Cryopreservation of Mesenchymal Stem Cells Using Medical Grade Ice Nucleation Inducer
Abstract
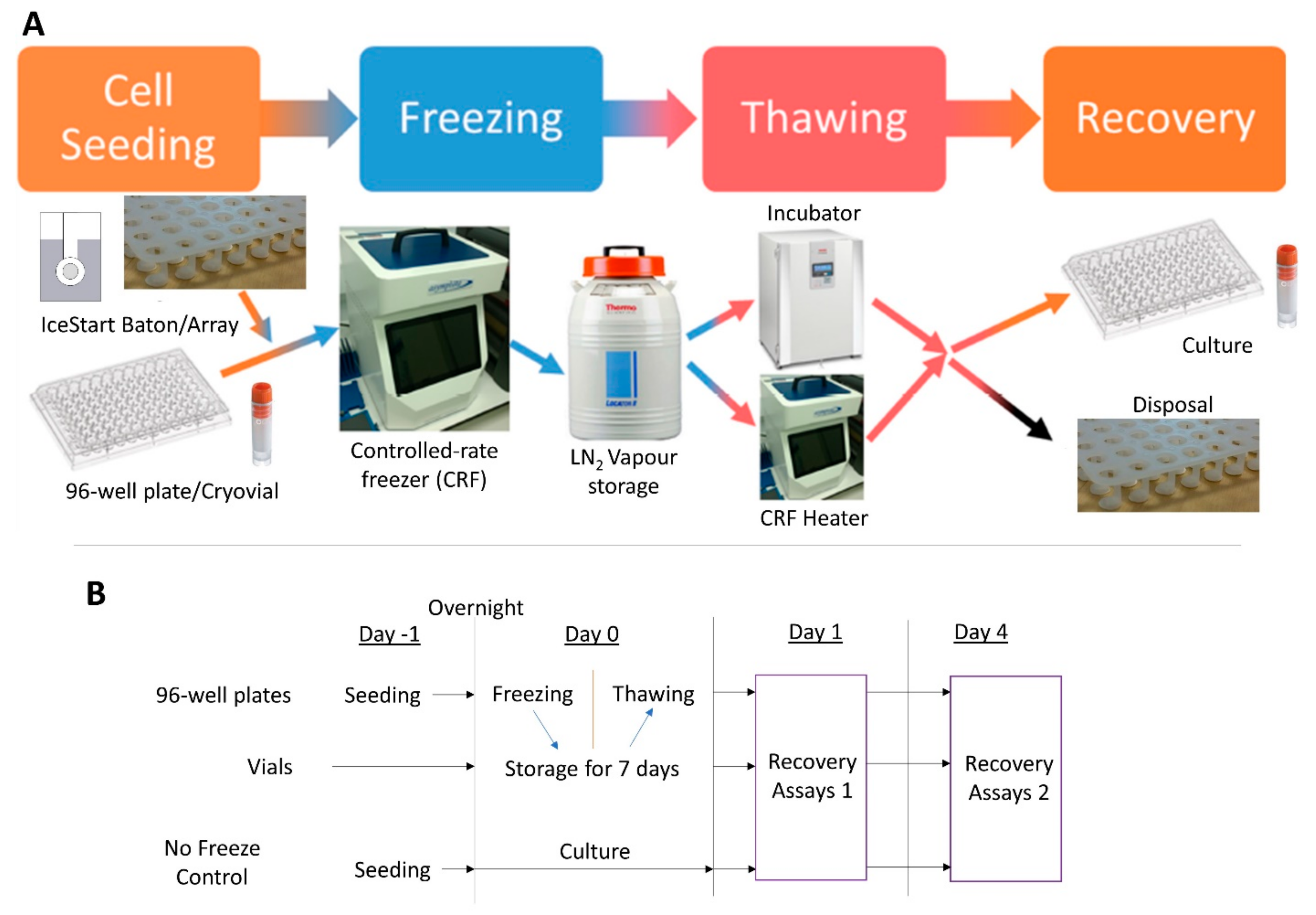
1. Introduction
2. Results
2.1. Ice Nucleation Temperature
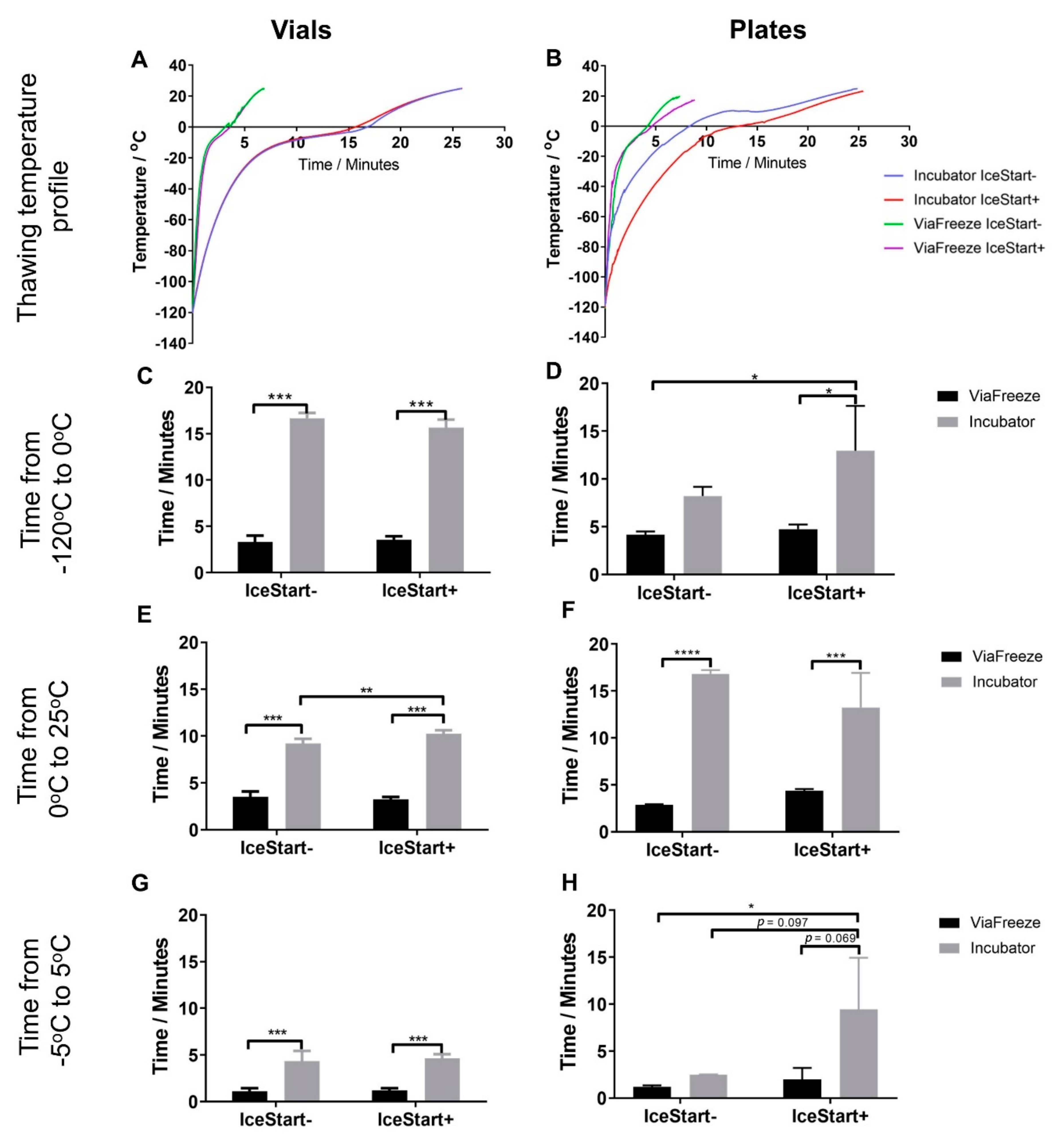
2.2. Effect of Thawing with Ice Nucleation Device
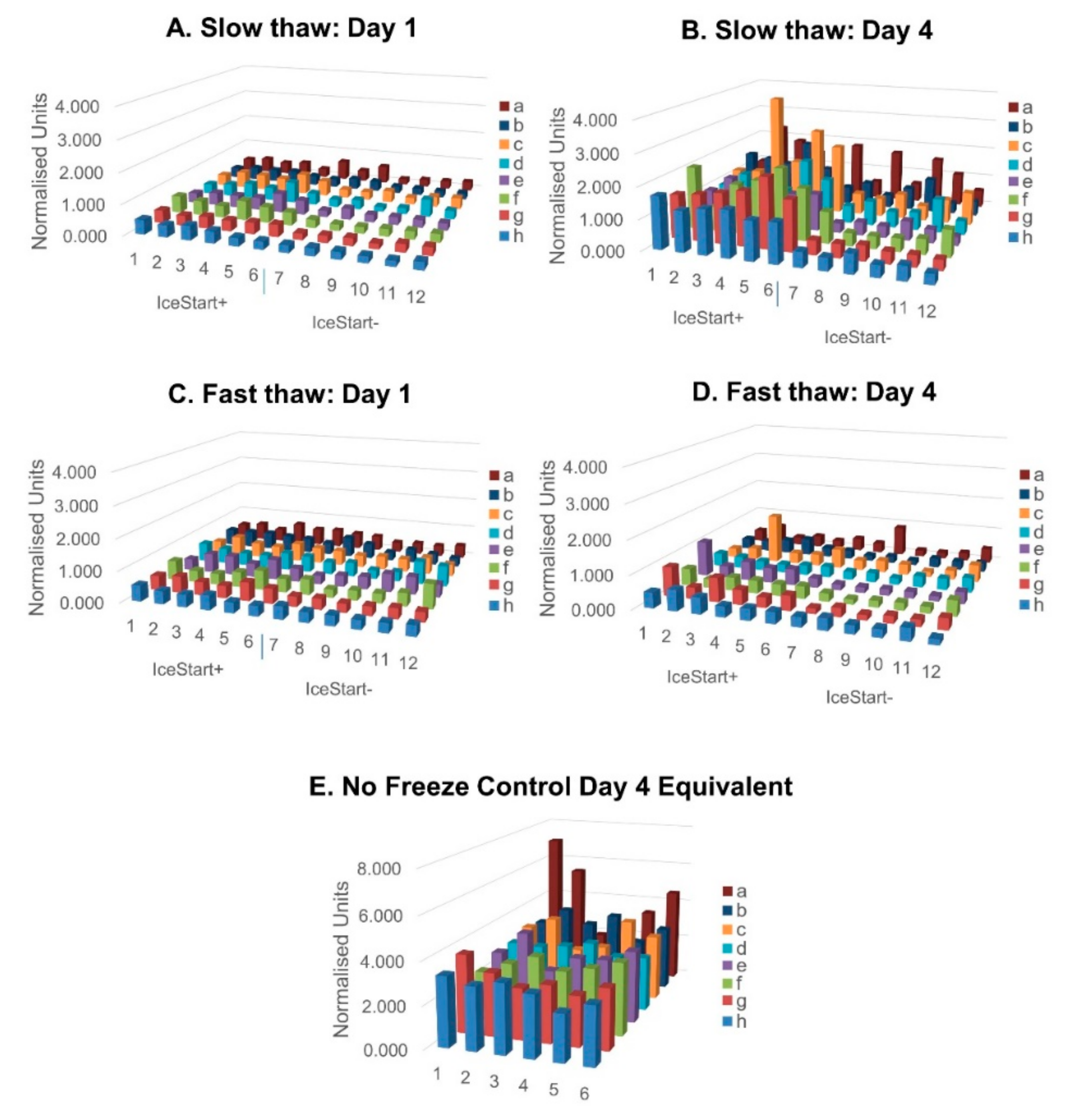
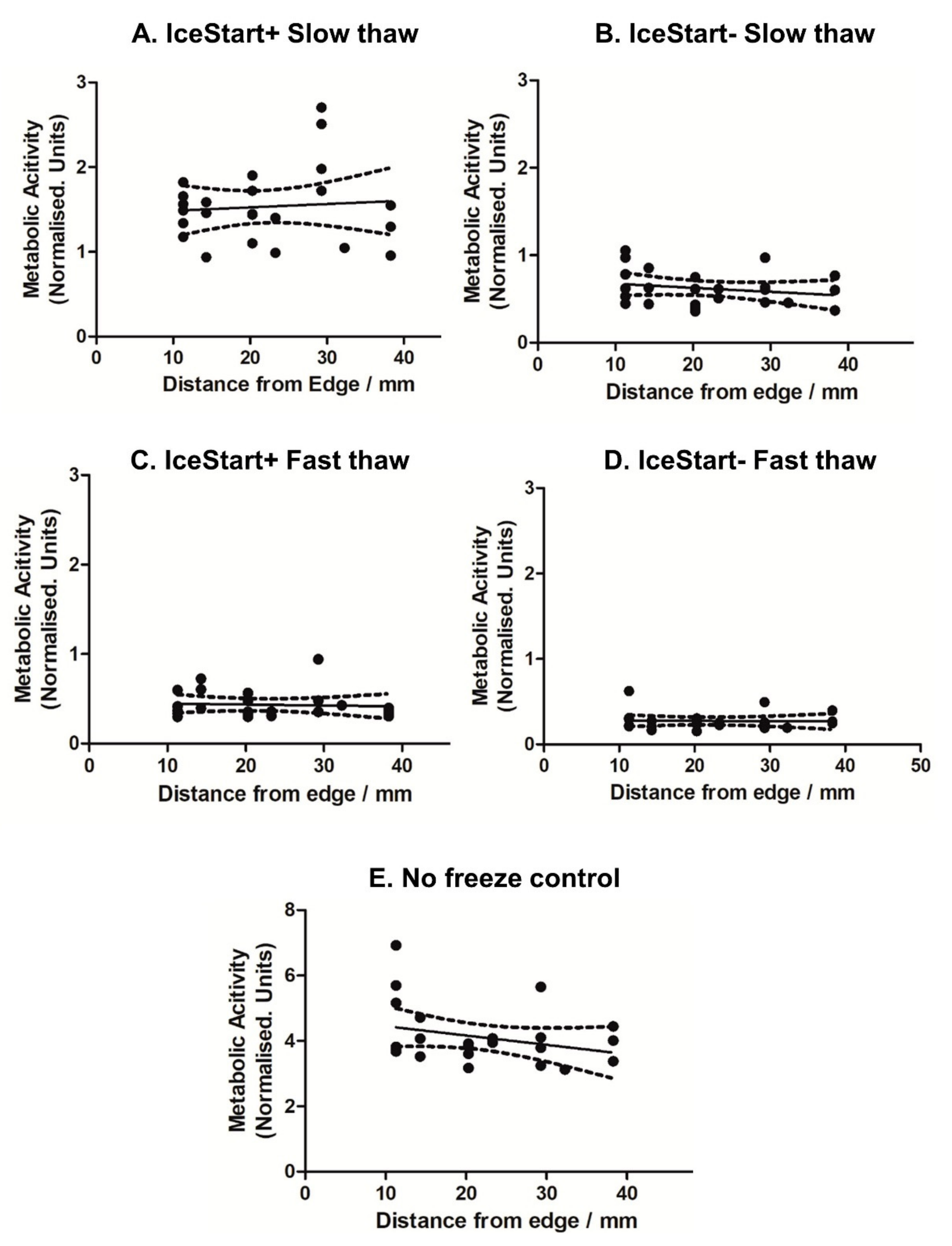
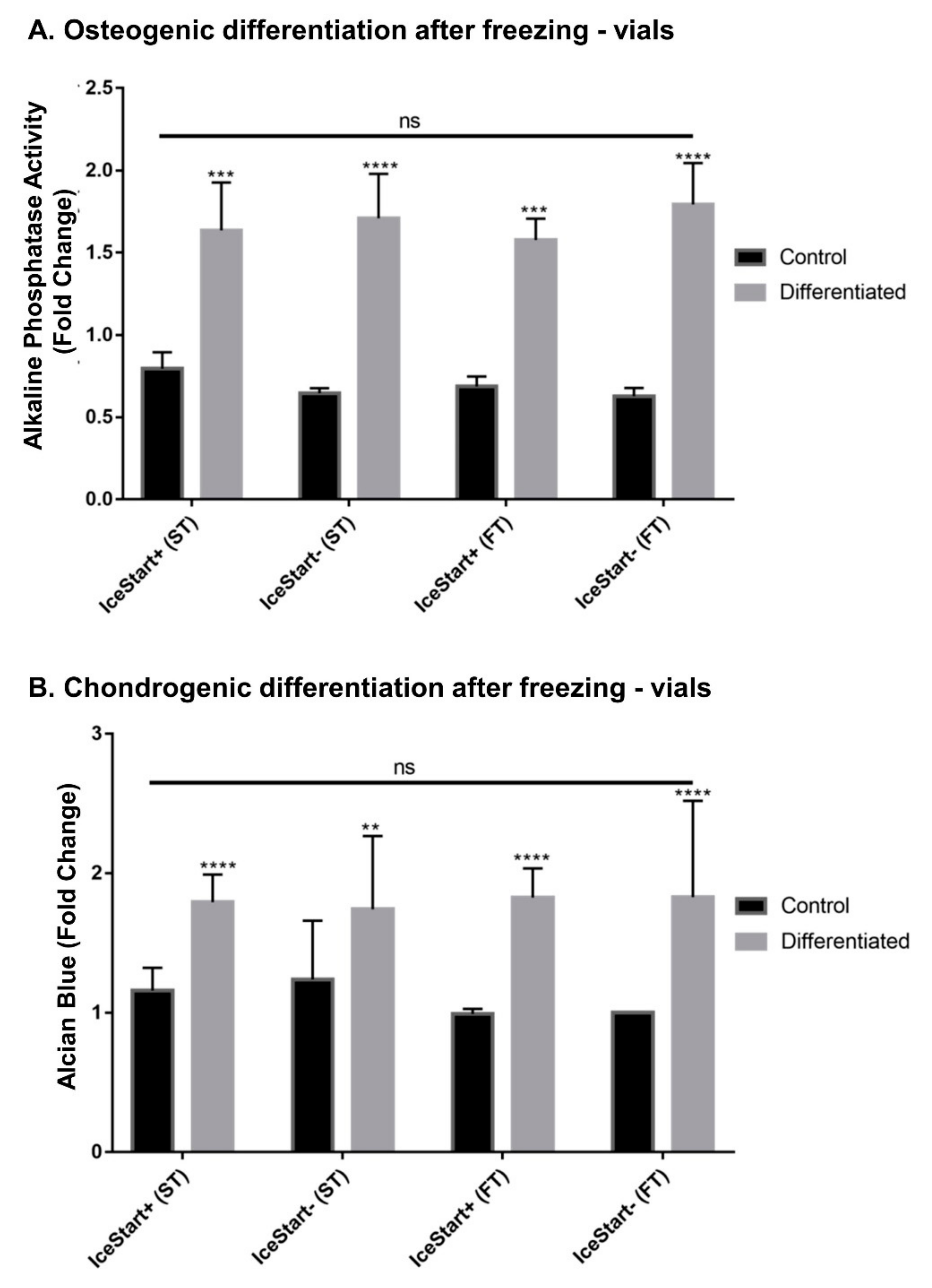
2.3. Differentiation Ability of MSC after Freezing with IceStart
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cryopreservation
4.2.1. Freezing Protocol
4.2.2. Plates
4.2.3. Vials
4.2.4. Thawing Protocol
4.3. Cell Recovery (Metabolic Activity)
4.4. Differentiation
4.4.1. Osteogenic Differentiation
4.4.2. Adipogenic Differentiation
4.4.3. Chondrogenic Differentiation
4.5. Temperature Profiles
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Cuellar, A.P.; Kang, J.H.; Jeung, E.B.; Choi, K.C. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol. Ther. 2019, 27, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Shariatzadeh, M.; Song, J.; Wilson, S.L. The efficacy of different sources of mesenchymal stem cells for the treatment of knee osteoarthritis. Cell Tissue Res. 2019. [Google Scholar] [CrossRef]
- Schulman, I.H.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell Therapy for Aging Frailty. Front. Nutr. 2018, 5, 2–11. [Google Scholar] [CrossRef]
- Kang, J.M.; Yeon, B.K.; Cho, S.J.; Suh, Y.H. Stem Cell Therapy for Alzheimer’s Disease: A Review of Recent Clinical Trials. J. Alzheimer’s Dis. 2016, 54, 879–889. [Google Scholar] [CrossRef]
- Hunt, C.J. Technical Considerations in the Freezing, Low-Temperature Storage and Thawing of Stem Cells for Cellular Therapies. Transfus. Med. Hemother. 2019, 46, 134–150. [Google Scholar] [CrossRef]
- Moll, G.; Geißler, S.; Catar, R.; Ignatowicz, L.; Hoogduijn, M.J.; Strunk, D.; Bieback, K.; Ringdén, O. Cryopreserved or Fresh Mesenchymal Stromal Cells: Only a Matter of Taste or Key to Unleash the Full Clinical Potential of MSC Therapy? In Advances in Experimental Medicine and Biology; Karimi-Busheri, F., Weinfeld, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; Volume 951, pp. 77–98. ISBN 978-3-319-45455-9. [Google Scholar]
- Bahsoun, S.; Coopman, K.; Akam, E.C. The impact of cryopreservation on bone marrow-derived mesenchymal stem cells: A systematic review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef]
- Baust, J.M.; Van Buskirk, R.; Baust, J.G. Modulation of the cryopreservation cap: Elevated survival with reduced dimethyl sulfoxide concentration. Cryobiology 2002, 45, 97–108. [Google Scholar] [CrossRef]
- Baust, J.G.; Gao, D.; Baust, J.M. Cryopreservation. Organogenesis 2009, 5, 90–96. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.Y. Control of ice nucleation: Freezing and antifreeze strategies. Chem. Soc. Rev. 2018, 47, 7116–7139. [Google Scholar] [CrossRef]
- Weng, L.; Tessier, S.N.; Swei, A.; Stott, S.L.; Toner, M. Controlled ice nucleation using freeze-dried Pseudomonas syringae encapsulated in alginate beads. Cryobiology 2017, 75, 1–6. [Google Scholar] [CrossRef] [PubMed]
- John Morris, G.; Acton, E. Controlled ice nucleation in cryopreservation—A review. Cryobiology 2013, 66, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Maki, L.R.; Galyan, E.L.; Chang-Chien, M.M.; Caldwell, D.R. Ice nucleation induced by pseudomonas syringae. Appl. Microbiol. 1974, 28, 456–459. [Google Scholar] [CrossRef]
- Marcolli, C. Ice nucleation triggered by negative pressure. Sci. Rep. 2017, 7, 16634. [Google Scholar] [CrossRef] [PubMed]
- Zaragotas, D.; Liolios, N.T.; Anastassopoulos, E. Supercooling, ice nucleation and crystal growth: A systematic study in plant samples. Cryobiology 2016, 72, 239–243. [Google Scholar] [CrossRef]
- Lauterboeck, L.; Hofmann, N.; Mueller, T.; Glasmacher, B. Active control of the nucleation temperature enhances freezing survival of multipotent mesenchymal stromal cells. Cryobiology 2015, 71, 384–390. [Google Scholar] [CrossRef]
- Massie, I.; Selden, C.; Hodgson, H.; Fuller, B.; Gibbons, S.; Morris, G.J. GMP Cryopreservation of Large Volumes of Cells for Regenerative Medicine: Active Control of the Freezing Process. Tissue Eng. Part C Methods 2014, 20, 693–702. [Google Scholar] [CrossRef]
- Petersen, A.; Schneider, H.; Rau, G.; Glasmacher, B. A new approach for freezing of aqueous solutions under active control of the nucleation temperature. Cryobiology 2006, 53, 248–257. [Google Scholar] [CrossRef]
- Prickett, R.C.; Marquez-Curtis, L.A.; Elliott, J.A.W.; McGann, L.E. Effect of supercooling and cell volume on intracellular ice formation. Cryobiology 2015, 70, 156–163. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Balasubramanian, S.K.; Ongstad, E.L.; Zec, H.C.; Bischof, J.C. Effects of freezing on membranes and proteins in LNCaP prostate tumor cells. Biochim. Biophys. Acta Biomembr. 2007, 1768, 728–736. [Google Scholar] [CrossRef]
- Baboo, J.; Kilbride, P.; Delahaye, M.; Milne, S.; Fonseca, F.; Blanco, M.; Meneghel, J.; Nancekievill, A.; Gaddum, N.; Morris, G.J. The Impact of Varying Cooling and Thawing Rates on the Quality of Cryopreserved Human Peripheral Blood T Cells. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Gurina, T.M.; Pakhomov, A.V.; Polyakova, A.L.; Legach, E.I.; Bozhok, G.A. The development of the cell cryopreservation protocol with controlled rate thawing. Cell Tissue Bank. 2016, 17, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Cheeseman, E.; Harriman, J.; Glen, K.E.; McCall, M.; Thomas, R. A new automated vial thawer controls the thawing of cryopreserved mesenchymal stem cells to achieve high cell viability and growth potential. Cytotherapy 2017, 19, S121–S122. [Google Scholar] [CrossRef]
- Saragusty, J.; Osmers, J.H.; Hildebrandt, T.B. Controlled ice nucleation-Is it really needed for large-volume sperm cryopreservation? Theriogenology 2016, 85, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Di Emidio, G.; Vento, M.; Ciriminna, R.; Artini, P.G. Cryopreservation and oxidative stress in reproductive cells. Gynecol. Endocrinol. 2010, 26, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Stacey, G.N.; Dowall, S. Cryopreservation of Primary Animal Cell Cultures. In Cryopreservation and Freeze-Drying Protocols; Day, J.G., Stacey, G.N., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 271–281. [Google Scholar]
- Liu, B.; McGrath, J. Freezing osteoblast cells attached to hydroxyapatite discs and glass coverslips: Mechanisms of damage. Sci. China Ser. E Technol. Sci. 2007, 50, 248–256. [Google Scholar] [CrossRef]
- Patapoff, T.W.; Overcashier, D.E. The importance of freezing on lyophilization cycle development. Biopharm 2002, 15, 16–21. [Google Scholar]
- Searles, J.A. Freezing and Annealing Phenomena in Lyophilization. In Freeze Drying/Lyophilization of Pharmaceutical and Biological Products; Rey, L., May, J.C., Eds.; Informa Healthcare: London, UK, 2010; Volume 206, pp. 52–81. ISBN 9781439825754. [Google Scholar]
- Kilbride, P.; Morris, G.J.; Milne, S.; Fuller, B.; Skepper, J.; Selden, C. A scale down process for the development of large volume cryopreservation. Cryobiology 2014, 69, 367–375. [Google Scholar] [CrossRef]
- Pruppacher, H.R.; Klett, J.D. Microphysics of Clouds and Precipitation, 2nd ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; ISBN 0-79-234211-1. [Google Scholar]
- Campbell, L.H.; Brockbank, K.G.M. Cryopreservation of Adherent Cells on a Fixed Substrate. In Recent Advances in Cryopreservation; InTechOnline: Mumbai, India, 2014; p. 13. [Google Scholar]
- Sugawara, Y.; Ichi-ishi, A. Application of “CryoSeeds” in the Cryopreservation of Cultured Plant Cells and Tissues. Plant Tissue Cult. Lett. 1992, 9, 47–50. [Google Scholar] [CrossRef][Green Version]
- Trad, F.S.; Toner, M.; Biggers, J.D. Effects of cryoprotectants and ice-seeding temperature on intracellular freezing and survival of human oocytes. Hum. Reprod. 1999, 14, 1569–1577. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, G.; Zhang, Y.; Xu, J.; Toth, T.L.; He, X. Predehydration and Ice Seeding in the Presence of Trehalose Enable Cell Cryopreservation. ACS Biomater. Sci. Eng. 2017, 3, 1758–1768. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, D.H.; Macaulay, M.N.; Mackenzie, A.P. Supercooling and nucleation of ice in single cells. Cryobiology 1975, 12, 328–339. [Google Scholar] [CrossRef]
- Franks, F.; Mathias, S.F.; Galfre, P.; Webster, S.D.; Brown, D. Ice nucleation and freezing in undercooled cells. Cryobiology 1983, 20, 298–309. [Google Scholar] [CrossRef]
- Tipler, P.A.; Mosca, G.P. Physics for Scientists and Engineers, 6th ed.; W.H. Freeman & Co Ltd.: New York, NY, USA, 2007; ISBN 978-1429201247. [Google Scholar]
- Kilbride, P.; Lamb, S.; Gibbons, S.; Bundy, J.; Erro, E.; Selden, C.; Fuller, B.; Morris, J. Cryopreservation and re-culture of a 2.3 litre biomass for use in a bioartificial liver device. PLoS ONE 2017, 12, 1–18. [Google Scholar] [CrossRef]
- Kilbride, P.; Mahbubani, K.T.; Lamb, S.; Morris, G.J. Cryopreservation of adherent cells in 96 well plates. In Proceedings of the 2nd IIR Workshop on Cold Applications in Life Sciences, Dresden, Germany, 8–9 September 2016; pp. 19–23. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wragg, N.M.; Tampakis, D.; Stolzing, A. Cryopreservation of Mesenchymal Stem Cells Using Medical Grade Ice Nucleation Inducer. Int. J. Mol. Sci. 2020, 21, 8579. https://doi.org/10.3390/ijms21228579
Wragg NM, Tampakis D, Stolzing A. Cryopreservation of Mesenchymal Stem Cells Using Medical Grade Ice Nucleation Inducer. International Journal of Molecular Sciences. 2020; 21(22):8579. https://doi.org/10.3390/ijms21228579
Chicago/Turabian StyleWragg, Nicholas M., Dimitris Tampakis, and Alexandra Stolzing. 2020. "Cryopreservation of Mesenchymal Stem Cells Using Medical Grade Ice Nucleation Inducer" International Journal of Molecular Sciences 21, no. 22: 8579. https://doi.org/10.3390/ijms21228579
APA StyleWragg, N. M., Tampakis, D., & Stolzing, A. (2020). Cryopreservation of Mesenchymal Stem Cells Using Medical Grade Ice Nucleation Inducer. International Journal of Molecular Sciences, 21(22), 8579. https://doi.org/10.3390/ijms21228579




