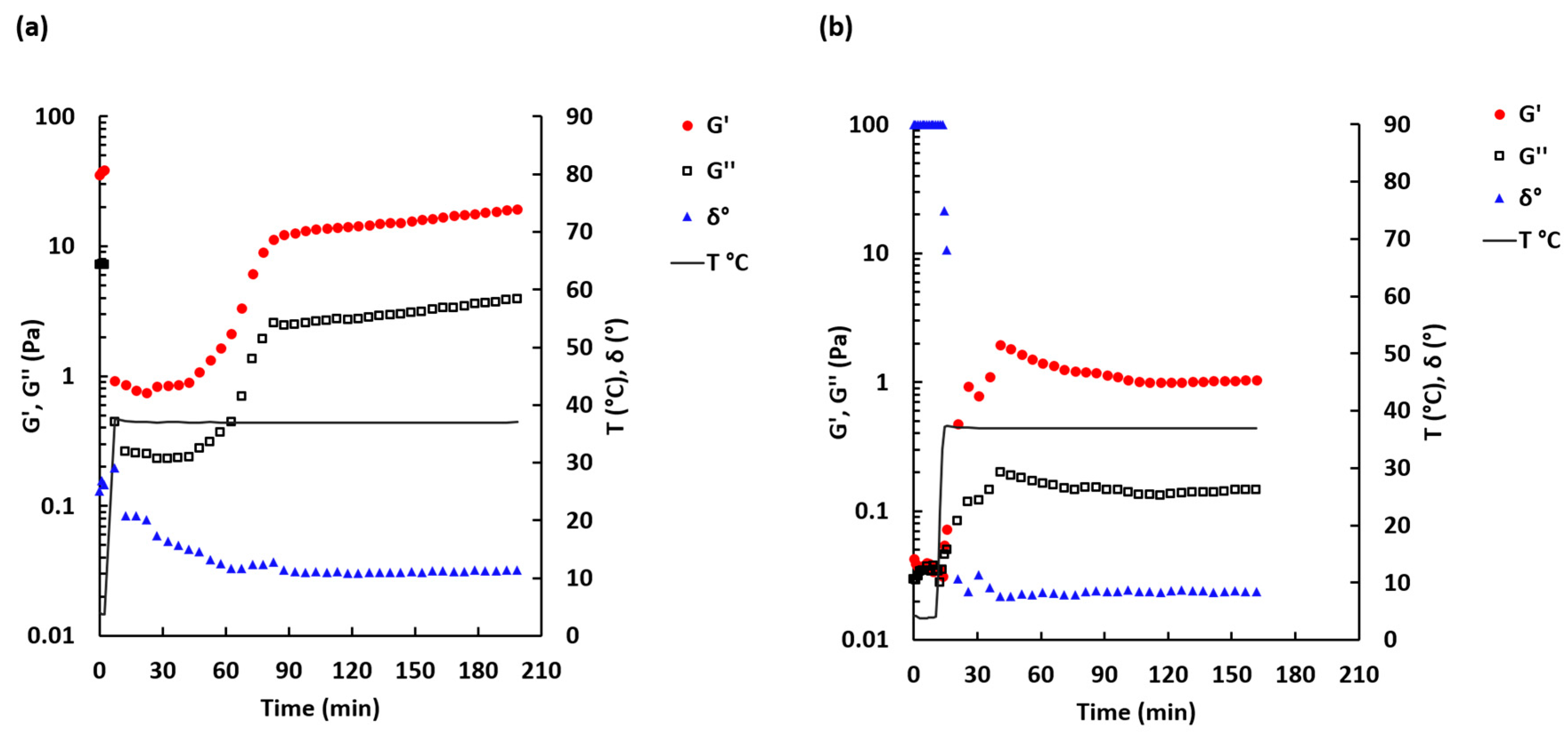
2.2. Rheological Properties
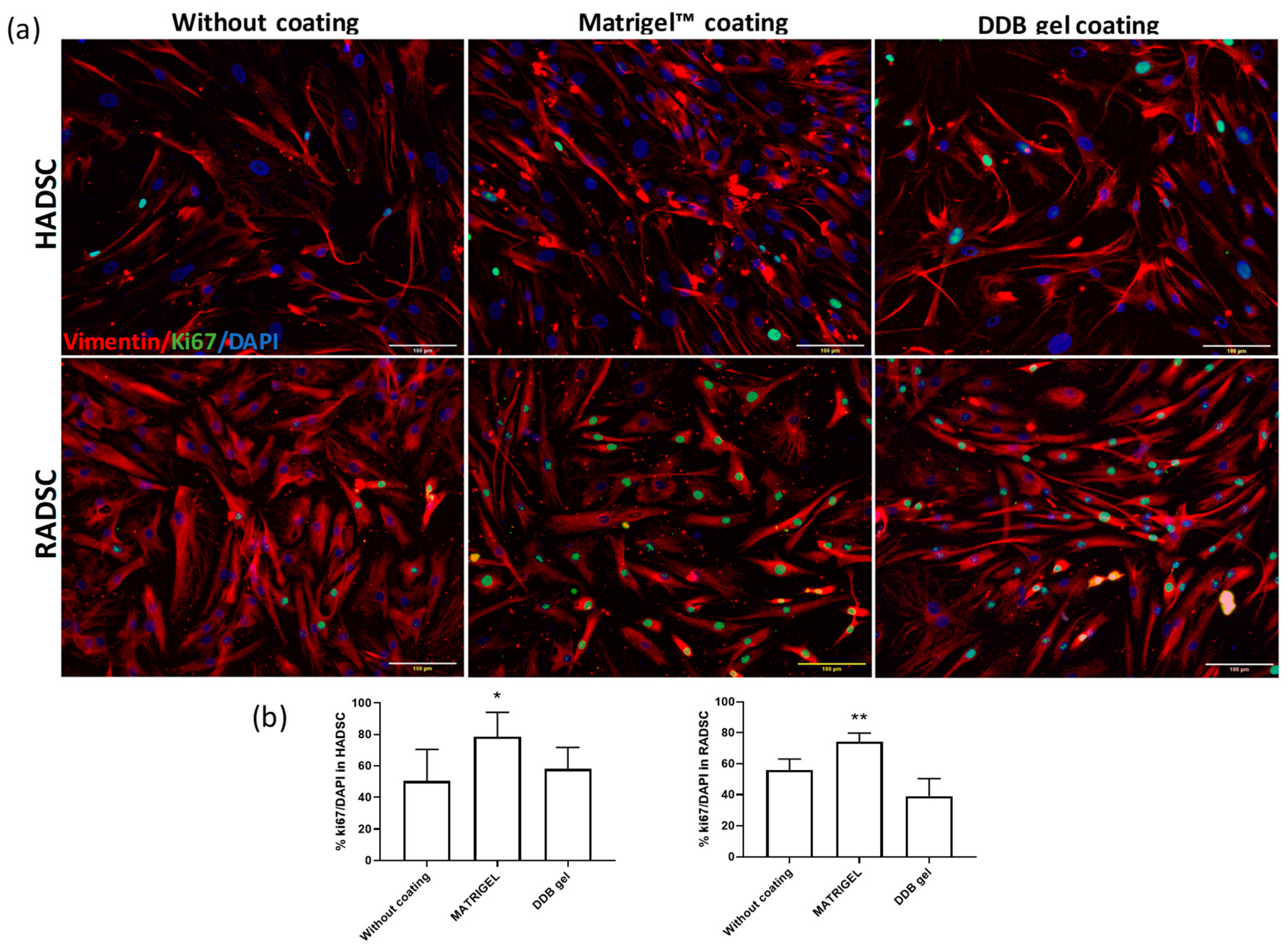
2.3. Cell Proliferation of ADSC
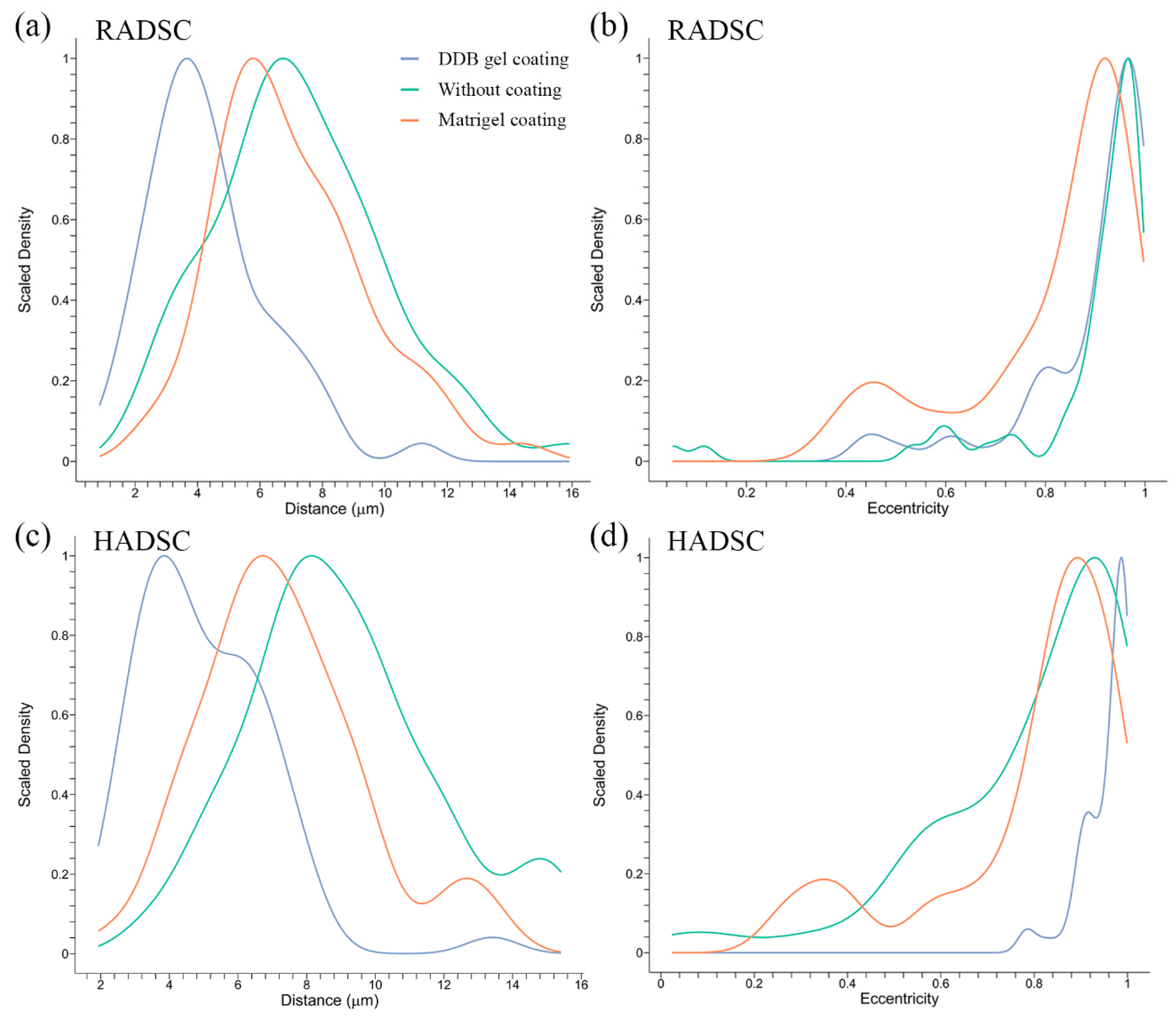
2.4. Cell Distances and Eccentricity
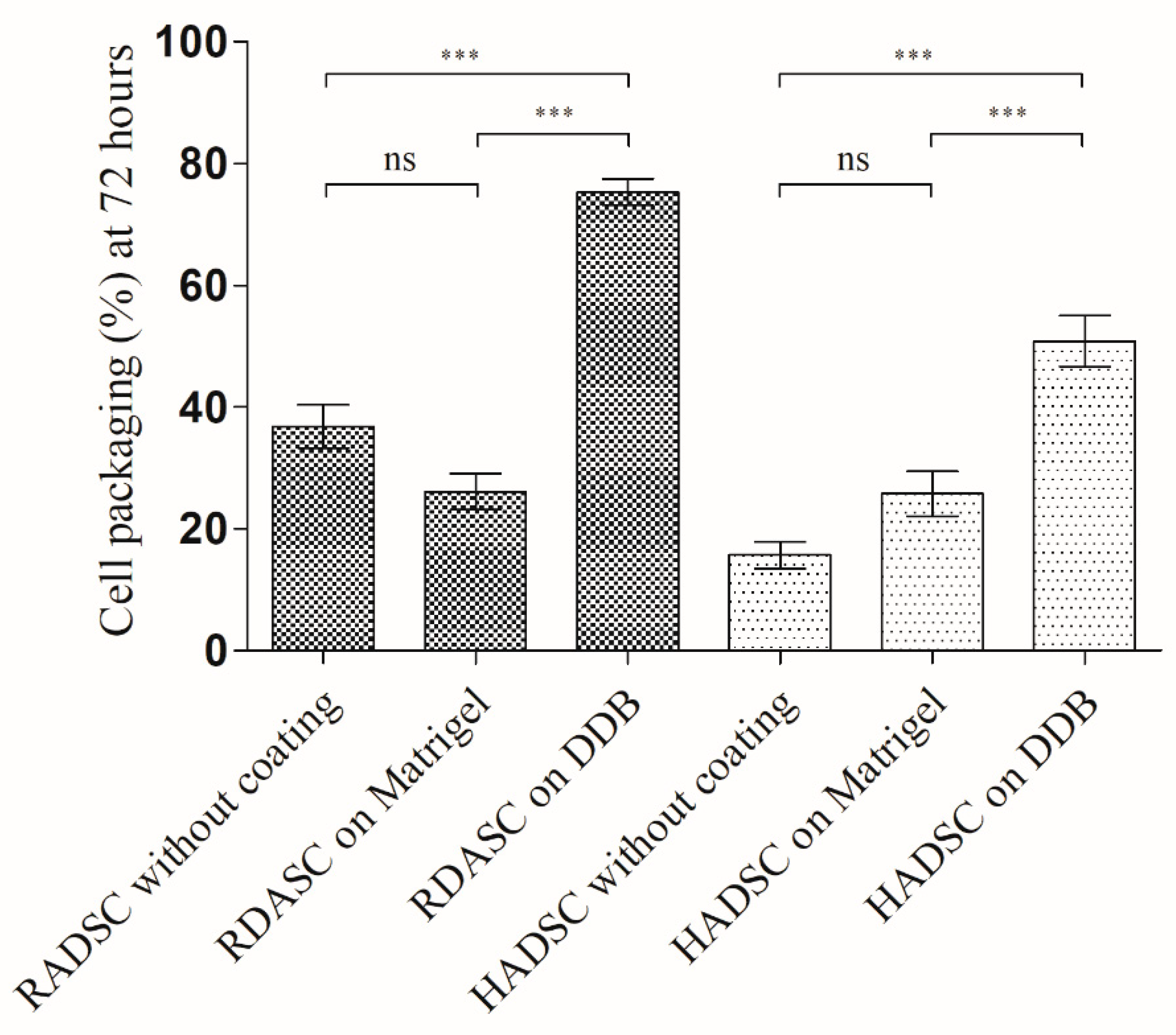
2.5. Cell Packaging Analysis
2.6. DDB Gel Favors Cell Smooth Muscle Maturation Stage
3. Materials and Methods
3.1. Bladder Decellularization
3.2. Enzymatic Digestion and Solubilization
3.3. Fourier Transform Infrared Spectroscopy
3.4. Rheological Characterization
3.5. Human and Rabbit ADSC Isolation
3.6. Cell Proliferation and Differentiation Analysis
3.7. Mathematical Analysis of Microscopy Images
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Volk, S.W.; Theoret, C. Translating stem cell therapies: The role of companion animals in regenerative medicine. Wound Repair Regen. 2013, 21, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering approaches for bladder regeneration. Int. J. Mol. Sci. 2018, 19, 1796. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Lin, D.L.; Hanzlicek, B.; Balog, B.; Penn, M.S.; Kiedrowski, M.J.; Hu, Z.; Ye, Z.; Zhu, H.; Damaser, M.S. Mesenchymal stem cells and their secretome partially restore nerve and urethral function in a dual muscle and nerve injury stress urinary incontinence model. AJP Ren. Physiol. 2015, 308, F92–F100. [Google Scholar] [CrossRef] [PubMed]
- Zhe, Z.; Jun, D.; Yang, Z.; Mingxi, X.; Ke, Z.; Ming, Z.; Zhong, W.; Mujun, L. Bladder Acellular Matrix Grafts Seeded with Adipose-Derived Stem Cells and Incubated Intraperitoneally Promote the Regeneration of Bladder Smooth Muscle and Nerve in a Rat Model of Bladder Augmentation. Stem Cells Dev. 2016, 25, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, S.; Yang, R.; Zou, Q.; Zhang, K.; Tian, Q.; Zhao, W.; Zong, L.; Fu, Q. Bioengineered bladder patches constructed from multilayered adipose-derived stem cell sheets for bladder regeneration. Acta Biomater. 2018, 85, 131–141. [Google Scholar] [CrossRef]
- Jack, G.S.; Zhang, R.; Lee, M.; Xu, Y.; Wu, B.M.; Rodríguez, L.V. Urinary bladder smooth muscle engineered from adipose stem cells and a three dimensional synthetic composite. Biomaterials 2009, 30, 3259–3270. [Google Scholar] [CrossRef]
- Shi, J.G.; Fu, W.J.; Wang, X.X.; Xu, Y.D.; Li, G.; Hong, B.F.; Hu, K.; Cui, F.Z.; Wang, Y.; Zhang, X. Transdifferentiation of human adipose-derived stem cells into urothelial cells: Potential for urinary tract tissue engineering. Cell Tissue Res. 2012, 347, 737–746. [Google Scholar] [CrossRef]
- Tran, C.; Damaser, M.S. The potential role of stem cells in the treatment of urinary incontinence. Ther. Adv. Urol. 2015, 7, 22–40. [Google Scholar] [CrossRef]
- Stangel-Wojcikiewicz, K.; Jarocha, D.; Piwowar, M.; Jach, R.; Uhl, T.; Basta, A.; Majka, M. Autologous muscle-derived cells for the treatment of female stress urinary incontinence: A 2-year follow-up of a polish investigation. Neurourol. Urodyn. 2014, 33, 324–330. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Carthew, J.; Lim, R.; Frith, J.E. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017, 26, 617–631. [Google Scholar] [CrossRef]
- Moreno-Manzano, V.; Mellado-López, M.; Morera-Esteve, M.J.; Alastrue-Agudo, A.; Bisbal-Velasco, V.; Forteza-Vila, J.; Serrano-Aroca, Á.; Vera-Donoso, C.D. Human adipose-derived mesenchymal stem cells accelerate decellularized neobladder regeneration. Regen Biomater. 2019, 7, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Ajalloueian, F.; Lemon, G.; Hilborn, J.; Chronakis, I.S.; Fossum, M. Bladder biomechanics and the use of scaffolds for regenerative medicine in the urinary bladder. Nat. Rev. Urol. 2018, 15, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Parekh, A.; Cigan, A.D.; Wognum, S.; Heise, R.L.; Chancellor, M.B.; Sacks, M.S. Ex vivo deformations of the urinary bladder wall during whole bladder filling: Contributions of extracellular matrix and smooth muscle. J. Biomech. 2010, 43, 1708–1716. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.A.; Hwei, A.N.M.; Ho, C.C.K.; Sagap, I.; Idrus, R.B.H.; Zainuddin, Z.M.D. Human adipose tissue derived stem cells as a source of smooth muscle cells in the regeneration of muscular layer of urinary bladder wall. Malaysian J. Microsc. 2013, 9, 149–153. [Google Scholar]
- Salemi, S.; Tremp, M.; Plock, J.A.; Andersson, K.E.; Gobet, R.; Sulser, T.; Eberli, D. Differentiated adipose-derived stem cells for bladder bioengineering. Scand. J. Urol. 2015, 49, 407–414. [Google Scholar] [CrossRef]
- Zhao, Z.; Yu, H.; Xiao, F.; Wang, X.; Yang, S.; Li, S. Differentiation of adipose-derived stem cells promotes regeneration of smooth muscle for ureteral tissue engineering. J. Surg. Res. 2012, 178, 55–62. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, J.A.; Houreld, N.; Abrahamse, H. Adipose derived stem cells and smooth muscle cells: Implications for rsegenerative medicine. Stem Cell Rev. Rep. 2009, 5, 256–265. [Google Scholar] [CrossRef]
- Wang, C.; Cen, L.; Yin, S.; Liu, Q.; Liu, W.; Cao, Y.; Cui, L. A small diameter elastic blood vessel wall prepared under pulsatile conditions from polyglycolic acid mesh and smooth muscle cells differentiated from adipose-derived stem cells. Biomaterials 2010, 31, 621–630. [Google Scholar] [CrossRef]
- Harris, L.J.; Abdollahi, H.; Zhang, P.; McIlhenny, S.; Tulenko, T.N.; DiMuzio, P.J. Differentiation of adult stem cells into smooth muscle for vascular tissue engineering. J. Surg. Res. 2011, 168, 306–314. [Google Scholar] [CrossRef]
- Lien, S.C.; Usami, S.; Chien, S.; Chiu, J.J. Phosphatidylinositol 3-kinase/Akt pathway is involved in transforming growth factor-β1-induced phenotypic modulation of 10T1/2 cells to smooth muscle cells. Cell. Signal. 2006, 18, 1270–1278. [Google Scholar] [CrossRef]
- Jeon, E.S.; Moon, H.J.; Lee, M.J.; Song, H.Y.; Kim, Y.M.; Cho, M.; Suh, D.-S.; Yoon, M.-S.; Chang, C.L.; Jung, J.S.; et al. Cancer-Derived Lysophosphatidic Acid Stimulates Differentiation of Human Mesenchymal Stem Cells to Myofibroblast-Like Cells. Stem Cells 2008, 26, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Gawaziuk, J.P.; Ma, X.; Sheikh, F.; Cheng, Z.Q.; Cattini, P.A.; Stephens, N.L. Transforming growth factor-β as a differentiating factor for cultured smooth muscle cells. Eur. Respir. J. 2007, 30, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Park, K.I.; Teng, Y.D.; Snyder, E.Y. The injured brain interacts reciprocally with neural stem cells supported by scaffolds to reconstitute lost tissue. Nat. Biotechnol. 2002, 20, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Weber, F.E.; Schmoekel, H.G.; Schense, J.C.; Kohler, T.; Müller, R.; Hubbell, J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 2003, 21, 513–518. [Google Scholar] [CrossRef]
- Rouet, V.; Hamma-Kourbali, Y.; Petit, E.; Panagopoulou, P.; Katsoris, P.; Barritault, D.; Caruelle, J.P.; Courty, J. A synthetic glycosaminoglycan mimetic binds vascular endothelial growth factor and modulates angiogenesis. J. Biol. Chem. 2005, 280, 32792–32800. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv. Drug Deliv. Rev. 2014, 79, 3–18. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Benton, G.; George, J.; Kleinman, H.K.; Arnaoutova, I.P. Advancing science and technology via 3D culture on basement membrane matrix. J. Cell. Physiol. 2009, 221, 18–25. [Google Scholar] [CrossRef]
- Wua, J.; Ding, Q.; Dutta, A.; Wang, Y.; Huang, Y.H.; Wenga, H.; Tang, L.; Hong, Y. An injectable extracellular matrix derived hydrogel for meniscus repair and regeneration. Acta Biomater. 2015, 16, 49–59. [Google Scholar] [CrossRef] [PubMed]
- DeQuach, J.A.; Mezzano, V.; Miglani, A.; Lange, S.; Keller, G.M.; Sheikh, F.; Christman, K.L. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS ONE 2010, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Seif-Naraghi, S.B.; Horn, D.; Schup-Magoffin, P.J.; Christman, K.L. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012, 8, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Freytes, D.O.; Martin, J.; Velankar, S.S.; Lee, A.S.; Badylak, S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 2008, 29, 1630–1637. [Google Scholar] [CrossRef]
- Kamatari, Y.O.; Dobson, C.M.; Konno, T. Structural dissection of alkaline-denatured pepsin. Spectroscopy 2004, 18, 227–236. [Google Scholar] [CrossRef]
- Wu, X.; Cai, L.; Cao, A.; Wang, Y.; Li, T.; Li, J. Comparative study on acid-soluble and pepsin-soluble collagens from skin and swim bladder of grass carp (Ctenopharyngodon idella). J. Sci. Food Agric. 2016, 96, 815–821. [Google Scholar] [CrossRef]
- Kaewdang, O.; Benjakul, S.; Kaewmanee, T.; Kishimura, H. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem. 2014, 155, 264–270. [Google Scholar] [CrossRef]
- Chou, M.T.; Chang, S.N.; Ke, C.; Chang, H.I.; Sung, M.L.; Kuo, H.C.; Chen, C.N. The proliferation and differentiation of placental-derived multipotent cells into smooth muscle cells on fibrillar collagen. Biomaterials 2010, 31, 4367–4375. [Google Scholar] [CrossRef]
- Zheng, L.; Fan, H.S.; Sun, J.; Chen, X.N.; Wang, G.; Zhang, L.; Fan, Y.J.; Zhang, X.D. Chondrogenic differentiation of mesenchymal stem cells induced by collagen-based hydrogel: An in vivo study. J. Biomed. Mater. Res. Part A 2010, 93, 783–792. [Google Scholar]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-α2β1 integrin interaction. J. Cell. Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Briquez, P.S.; Hubbell, J.A.; Martino, M.M. Extracellular Matrix-Inspired Growth Factor Delivery Systems for Skin Wound Healing. Adv. Wound Care 2015, 4, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Med, R. The role of small molecules in musculoskeletal regeneration. Regen. Med. 2016, 7, 535–549. [Google Scholar]
- Doyle, B.B.; Bendit, E.G.; Blout, E.R. Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolymers 1975, 14, 937–957. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Abe, Y.; Krimm, S. Normal vibrations of crystalline polyglycine I. Biopolymers 1972, 11, 1817–1839. [Google Scholar] [CrossRef]
- Payne, K.J.; Veis, A. Fourier transform ir spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies. Biopolymers 1988, 27, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Massensini, A.R.; Ghuman, H.; Saldin, L.T.; Medberry, C.J.; Keane, T.J.; Nicholls, F.J.; Velankar, S.S.; Badylak, S.F.; Modo, M. Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater. 2015, 27, 116–130. [Google Scholar] [CrossRef]
- Wolf, M.T.; Daly, K.A.; Brennan-Pierce, E.P.; Johnson, S.A.; Carruthers, C.A.; D’Amore, A.; Nagarkar, S.P.; Velankar, S.S.; Badylak, S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 2012, 33, 7028–7038. [Google Scholar] [CrossRef]
- Matrigel Matrix|Extracellular Matrix|Corning. Available online: https://www.corning.com/ (accessed on 12 October 2020).
- Grabowska, I.; Zimowska, M.; Maciejewska, K.; Jablonska, Z.; Bazga, A.; Ozieblo, M.; Streminska, W.; Bem, J.; Brzoska, E.; Ciemerych, M.A. Adipose tissue-derived stromal cells in matrigel impacts the regeneration of severely damaged skeletal muscles. Int. J. Mol. Sci. 2019, 20, 3313. [Google Scholar] [CrossRef]
- Chicharro, D.; Carrillo, J.M.; Rubio, M.; Cugat, R.; Cuervo, B.; Guil, S.; Forteza, J.; Moreno, V.; Vilar, J.M.; Sopena, J. Combined plasma rich in growth factors and adipose-derived mesenchymal stem cells promotes the cutaneous wound healing in rabbits. BMC Vet. Res. 2018, 14, 288. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Criado, I.; Meseguer-Ripolles, J.; Mellado-López, M.; Alastrue-Agudo, A.; Griffeth, R.J.; Forteza-Vila, J.; Cugat, R.; García, M.; Moreno-Manzano, V. Human Suprapatellar Fat Pad-Derived Mesenchymal Stem Cells Induce Chondrogenesis and Cartilage Repair in a Model of Severe Osteoarthritis. Stem Cells Int. 2017, 2017, 4758930. [Google Scholar] [CrossRef] [PubMed]
- Mellado-López, M.; Griffeth, R.J.; Meseguer-Ripolles, J.; Cugat, R.; García, M.; Moreno-Manzano, V. Plasma Rich in Growth Factors Induces Cell Proliferation, Migration, Differentiation, and Cell Survival of Adipose-Derived Stem Cells. Stem Cells Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; Forsythe, A.B. The Small Sample Behavior of Some Statistics Which Test the Equality of Several Means. Technometrics 1974, 16, 129. [Google Scholar] [CrossRef]
- Games, P.A.; Howell, J.F. Pairwise Multiple Comparison Procedures with Unequal N’s and/or Variances: A Monte Carlo Study. J. Educ. Stat. 1976, 1, 113. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Manzano, V.; Zaytseva-Zotova, D.; López-Mocholí, E.; Briz-Redón, Á.; Løkensgard Strand, B.; Serrano-Aroca, Á. Injectable Gel Form of a Decellularized Bladder Induces Adipose-Derived Stem Cell Differentiation into Smooth Muscle Cells In Vitro. Int. J. Mol. Sci. 2020, 21, 8608. https://doi.org/10.3390/ijms21228608
Moreno-Manzano V, Zaytseva-Zotova D, López-Mocholí E, Briz-Redón Á, Løkensgard Strand B, Serrano-Aroca Á. Injectable Gel Form of a Decellularized Bladder Induces Adipose-Derived Stem Cell Differentiation into Smooth Muscle Cells In Vitro. International Journal of Molecular Sciences. 2020; 21(22):8608. https://doi.org/10.3390/ijms21228608
Chicago/Turabian StyleMoreno-Manzano, Victoria, Daria Zaytseva-Zotova, Eric López-Mocholí, Álvaro Briz-Redón, Berit Løkensgard Strand, and Ángel Serrano-Aroca. 2020. "Injectable Gel Form of a Decellularized Bladder Induces Adipose-Derived Stem Cell Differentiation into Smooth Muscle Cells In Vitro" International Journal of Molecular Sciences 21, no. 22: 8608. https://doi.org/10.3390/ijms21228608
APA StyleMoreno-Manzano, V., Zaytseva-Zotova, D., López-Mocholí, E., Briz-Redón, Á., Løkensgard Strand, B., & Serrano-Aroca, Á. (2020). Injectable Gel Form of a Decellularized Bladder Induces Adipose-Derived Stem Cell Differentiation into Smooth Muscle Cells In Vitro. International Journal of Molecular Sciences, 21(22), 8608. https://doi.org/10.3390/ijms21228608





