Aesculetin Inhibits Osteoclastic Bone Resorption through Blocking Ruffled Border Formation and Lysosomal Trafficking
Abstract
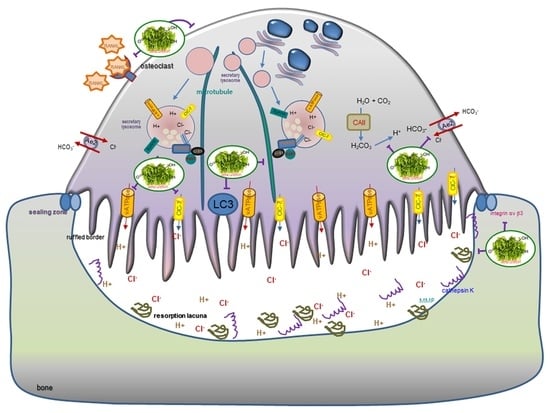
1. Introduction
2. Results
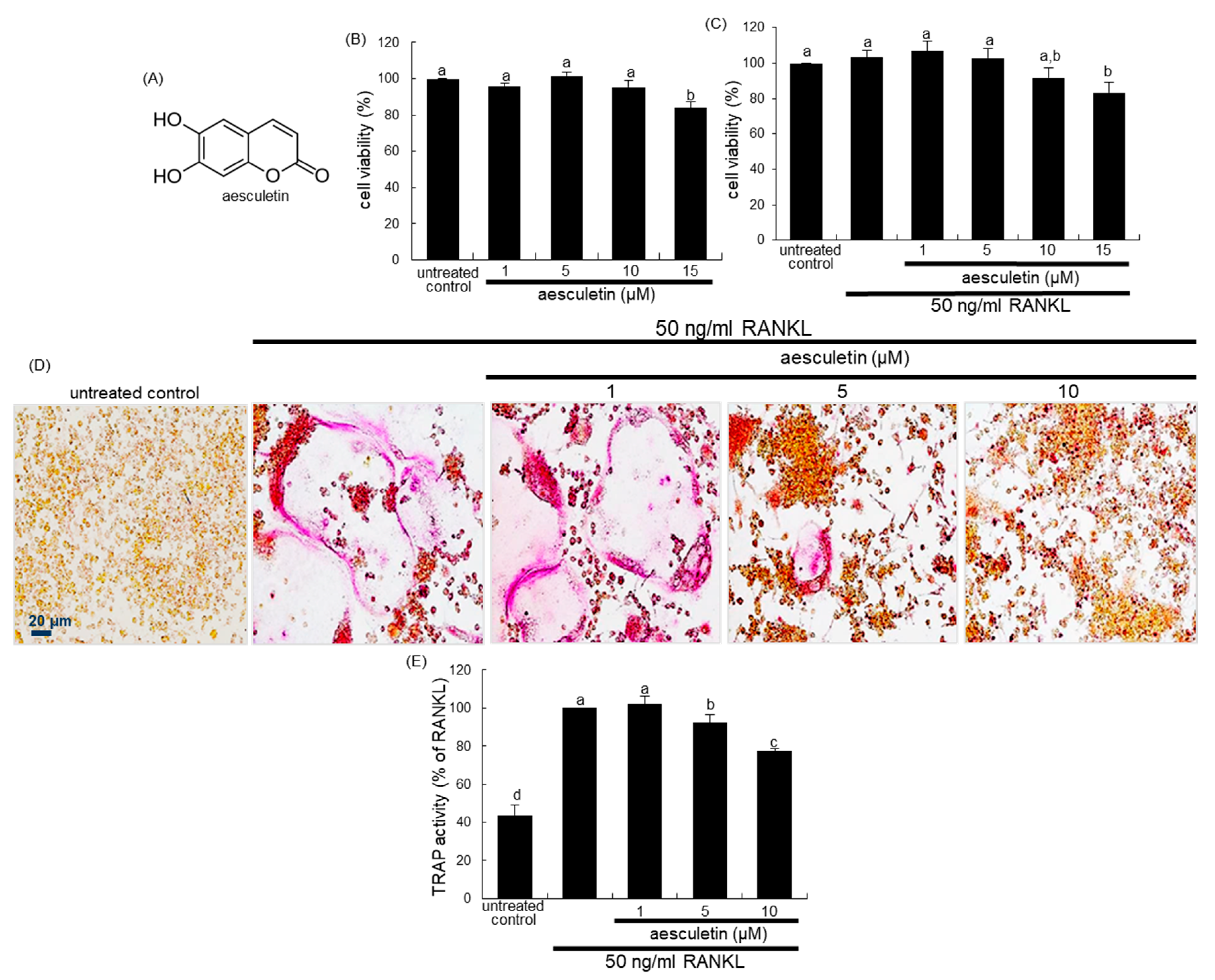
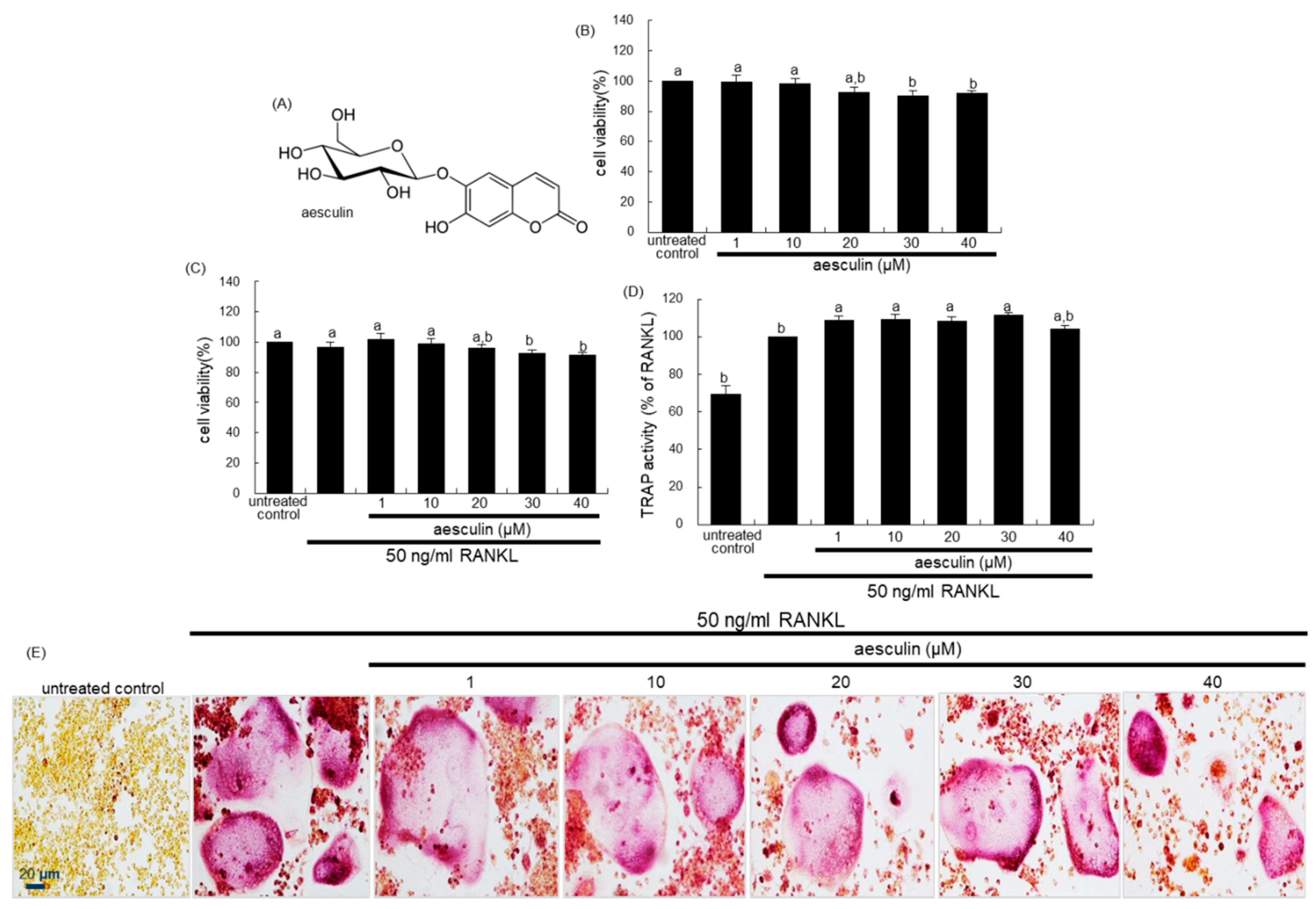
2.1. Blockade of RANKL-Induced Osteoclast Differentiation by Aesculetin
2.2. Inhibition of Lacunar Acidification and Bone Resorption by Aesculetin
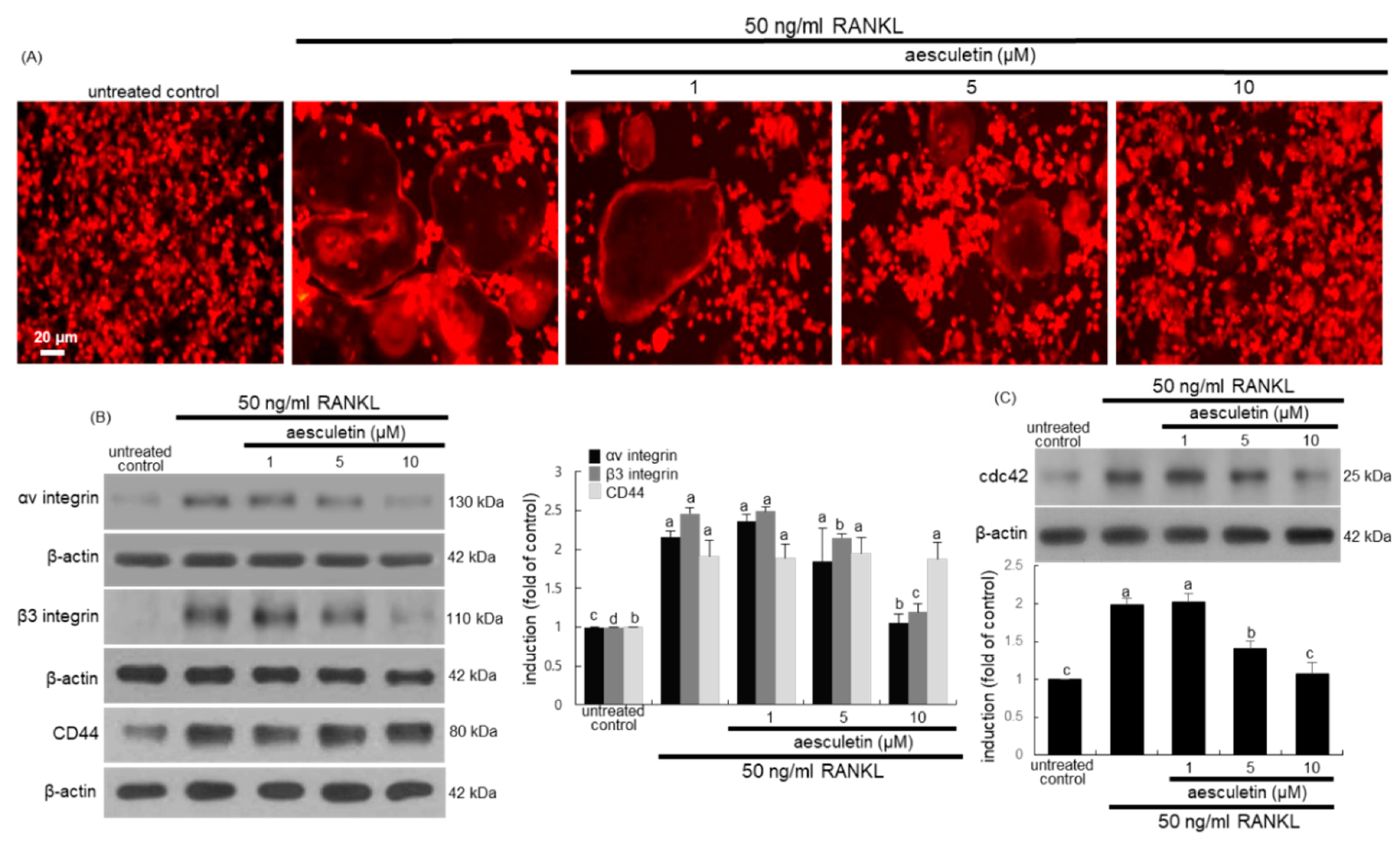
2.3. Inhibitory Effects of Aesculetin on Actin Ring Formation
2.4. Disruption of Osteoclastic Cytoskeletal Arrangement by Aesculetin
2.5. Blockade of Lysosome Positioning to Microtubules by Aesculetin
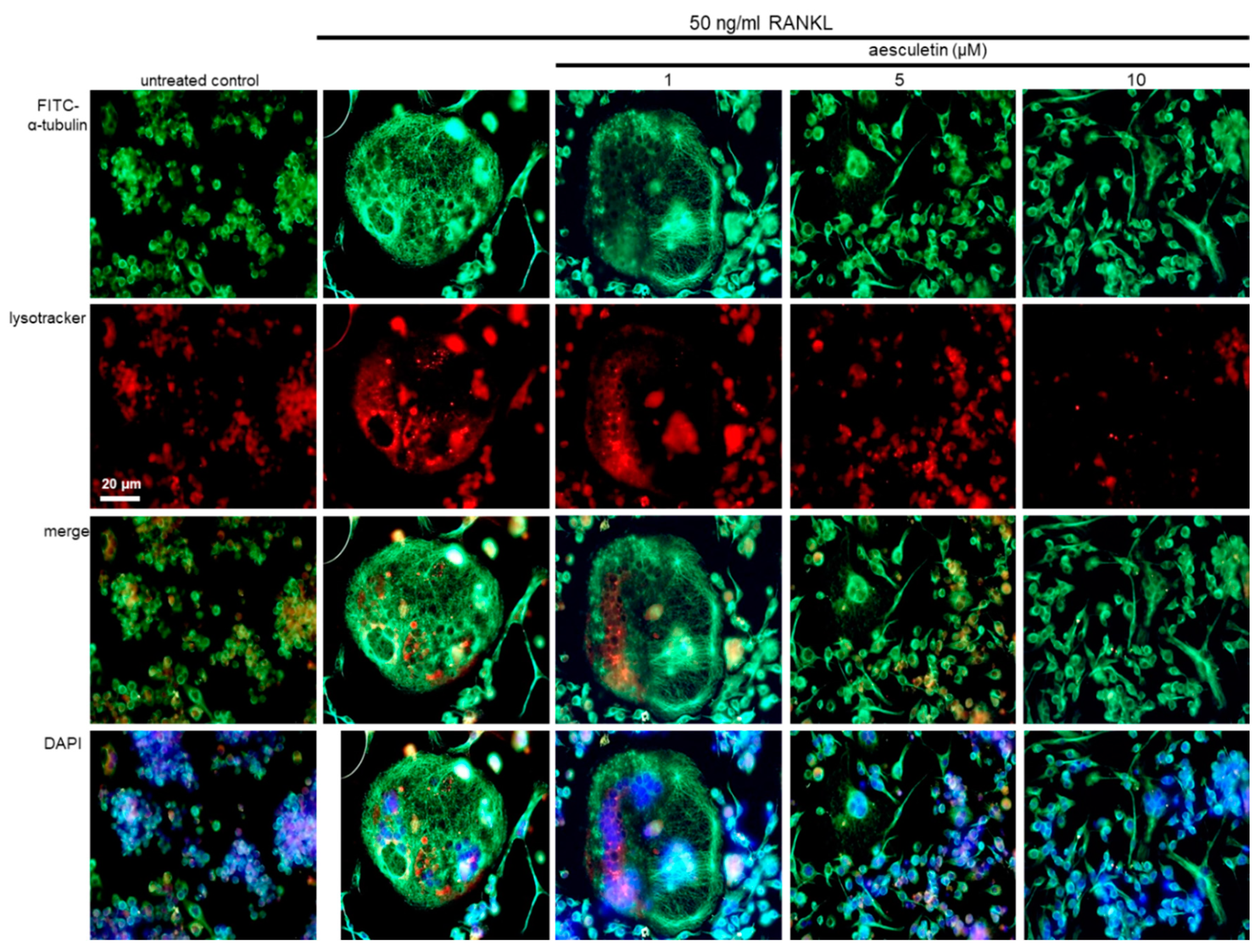
2.6. Disruption of Trafficking of Lysosomes into Ruffled Border by Aesculetin
3. Materials and Methods
3.1. Materials
3.2. Osteoclast Differentiation of Raw 264.7 Cells
3.3. Measurement of Tartrate-Resistant Acid Phosphatase (TRAP) Activity
3.4. Western Blot Analysis
3.5. Actin Ring Staining
3.6. Immunochemical Staining
3.7. Statistical Analysis
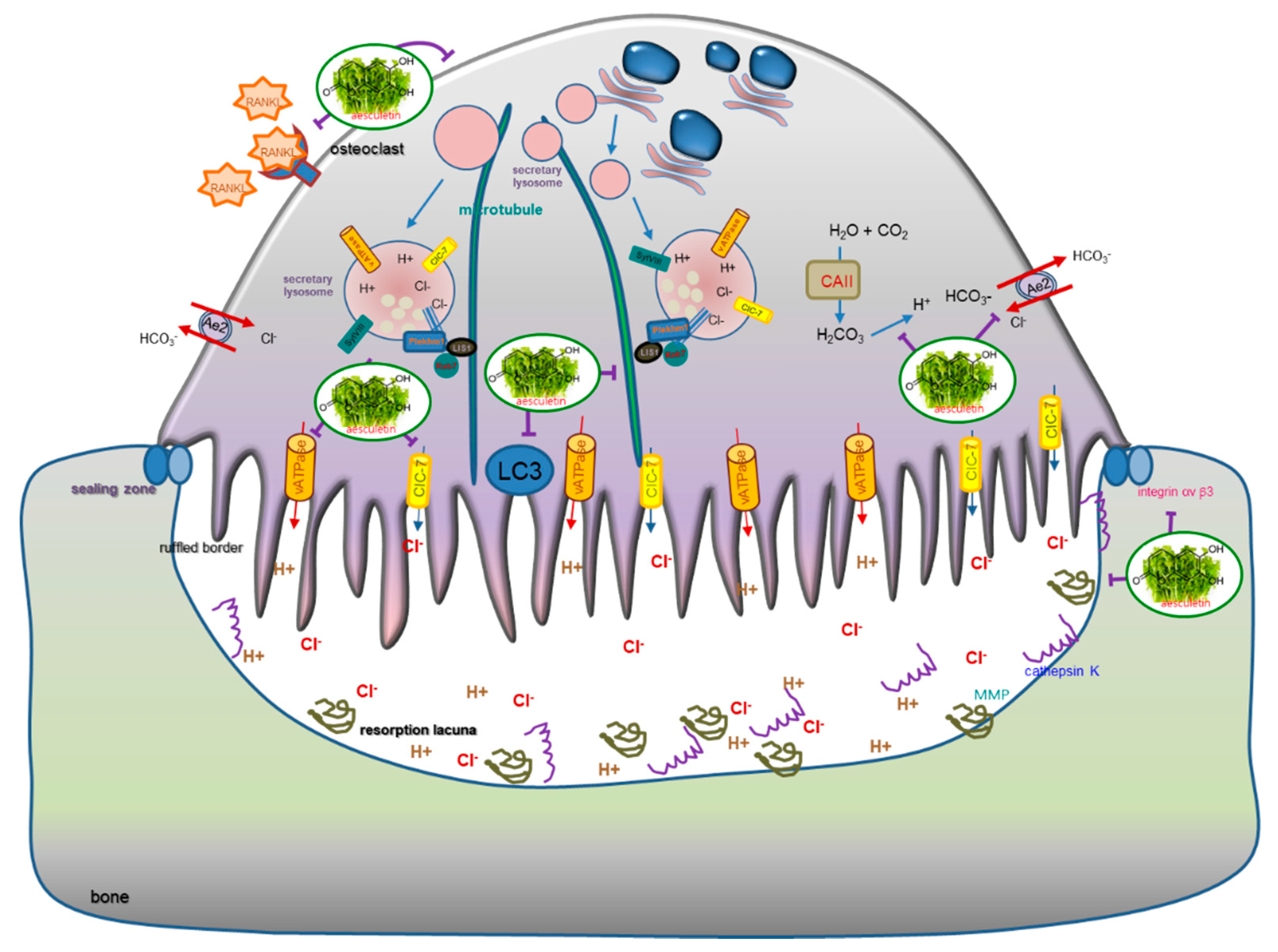
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ae2 | anion exchange protein 2 |
| Atg | autophagy-related proteins |
| CAII | carbonic anhydrase II |
| ClC-7 | chloride channel 7 |
| LAMP2 | lysosome-associated membrane protein 2 |
| LC3 | light chain 3 |
| LIS1 | lissencephaly-1 |
| OPG | osteoprotegerin |
| PLEKHM1 | Pleckstrin homology domain-containing protein family member 1 |
| MMP | matrix metalloproteinase |
| RANK | receptor activator of nuclear factor-κB |
| RANKL | RANK ligand |
| Syt VII | synaptotagmin VII |
| TRAP | tartrate-resistance acid phosphatase |
| V-ATPase | vacuolar-type H(+)-ATPase |
References
- Kenkre, J.S.; Bassett, J.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, Y.; Pacios, S.; Li, S.; Graves, D.T. Cellular and molecular aspects of bone remodeling. Front. Oral Biol. 2015, 18, 9–16. [Google Scholar] [CrossRef]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nat. Cell Biol. 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef]
- Kenny, A.M.; Raisz, L.G. Mechanisms of bone remodeling: Implications for clinical practice. J. Reprod. Med. 2002, 47, 63–70. [Google Scholar]
- Cosman, F. Anabolic and antiresorptive therapy for osteoporosis: Combination and sequential approaches. Curr. Osteoporos. Rep. 2014, 12, 385–395. [Google Scholar] [CrossRef]
- Anagnostis, P.; Gkekas, N.K.; Potoupnis, M.; Kenanidis, E.; Tsiridis, E.; Goulis, D.G. New therapeutic targets for osteoporosis. Maturitas 2019, 120, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Papapoulos, S.; Makras, P. Selection of antiresorptive or anabolic treatments for postmenopausal osteoporosis. Nat. Clin. Pract. Endocrinol. Metab. 2008, 4, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.Y.; Ribet, A.B.P.; Pavlos, N.J. Membrane trafficking in osteoclasts and implications for osteoporosis. Biochem. Soc. Trans. 2019, 47, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Väänänen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. J. Cell Sci. 2000, 113, 377–381. [Google Scholar] [PubMed]
- Stenbeck, G. Formation and function of the ruffled border in osteoclasts. Semin. Cell Dev. Biol. 2002, 13, 285–292. [Google Scholar] [CrossRef]
- Rousselle, A.-V.; Heymann, D. Osteoclastic acidification pathways during bone resorption. Bone 2002, 30, 533–540. [Google Scholar] [CrossRef]
- Henriksen, K.; Sørensen, M.G.; Nielsen, R.H.; Gram, J.; Schaller, S.; Dziegiel, M.H.; Everts, V.; Bollerslev, J.; A Karsdal, M. Degradation of the organic phase of bone by osteoclasts: A secondary role for lysosomal scidification. J. Bone Miner. Res. 2005, 21, 58–66. [Google Scholar] [CrossRef]
- Josephsen, K.; Praetorius, J.; Frische, S.; Gawenis, L.R.; Kwon, T.-H.; Agre, P.; Nielsen, S.; Fejerskov, O. Targeted disruption of the Cl-/HCO3- exchanger Ae2 results in osteopetrosis in mice. Proc. Natl. Acad. Sci. USA 2009, 106, 1638–1641. [Google Scholar] [CrossRef]
- Kornak, U.; Kasper, D.; Bösl, M.R.; Kaiser, E.; Schweizer, M.; Schulz, A.; Friedrich, W.; Delling, G.; Jentsch, T.J. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 2001, 104, 205–215. [Google Scholar] [CrossRef]
- Lacombe, J.; Karsenty, G.; Ferron, M. Regulation of lysosome biogenesis and functions in osteoclasts. Cell Cycle 2013, 12, 2744–2752. [Google Scholar] [CrossRef]
- Zhao, H.; Ito, Y.; Chappel, J.; Andrews, N.; Ross, F.P.; Teitelbaum, S.L. How do bone cells secrete proteins? Adv. Exp. Med. Biol. 2010, 658, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Fowler, T.W.; Pavlos, N.J.; Ng, P.Y.; Liang, K.; Feng, Y.; Zheng, M.; Kurten, R.; Manolagas, S.C.; Zhao, H. LIS1 regulates osteoclast formation and function through its interactions with Dynein/Dynactin and Plekhm1. PLoS ONE 2011, 6, e27285. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-H.; Yoon, S.-Y.; Choi, B.; Sohn, N.H.; Yoon, K.-H.; Kim, W.-J.; Kim, D.-H.; Chang, E.-J. Microtubule-associated protein light chain 3 regulates Cdc42-dependent actin ring formation in osteoclast. Int. J. Biochem. Cell Biol. 2012, 44, 989–997. [Google Scholar] [CrossRef] [PubMed]
- DeSelm, C.J.; Miller, B.C.; Zou, W.; Beatty, W.L.; van Meel, E.; Takahata, Y.; Klumperman, J.; Tooze, S.A.; Teitelbaum, S.L.; Virgin, H.W. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev. Cell 2011, 21, 966–974. [Google Scholar] [CrossRef]
- Đudarić, L.; Fužinac-Smojver, A.; Muhvić, D.; Giacometti, J. The role of polyphenols on bone metabolism in osteoporosis. Food Res. Int. 2015, 77, 290–298. [Google Scholar] [CrossRef]
- Horcajada, M.N.; Offord, E. Naturally plant-derived compounds: Role in bone anabolism. Curr. Mol. Pharmacol. 2012, 5, 205–218. [Google Scholar] [CrossRef]
- Antika, L.D.; Kim, Y.-H.; Kang, M.-K.; Park, S.-H.; Lee, E.-J.; Choi, Y.-J.; Kang, Y.-H. Dietary compound gossypetin inhibits bone resorption through down-regulating lysosomal cathepsin K activity and autophagy-related protein induction in actin ring-bearing osteoclasts. J. Funct. Foods 2016, 24, 390–402. [Google Scholar] [CrossRef]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef]
- Sundaram, K.; Nishimura, R.; Senn, J.; Youssef, R.F.; London, S.D.; Reddy, S.V. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007, 313, 168–178. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Y.; Du, Q.-S.; Wu, X.-J.; Feng, X.; Mei, L.; McDonald, J.M.; Xiong, W.-C. Regulation of the formation of osteoclastic actin rings by proline-rich tyrosine kinase 2 interacting with gelsolin. J. Cell Biol. 2003, 160, 565–575. [Google Scholar] [CrossRef]
- Nakamura, I.; Pilkington, M.F.; Lakkarkorpi, P.T.; Lipfert, L.; Sims, S.M.; Dixon, S.J.; Rodan, G.A.; Duong, L.T. Role of αvβ3 integrin in osteoclast migration and formation of sealing zone. J. Cell. Sci. 1999, 112, 3985–3993. [Google Scholar] [PubMed]
- Jurdic, P.; Saltel, F.; Chabadel, A.; Destaing, O. Podosome and sealing zone: Specificity of the osteoclast model. Eur. J. Cell Biol. 2006, 85, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Chabadel, A.; Bañon-Rodríguez, I.; Cluet, D.; Rudkin, B.B.; Wehrle-Haller, B.; Genot, E.; Jurdic, P.; Anton, I.M.; Saltel, F. CD44 and beta3 integrin organize two functionally distinct actin-based domains in osteoclasts. Mol. Biol. Cell. 2007, 18, 4899–4910. [Google Scholar] [CrossRef] [PubMed]
- Itzstein, C.; Coxon, F.P.; Rogers, M.J. The regulation of osteoclast function and bone resorption by small GTPases. Small GTPases 2011, 2, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Mizoguchi, T.; Sato, N.; Yamaki, M.; Kobayashi, Y.; Yamauchi, H.; Ozawa, H.; Udagawa, N.; Takahashi, N. Coordination of microtubules and the actin cytoskeleton is important in osteoclast function, but calcitonin disrupts sealing zones without affecting microtubule networks. Bone 2006, 39, 684–693. [Google Scholar] [CrossRef]
- Zhao, H.; Ito, Y.; Chappel, J.; Andrews, N.W.; Teitelbaum, S.L.; Ross, F.P. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev. Cell 2008, 14, 914–925. [Google Scholar] [CrossRef]
- Fujiwara, T.; Ye, S.; Castro-Gomes, T.; Winchell, C.G.; Andrews, N.W.; Voth, D.E.; Varughese, K.I.; Mackintosh, S.G.; Feng, Y.; Pavlos, N.; et al. PLEKHM1/DEF8/RAB7 complex regulates lysosome positioning and bone homeostasis. JCI Insight 2016, 1, e86330. [Google Scholar] [CrossRef]
- Wang, X.; Diao, L.; Sun, D.; Wang, D.; Zhu, J.; He, Y.; Liu, Y.; Xu, H.; Zhang, Y.; Liu, J.; et al. OsteoporosAtlas: A human osteoporosis-related gene database. PeerJ 2019, 7, e6778. [Google Scholar] [CrossRef]
- Eriksen, E.F. Cellular mechanisms of bone remodeling. Rev. Endocr. Metab. Disord. 2010, 11, 219–227. [Google Scholar] [CrossRef]
- Crane, J.L.; Cao, X. Bone marrow mesenchymal stem cells and TGF-β signaling in bone remodeling. J. Clin. Investig. 2014, 124, 466–472. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Tapking, C.; Borrelli, M.R.; Popp, D.; Duscher, D.; Maan, Z.N.; Chelliah, M.P.; Li, J.; Harati, K.; Wallner, C.; et al. Wnt pathway in bone repair and regeneration—What do we know so far. Front. Cell. Dev. Biol. 2019, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.A.; Karsenty, G. Molecular bases of the regulation of bone remodeling by the canonical Wnt signaling pathway. Curr. Top. Dev. Biol. 2006, 73, 43–84. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Anti-resorptive therapies for osteoporosis. Semin. Cell Dev. Biol. 2008, 19, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, V.; Sarkar, M.; Chaubey, P.; Khan, T.; Sherje, A.; Patel, K.; Dravyakar, B. Bone health and natural products- An insight. Front. Pharmacol. 2018, 9, 981. [Google Scholar] [CrossRef]
- Baek, J.M.; Park, S.-H.; Cheon, Y.-H.; Ahn, S.-J.; Lee, M.S.; Oh, J.; Kim, J.-Y. Esculetin attenuates receptor activator of nuclear factor kappa-B ligand-mediated osteoclast differentiation through c-Fos/nuclear factor of activated T-cells c1 signaling pathway. Biochem. Biophys. Res. Commun. 2015, 461, 334–341. [Google Scholar] [CrossRef]
- Kim, J.-L.; Kang, M.-K.; Gong, J.-H.; Park, S.-H.; Han, S.-Y.; Kang, Y.-H. Novel antiosteoclastogenic activity of phloretin antagonizing RANKL-induced osteoclast differentiation of murine macrophages. Mol. Nutr. Food Res. 2012, 56, 1223–1233. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, J.-L.; Lee, E.-J.; Park, S.-H.; Han, S.-Y.; Kang, S.A.; Kang, Y.-H. Fisetin antagonizes cell fusion, cytoskeletal organization and bone resorption in RANKL-differentiated murine macrophages. J. Nutr. Biochem. 2014, 25, 295–303. [Google Scholar] [CrossRef]
- Anderegg, F.; Geblinger, D.; Horvath, P.; Charnley, M.; Textor, M.; Addadi, L.; Geiger, B. Substrate adhesion regulates sealing zone architecture and dynamics in cultured osteoclasts. PLoS ONE 2011, 6, e28583. [Google Scholar] [CrossRef]
- Ito, Y.; Teitelbaum, S.L.; Zou, W.; Zheng, Y.; Johnson, J.F.; Chappel, J.; Ross, F.P.; Zhao, H. Cdc42 regulates bone modeling and remodeling in mice by modulating RANKL/M-CSF signaling and osteoclast polarization. J. Clin. Investig. 2010, 120, 1981–1993. [Google Scholar] [CrossRef]
- Bestebroer, J.; V’Kovski, P.; Mauthe, M.; Reggiori, F. Hidden behind autophagy: The unconventional roles of ATG proteins. Traffic 2013, 14, 1029–1041. [Google Scholar] [CrossRef]
- Zhao, H. Membrane trafficking in osteoblasts and osteoclasts: New avenues for understanding and treating skeletal diseases. Traffic 2012, 13, 1307–1314. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, W.; Lee, E.-J.; Kang, M.-K.; Kim, Y.-H.; Kim, D.Y.; Oh, H.; Kim, S.-I.; Oh, S.Y.; Kang, Y.-H. Aesculetin Inhibits Osteoclastic Bone Resorption through Blocking Ruffled Border Formation and Lysosomal Trafficking. Int. J. Mol. Sci. 2020, 21, 8581. https://doi.org/10.3390/ijms21228581
Na W, Lee E-J, Kang M-K, Kim Y-H, Kim DY, Oh H, Kim S-I, Oh SY, Kang Y-H. Aesculetin Inhibits Osteoclastic Bone Resorption through Blocking Ruffled Border Formation and Lysosomal Trafficking. International Journal of Molecular Sciences. 2020; 21(22):8581. https://doi.org/10.3390/ijms21228581
Chicago/Turabian StyleNa, Woojin, Eun-Jung Lee, Min-Kyung Kang, Yun-Ho Kim, Dong Yeon Kim, Hyeongjoo Oh, Soo-Il Kim, Su Yeon Oh, and Young-Hee Kang. 2020. "Aesculetin Inhibits Osteoclastic Bone Resorption through Blocking Ruffled Border Formation and Lysosomal Trafficking" International Journal of Molecular Sciences 21, no. 22: 8581. https://doi.org/10.3390/ijms21228581
APA StyleNa, W., Lee, E.-J., Kang, M.-K., Kim, Y.-H., Kim, D. Y., Oh, H., Kim, S.-I., Oh, S. Y., & Kang, Y.-H. (2020). Aesculetin Inhibits Osteoclastic Bone Resorption through Blocking Ruffled Border Formation and Lysosomal Trafficking. International Journal of Molecular Sciences, 21(22), 8581. https://doi.org/10.3390/ijms21228581