Partial Sperm beta1 Integrin Subunit Deletion Proves Its Involvement in Mouse Gamete Adhesion/Fusion
Abstract
1. Introduction
2. Results
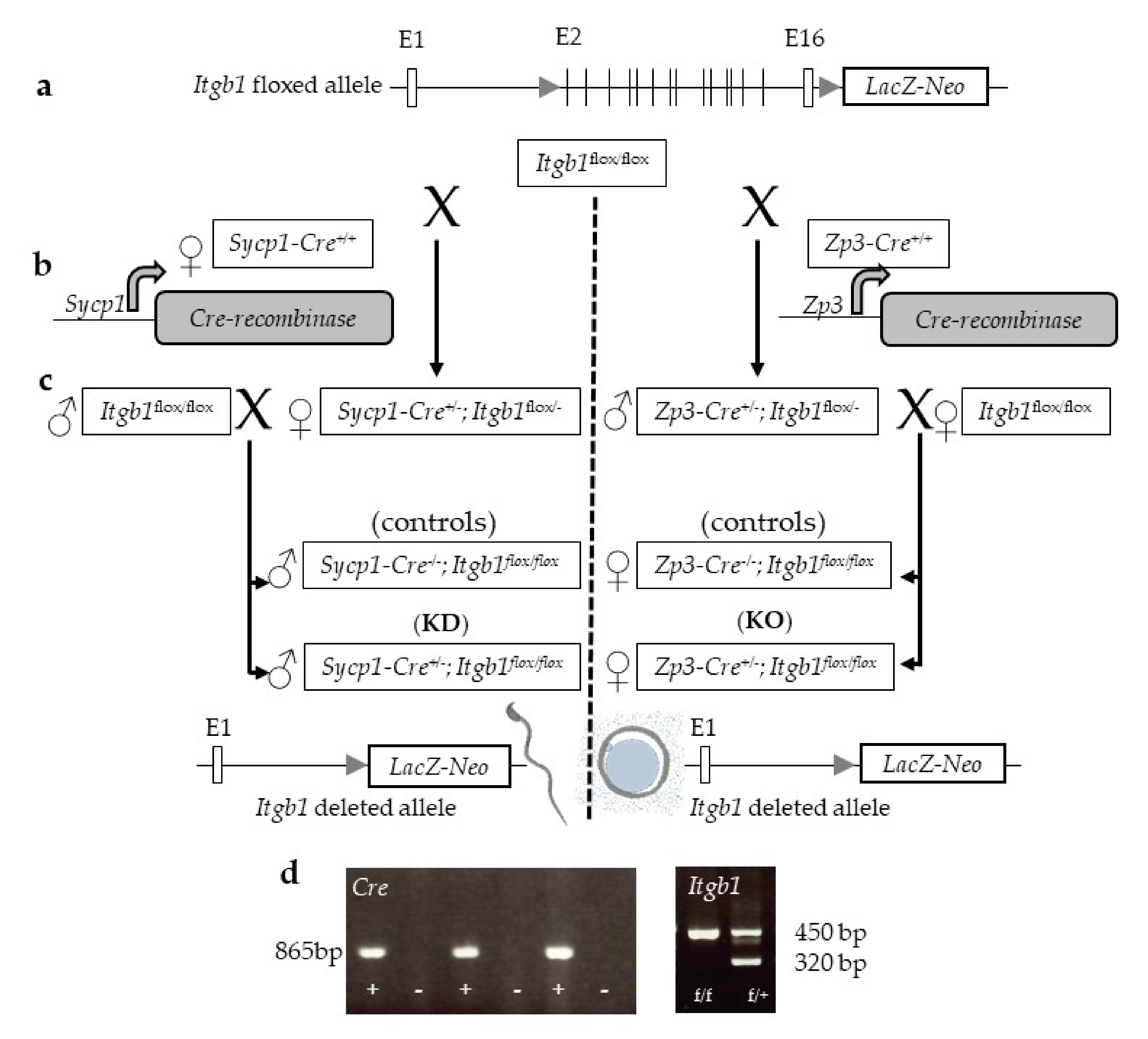
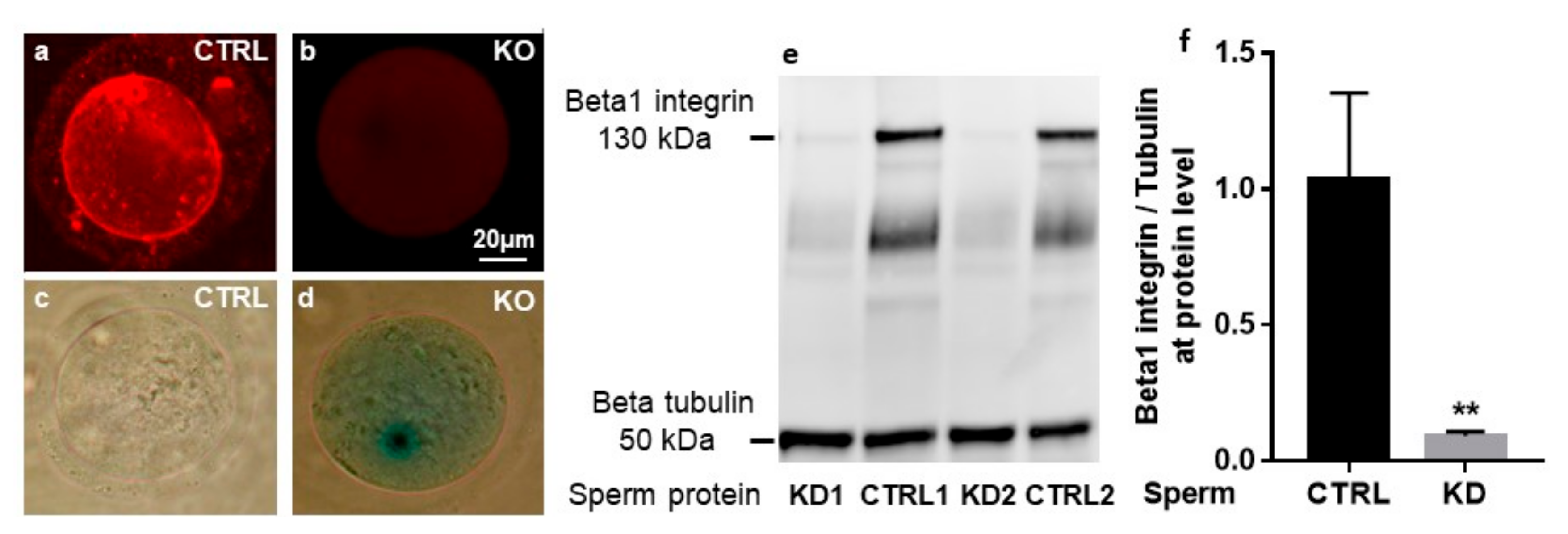
2.1. Cre-Mediated Deletion of the Itgb1 Gene in Oocytes and Sperm
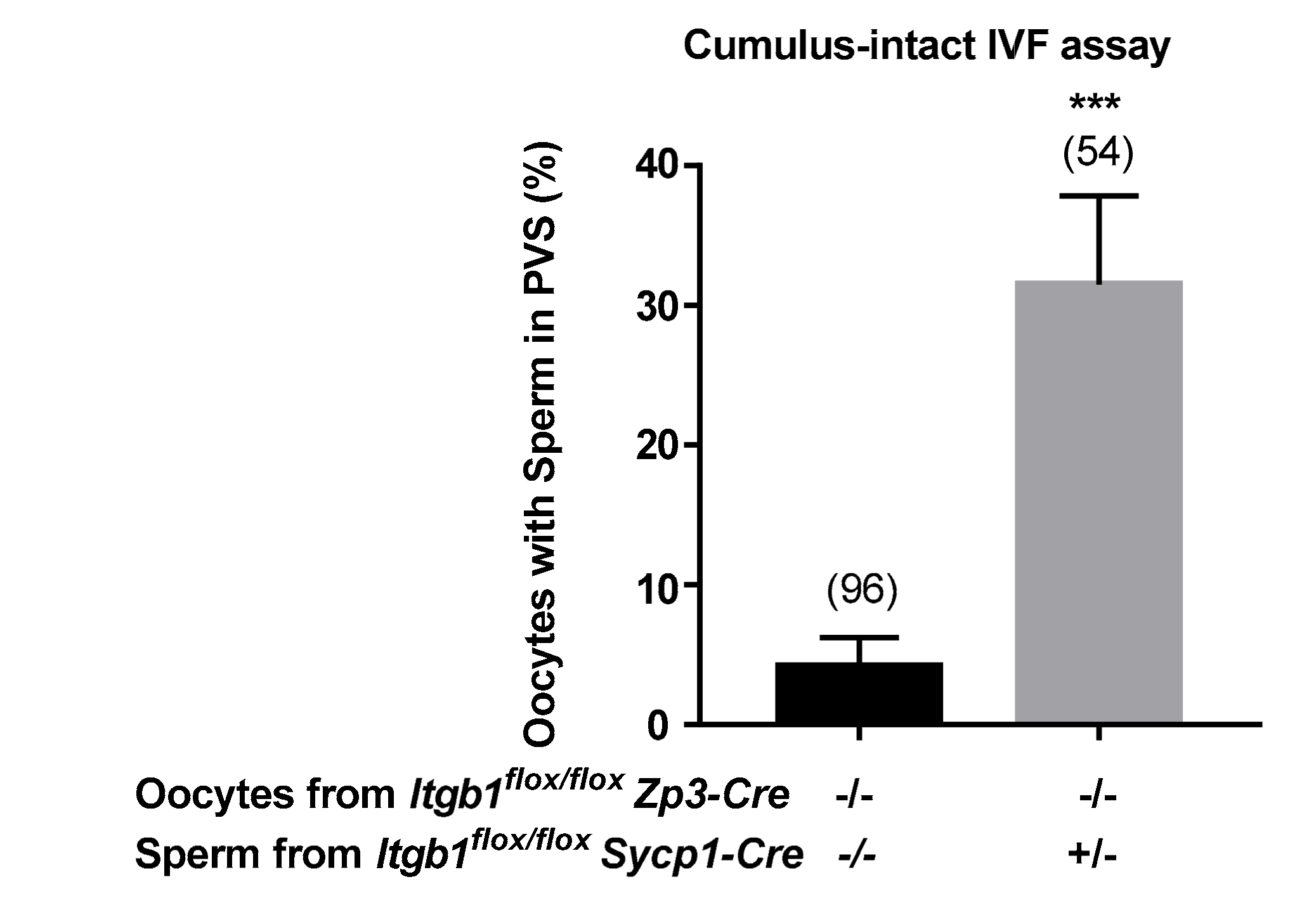
2.2. In Vivo and in Vitro Evaluation of the Fertilizing Ability of Sycp1-Cre +/− Itgb1 flox/flox Sperm and Zp3-Cre +/− Itgb1 flox/flox Oocytes
2.3. In vitro Accumulation of Sperm from Sycp1-Cre +/− Itgb1 flox/flox Males in the Perivitelline Space of the Oocytes
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Generation of Oocyte and Sperm Itgb1 Conditional Knockout Mice
4.3. Immunofluorescence and Beta-galactosidase Staining of Mouse Oocytes
4.4. Western Blot Analysis and Quantification of Sperm β1 Integrin
4.5. In Vivo Mating, Gamete Preparation and in Vitro Fertilization
4.6. Gamete Preparation and in Vitro Fertilization
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Acrosome reaction |
| CTRL | control |
| DAPI | 4′, 6-diamidino-2-phenylindole |
| IVF | In Vitro fertilization |
| KD | knockdown |
| KO | knockout |
| ZP | Zona pellucida |
References
- Almeida, E.A.; Huovila, A.P.; Sutherland, A.E.; Stephens, L.E.; Calarco, P.G.; Shaw, L.M.; Mercurio, A.M.; Sonnenberg, A.; Primakoff, P.; Myles, D.G.; et al. Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell 1995, 81, 1095–1104. [Google Scholar] [CrossRef]
- Miller, B.J.; Georges-Labouesse, E.; Primakoff, P.; Myles, D.G. Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is CD9-dependent. J. Cell Biol. 2000, 149, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Y.; Brakebusch, C.; Fassler, R.; Kreidberg, J.A.; Primakoff, P.; Myles, D.G. None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm-egg binding and fusion. Dev. Biol. 2003, 254, 226–237. [Google Scholar] [CrossRef]
- Barraud-Lange, V.; Naud-Barriant, N.; Saffar, L.; Gattegno, L.; Ducot, B.; Drillet, A.S.; Bomsel, M.; Wolf, J.P.; Ziyyat, A. Alpha6beta1 integrin expressed by sperm is determinant in mouse fertilization. BMC Dev. Biol. 2007, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Boissonnas, C.C.; Montjean, D.; Lesaffre, C.; Auer, J.; Vaiman, D.; Wolf, J.P.; Ziyyat, A. Role of sperm alphavbeta3 integrin in mouse fertilization. Dev. Dyn. 2010, 239, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Glander, H.J.; Dethloff, J. Evidence of beta 1 integrins and fibronectin on spermatogenic cells in human testis. Hum. Reprod. 1993, 8, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.R.; Rajeev, S.K.; Gupta, V. Alpha 6 beta 1 Integrin is a potential clinical marker for evaluating sperm quality in men. Fertil. Steril. 2003, 79 (Suppl. 3), 1590–1596. [Google Scholar] [CrossRef]
- Klentzeris, L.D.; Fishel, S.; McDermott, H.; Dowell, K.; Hall, J.; Green, S. A positive correlation between expression of beta 1-integrin cell adhesion molecules and fertilizing ability of human spermatozoa in vitro. Hum. Reprod. 1995, 10, 728–733. [Google Scholar] [CrossRef]
- Frolikova, M.; Valaskova, E.; Cerny, J.; Lumeau, A.; Sebkova, N.; Palenikova, V.; Sanches-Hernandez, N.; Pohlova, A.; Manaskova-Postlerova, P.; Dvorakova-Hortova, K. Addressing the Compartmentalization of Specific Integrin Heterodimers in Mouse Sperm. Int. J. Mol. Sci. 2019, 20, 1004. [Google Scholar] [CrossRef]
- Fusi, F.M.; Bernocchi, N.; Ferrari, A.; Bronson, R.A. Is vitronectin the velcro that binds the gametes together? Mol. Hum. Reprod. 1996, 2, 859–866. [Google Scholar] [CrossRef]
- Henkel, R.; Schaller, J.; Glander, H.J.; Schill, W.B. Low expression of adhesion molecules and matrix proteins in patients showing poor penetration in zona-free hamster oocytes. Mol. Hum. Reprod. 1996, 2, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.V.; Meherji, P.K.; Shahani, S.K. Integrin cell adhesion molecules on human spermatozoa. Indian. J. Exp. Biol. 1998, 36, 456–463. [Google Scholar] [PubMed]
- Xie, Y.; Yue, L.; He, Y.; Zhang, J.; Mao, R.; Lei, S.; Zheng, Y. Study on the effect of integrin on human sperm activation. SO Sichuan Da Xue Xue Bao Yi Xue Ban 2003, 34, 459–461. [Google Scholar] [PubMed]
- Goncalves, R.F.; Wolinetz, C.D.; Killian, G.J. Influence of arginine-glycine-aspartic acid (RGD), integrins (alpha(V) and alpha(5)) and osteopontin on bovine sperm-egg binding, and fertilization in vitro. Theriogenology 2007, 63, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.F.; Bertolla, R.P.; Mortara, R.A.; Barnabe, V.H. alpha(6), beta(1), and beta(3) integrins expressed by sperm may be involved in cattle fertilization. Reprod. Fertil. Dev. 2009, 21, 201. [Google Scholar] [CrossRef]
- Thys, M.; Nauwynck, H.; Maes, D.; Hoogewijs, M.; Vercauteren, D.; Favoreel, H.; Van Soom, A. Expression and putative function of fibronectin and its receptor (integrin alpha5 beta1) in male and female gametes during bovine fertilization in vitro. Reproduction 2009, 138, 471–482. [Google Scholar] [CrossRef]
- Osycka-Salut, C.E.; Martinez-Leon, E.; Gervasi, M.G.; Castellano, L.; Davio, C.; Chiarante, N.; Franchi, A.M.; Ribeiro, M.L.; Diaz, E.S.; Perez-Martinez, S. Fibronectin induces capacitation-associated events through the endocannabinoid system in bull sperm. Theriogenology 2020, 153, 91–101. [Google Scholar] [CrossRef]
- Barboni, B.; Lucidi, P.; Mattioli, M.; Berardinelli, P. VLA-6 integrin distribution and calcium signalling in capacitated boar sperm. Mol. Reprod. Dev. 2001, 59, 322–329. [Google Scholar] [CrossRef]
- Mattioli, M.; Lucidi, P.; Barboni, B. Expanded cumuli induce acrosome reaction in boar sperm. Mol. Reprod. Dev. 1998, 51, 445–453. [Google Scholar] [CrossRef]
- Barbaux, S.; Ialy-Radio, C.; Chalbi, M.; Dybal, E.; Homps-Legrand, M.; Do Cruzeiro, M.; Vaiman, D.; Wolf, J.P.; Ziyyat, A. Sperm SPACA6 protein is required for mammalian Sperm-Egg Adhesion/Fusion. Sci. Rep. 2020, 10, 5335. [Google Scholar] [CrossRef]
- Inoue, N.; Wada, I. Monitoring dimeric status of IZUMO1 during the acrosome reaction in living spermatozoon. Cell Cycle 2018, 17, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Satouh, Y.; Inoue, N.; Ikawa, M.; Okabe, M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J. Cell Sci. 2012, 125, 4985–4990. [Google Scholar] [CrossRef] [PubMed]
- Sebkova, N.; Ded, L.; Vesela, K.; Dvorakova-Hortova, K. Progress of sperm IZUMO1 relocation during spontaneous acrosome reaction. Reproduction 2014, 147, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, J.; Miranda, P.V.; Spiridonov, N.A.; Yoon, S.Y.; Fissore, R.A.; Johnson, G.R.; Visconti, P.E. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J. Cell Sci. 2009, 122, 2741–2749. [Google Scholar] [CrossRef] [PubMed]
- Frolikova, M.; Sebkova, N.; Ded, L.; Dvorakova-Hortova, K. Characterization of CD46 and beta1 integrin dynamics during sperm acrosome reaction. Sci. Rep. 2016, 6, 33714. [Google Scholar] [CrossRef]
- Fassler, R.; Meyer, M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995, 9, 1896–1908. [Google Scholar] [CrossRef]
- Stephens, L.E.; Sutherland, A.E.; Klimanskaya, I.V.; Andrieux, A.; Meneses, J.; Pedersen, R.A.; Damsky, C.H. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995, 9, 1883–1895. [Google Scholar] [CrossRef]
- Rasoulpour, R.J.; Boekelheide, K. The Sycp1-Cre transgenic mouse and male germ cell inhibition of NF-kappa b. J. Androl. 2006, 27, 729–733. [Google Scholar] [CrossRef][Green Version]
- Rassoulzadegan, M.; Magliano, M.; Cuzin, F. Transvection effects involving DNA methylation during meiosis in the mouse. EMBO J. 2002, 21, 440–450. [Google Scholar] [CrossRef]
- Braun, R.E.; Behringer, R.R.; Peschon, J.J.; Brinster, R.L.; Palmiter, R.D. Genetically haploid spermatids are phenotypically diploid. Nature 1989, 337, 373–376. [Google Scholar] [CrossRef]
- Vjugina, U.; Zhu, X.; Oh, E.; Bracero, N.J.; Evans, J.P. Reduction of Mouse Egg Surface Integrin Alpha9 Subunit (ITGA9) Reduces the Egg’s Ability to Support Sperm-Egg Binding and Fusion. Biol. Reprod. 2009, 80, 833–841. [Google Scholar] [CrossRef][Green Version]
- Desiderio, U.V.; Zhu, X.; Evans, J.P. ADAM2 Interactions with Mouse Eggs and Cell Lines Expressing alpha(4)/alpha(9) (ITGA4/ITGA9) Integrins: Implications for Integrin-Based Adhesion and Fertilization. PLoS ONE 2010, 5, e13744. [Google Scholar] [CrossRef]
- Baessler, K.A.; Lee, Y.; Sampson, N.S. Beta1 integrin is an adhesion protein for sperm binding to eggs. ACS Chem. Biol. 2009, 4, 357–366. [Google Scholar] [CrossRef]
- Le Naour, F.; Rubinstein, E.; Jasmin, C.; Prenant, M.; Boucheix, C. Severely reduced female fertility in CD9-deficient mice. Science 2000, 287, 319–321. [Google Scholar] [CrossRef]
- Miyado, K.; Yamada, G.; Yamada, S.; Hasuwa, H.; Nakamura, Y.; Ryu, F.; Suzuki, K.; Kosai, K.; Inoue, K.; Ogura, A.; et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science 2000, 287, 321–324. [Google Scholar] [CrossRef]
- Bianchi, E.; Doe, B.; Goulding, D.; Wright, G.J. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014, 508, 483–487. [Google Scholar] [CrossRef]
- Fujihara, Y.; Lu, Y.; Noda, T.; Oji, A.; Larasati, T.; Kojima-Kita, K.; Yu, Z.; Matzuk, R.M.; Matzuk, M.M.; Ikawa, M. Spermatozoa lacking Fertilization Influencing Membrane Protein (FIMP) fail to fuse with oocytes in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 9393–9400. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Isotani, A.; Okabe, M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005, 434, 234–238. [Google Scholar] [CrossRef]
- Noda, T.; Lu, Y.; Fujihara, Y.; Oura, S.; Koyano, T.; Kobayashi, S.; Matzuk, M.M.; Ikawa, M. Sperm proteins SOF1, TMEM95, and SPACA6 are required for sperm-oocyte fusion in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 11493–11502. [Google Scholar] [CrossRef]
- Sadate-Ngatchou, P.I.; Payne, C.J.; Dearth, A.T.; Braun, R.E. Cre recombinase activity specific to postnatal, premeiotic male germ cells in transgenic mice. Genesis 2008, 46, 738–742. [Google Scholar] [CrossRef]
- Hirota, T.; Blakeley, P.; Sangrithi, M.N.; Mahadevaiah, S.K.; Encheva, V.; Snijders, A.P.; ElInati, E.; Ojarikre, O.A.; de Rooij, D.G.; Niakan, K.K.; et al. SETDB1 Links the Meiotic DNA Damage Response to Sex Chromosome Silencing in Mice. Dev. Cell 2018, 47, 645–659.e6. [Google Scholar] [CrossRef]
- Suarez, S.S. Sperm transport and motility in the mouse oviduct: Observations in situ. Biol. Reprod. 1987, 36, 203–210. [Google Scholar] [CrossRef]
- Baillie, H.S.; Pacey, A.A.; Warren, M.A.; Scudamore, I.W.; Barratt, C.L. Greater numbers of human spermatozoa associate with endosalpingeal cells derived from the isthmus compared with those from the ampulla. Hum. Reprod. 1997, 12, 1985–1992. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Muro, Y.; Isotani, A.; Tokuhiro, K.; Takumi, K.; Adham, I.; Ikawa, M.; Okabe, M. Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol. Reprod. 2009, 81, 142–146. [Google Scholar] [CrossRef]
- Potocnik, A.J.; Brakebusch, C.; Fassler, R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 2000, 12, 653–663. [Google Scholar] [CrossRef]
- Vidal, F.; Sage, J.; Cuzin, F.; Rassoulzadegan, M. Cre expression in primary spermatocytes: A tool for genetic engineering of the germ line. Mol. Reprod. Dev. 1998, 51, 274–280. [Google Scholar] [CrossRef]
- Lewandoski, M.; Wassarman, K.M.; Martin, G.R. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr. Biol. 1997, 7, 148–151. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barraud-Lange, V.; Ialy-Radio, C.; Chalas, C.; Holtzmann, I.; Wolf, J.-P.; Barbaux, S.; Ziyyat, A. Partial Sperm beta1 Integrin Subunit Deletion Proves Its Involvement in Mouse Gamete Adhesion/Fusion. Int. J. Mol. Sci. 2020, 21, 8494. https://doi.org/10.3390/ijms21228494
Barraud-Lange V, Ialy-Radio C, Chalas C, Holtzmann I, Wolf J-P, Barbaux S, Ziyyat A. Partial Sperm beta1 Integrin Subunit Deletion Proves Its Involvement in Mouse Gamete Adhesion/Fusion. International Journal of Molecular Sciences. 2020; 21(22):8494. https://doi.org/10.3390/ijms21228494
Chicago/Turabian StyleBarraud-Lange, Virginie, Côme Ialy-Radio, Céline Chalas, Isabelle Holtzmann, Jean-Philippe Wolf, Sandrine Barbaux, and Ahmed Ziyyat. 2020. "Partial Sperm beta1 Integrin Subunit Deletion Proves Its Involvement in Mouse Gamete Adhesion/Fusion" International Journal of Molecular Sciences 21, no. 22: 8494. https://doi.org/10.3390/ijms21228494
APA StyleBarraud-Lange, V., Ialy-Radio, C., Chalas, C., Holtzmann, I., Wolf, J.-P., Barbaux, S., & Ziyyat, A. (2020). Partial Sperm beta1 Integrin Subunit Deletion Proves Its Involvement in Mouse Gamete Adhesion/Fusion. International Journal of Molecular Sciences, 21(22), 8494. https://doi.org/10.3390/ijms21228494




