3D-Reconstructed Retinal Pigment Epithelial Cells Provide Insights into the Anatomy of the Outer Retina
Abstract
1. Introduction
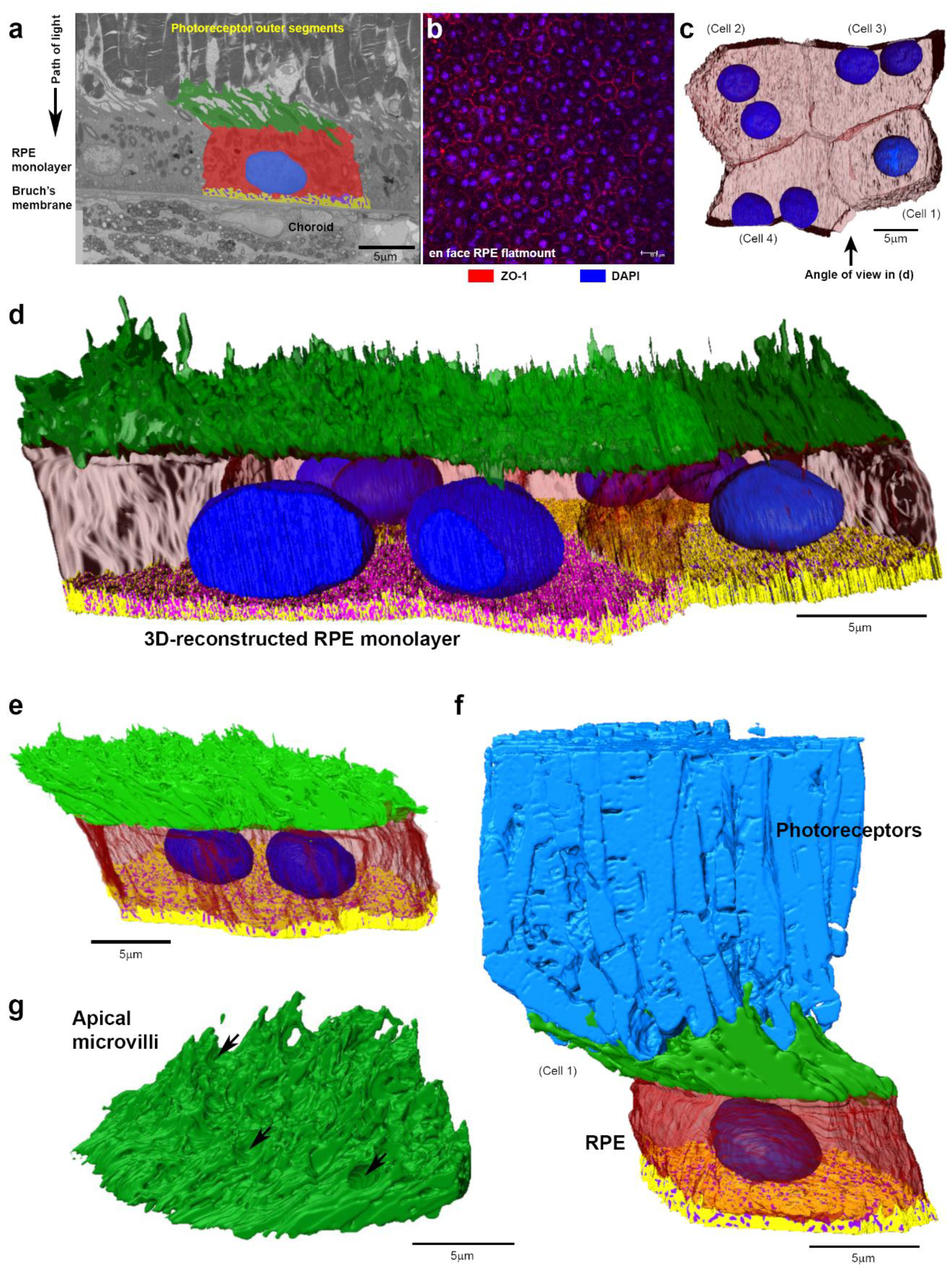
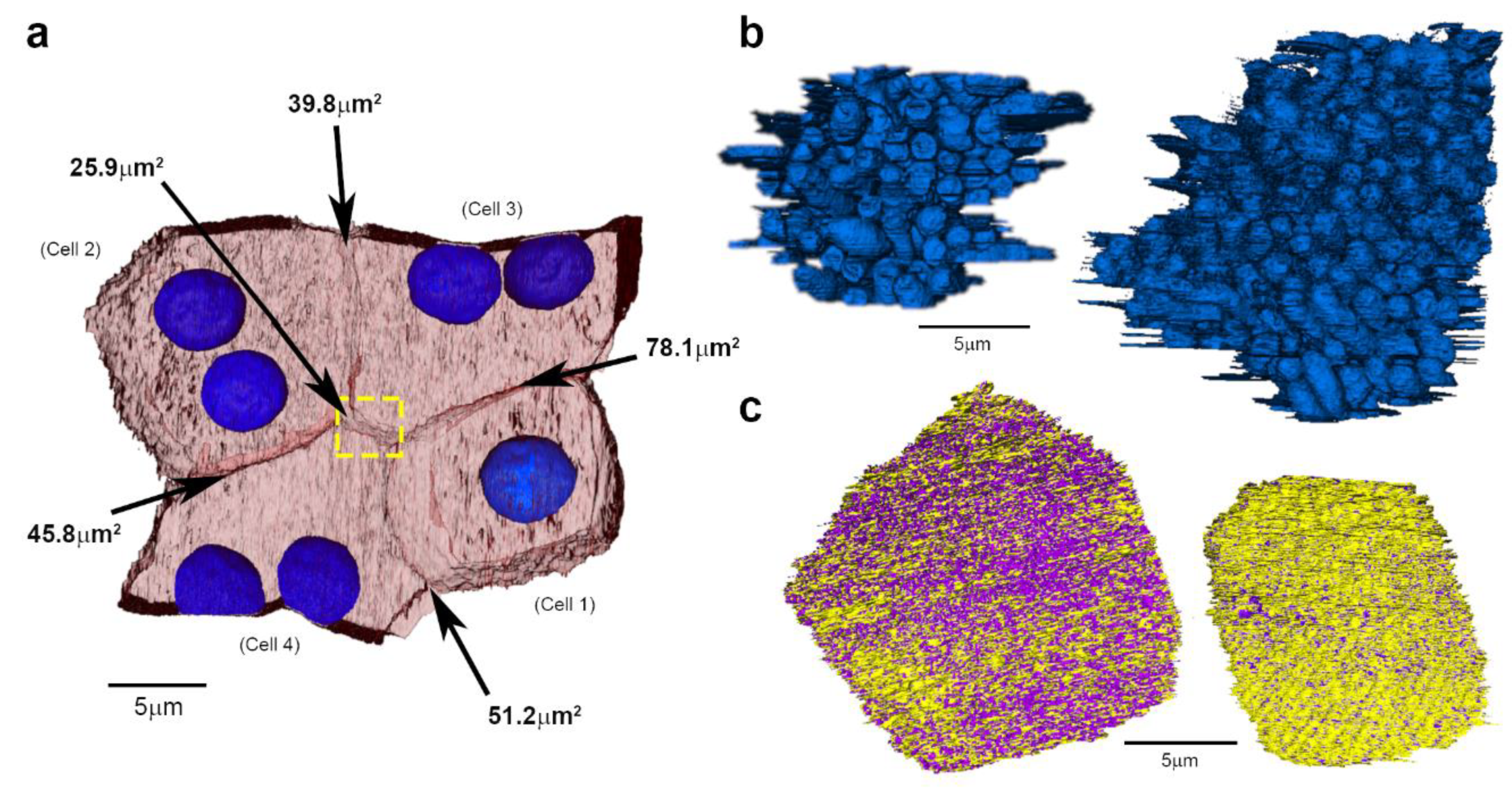
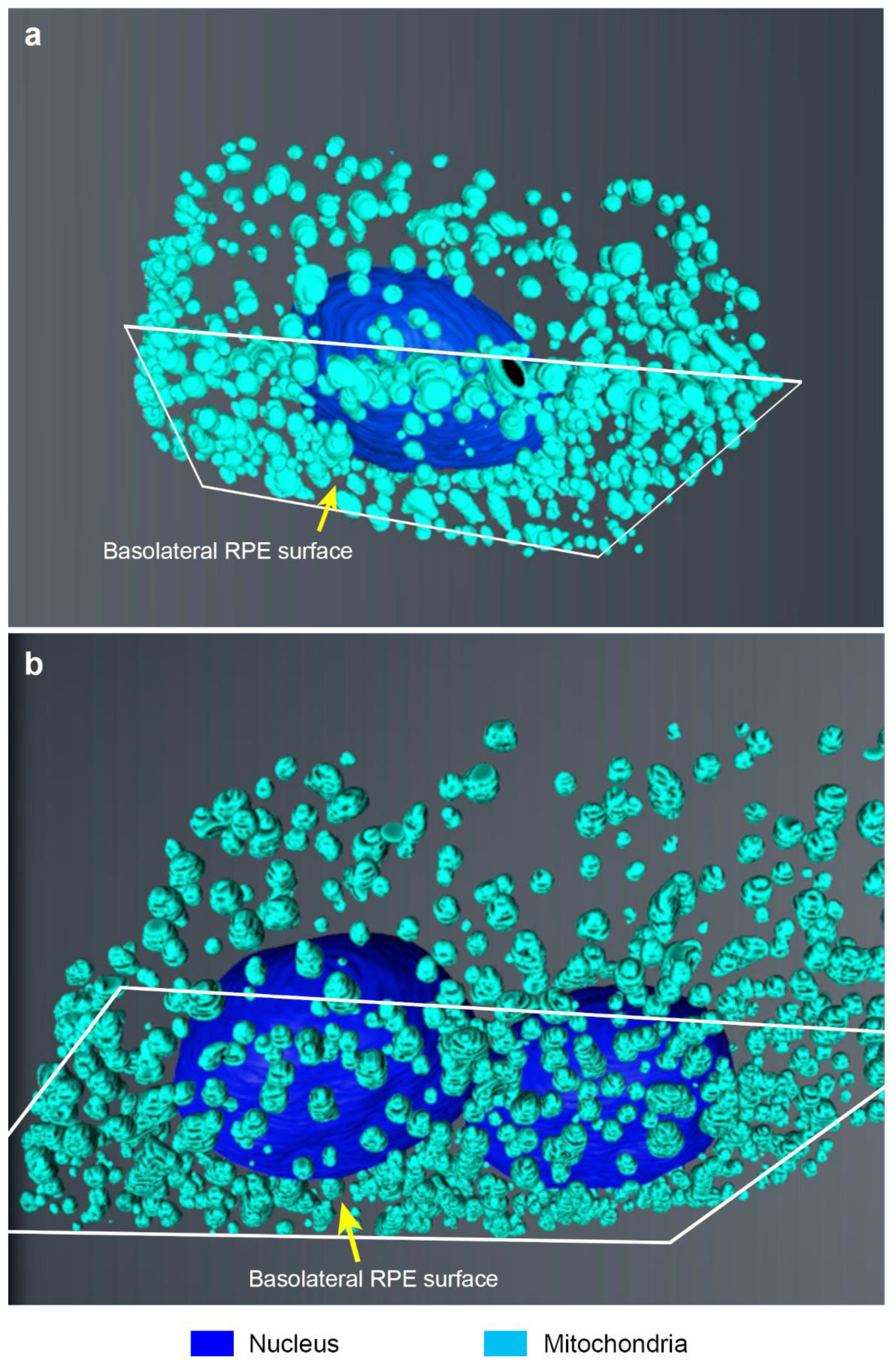
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Procedures
4.2. Preparation of Flatmounts
4.3. Preparation of Eyes for SBF-SEM
4.4. SBF-SEM
4.5. Segmentation and 3D Reconstructions
4.6. Analysis
| object1 = “Untitled”; |
| object2 = “Untitled”; |
| Dialog.create(“Measuring shared area between”); |
| Dialog.addString(“Object1:”, object1); |
| Dialog.addString(“Object2:”, object2); |
| Dialog.show(); |
| object1 = Dialog.getString(); |
| object2 = Dialog.getString(); |
| path = File.directory(); |
| title_orig = getTitle(); |
| getVoxelSize(width, height, depth, unit); |
| pixelWidth = width; |
| pixelDepth = depth; |
| pixelUnit = unit; |
| junctionArea_total = 0; |
| junctionArea_array = newArray(0); |
| imageNbr = nSlices(); |
| for(i = 1; i < imageNbr; i++){ |
| selectWindow(title_orig); |
| setSlice(i); |
| run(“Duplicate...”, “title = Object1.tif”); |
| setAutoThreshold(“Default”); |
| //run(“Threshold...”); |
| setAutoThreshold(“Default dark”); |
| setThreshold(1, 1); |
| setOption(“BlackBackground”, false); |
| run(“Convert to Mask”); |
| rename(“Object1_binary.tif”); |
| run(“Dilate”); |
| selectWindow(title_orig); |
| run(“Duplicate...”, “title = Object2.tif”); |
| setAutoThreshold(“Default”); |
| //run(“Threshold...”); |
| setAutoThreshold(“Default dark”); |
| setThreshold(2, 2); |
| setOption(“BlackBackground”, false); |
| run(“Convert to Mask”); |
| rename(“Object2_binary.tif”); |
| //run(“Dilate”); |
| //run(“Fill Holes”); |
| imageCalculator(“AND create”, “Object1_binary.tif”,”Object2_binary.tif”); |
| selectWindow(“Object1_binary.tif”); |
| close(); |
| selectWindow(“Object2_binary.tif”); |
| close(); |
| selectWindow(“Result of Object1_binary.tif”); |
| setAutoThreshold(“Default dark”); |
| //run(“Threshold...”); |
| setAutoThreshold(“Default”); |
| run(“Create Selection”); |
| getSelectionBounds(xRect0, yRect0, xWidth, yHeight); |
| selectionArray = newArray(xWidth*yHeight); |
| a = 0; |
| for(y = yRect0; y < yRect0 + yHeight; y++){ |
| for(x = xRect0; x < xRect0 + xWidth; x++){ |
| selectionArray[a++] = getPixel(x,y); |
| } |
| } |
| selectionArray2 = newArray(0); |
| for(c = 0; c < selectionArray.length; c++){ |
| if (selectionArray[c] > 254){ |
| selectionArray2 = Array.concat(selectionArray2, selectionArray[c]); |
| } |
| } |
| nbrPixel = selectionArray2.length; |
| junctionLength = nbrPixel * pixelWidth; |
| junctionArea = junctionLength * pixelDepth; |
| junctionArea_array = Array.concat(junctionArea_array, junctionArea); |
| junctionArea_total = junctionArea_total + junctionArea; |
| selectWindow(“Result of Object1_binary.tif”); |
| close(); |
| } |
| if (nResults > =0) { |
| run(“Clear Results”); |
| } |
| i = nResults; |
| for (n = 0; n < junctionArea_array.length; n++){ |
| setResult(“Slice”, i, n); |
| setResult(“Pixel_Unit”, 0, pixelUnit); |
| setResult(“Surf.Area_stack”, 0, junctionArea_total); |
| setResult(“Surf.Area_slices”, i, junctionArea_array[n]); |
| i = nResults; |
| } |
| updateResults; |
| saveAs(“Results”, path + “SharedSA_”+object1+”_”+object2+”.csv”); |
| run(“Close”); |
| selectWindow(title_orig); |
| close(); |
| exit(“ANALYSIS FINISHED”); |
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ts’o, M.O.; Friedman, E. The retinal pigment epithelium: III. Growth and development. Arch. Ophthalmol. (Chic.) 1968, 80, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Marmor, M.F.; Wolfensberger, T. The retinal pigment epithelium. In Function and Disease; Oxford University Press: New York, NY, USA, 1998; pp. 103–134. [Google Scholar]
- Bodenstein, L.; Sidman, R.L. Growth and development of the mouse retinal pigment epithelium. I. Cell and tissue morphometrics and topography of mitotic activity. Dev. Biol. 1987, 121, 192–204. [Google Scholar] [CrossRef]
- Ferrington, D.A.; Sinha, D.; Kaarniranta, K. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog. Retin. Eye Res. 2016, 51, 69–89. [Google Scholar] [CrossRef]
- Altunay, H. Fine structure of the retinal pigment epithelium, Bruch’s membrane and choriocapillaris in the camel. Anat. Histol. Embryol. 2007, 36, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Booij, J.C.; Baas, D.C.; Beisekeeva, J.; Gorgels, T.G.; Bergen, A.A. The dynamic nature of Bruch’s membrane. Prog. Retin. Eye Res. 2010, 29, 1–18. [Google Scholar] [CrossRef]
- Bonilha, V.L. Age and disease-related structural changes in the retinal pigment epithelium. Clin. Ophthalmol. 2008, 2, 413–424. [Google Scholar] [CrossRef]
- Christensen, D.R.G.; Brown, F.E.; Cree, A.J.; Ratnayaka, J.A.; Lotery, A.J. Sorsby fundus dystrophy—A review of pathology and disease mechanisms. Exp. Eye Res. 2017, 165, 35–46. [Google Scholar] [CrossRef]
- Michaelides, M.; Hunt, D.M.; Moore, A.T. The genetics of inherited macular dystrophies. J. Med. Genet. 2003, 40, 641–650. [Google Scholar] [CrossRef]
- Smith, D.; Starborg, T. Serial block face scanning electron microscopy in cell biology: Applications and technology. Tissue Cell 2019, 57, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Sterratt, D.C.; Lyngholm, D.; Willshaw, D.J.; Thompson, I.D. Standard anatomical and visual space for the mouse retina: Computational reconstruction and transformation of flattened retinae with the Retistruct package. PLoS Comput. Biol. 2013, 9, e1002921. [Google Scholar] [CrossRef]
- Volland, S.; Esteve-Rudd, J.; Hoo, J.; Yee, C.; Williams, D.S. A comparison of some organizational characteristics of the mouse central retina and the human macula. PLoS ONE 2015, 10, e0125631. [Google Scholar] [CrossRef]
- Mustafi, D.; Kevany, B.M.; Genoud, C.; Okano, K.; Cideciyan, A.V.; Sumaroka, A.; Roman, A.J.; Jacobson, S.G.; Engel, A.; Adams, M.D.; et al. Defective photoreceptor phagocytosis in a mouse model of enhanced S-cone syndrome causes progressive retinal degeneration. FASEB J. 2011, 25, 3157–3176. [Google Scholar] [CrossRef]
- Pollreisz, A.; Messinger, J.D.; Sloan, K.R.; Mittermueller, T.J.; Weinhandl, A.S.; Benson, E.K.; Kidd, G.J.; Schmidt-Erfurth, U.; Curcio, C.A. Visualizing melanosomes, lipofuscin, and melanolipofuscin in human retinal pigment epithelium using serial block face scanning electron microscopy. Exp. Eye Res. 2018, 166, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Volland, S.; Hughes, L.C.; Kong, C.; Burgess, B.L.; Linberg, K.A.; Luna, G.; Zhou, Z.H.; Fisher, S.K.; Williams, D.S. Three-dimensional organization of nascent rod outer segment disk membranes. Proc. Natl. Acad. Sci. USA 2015, 112, 14870–14875. [Google Scholar] [CrossRef]
- Hayes, M.J.; Burgoyne, T.; Wavre-Shapton, S.T.; Tolmachova, T.; Seabra, M.C.; Futter, C.E. Remodeling of the Basal Labyrinth of Retinal Pigment Epithelial Cells With Osmotic Challenge, Age, and Disease. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2515–2524. [Google Scholar] [CrossRef]
- Young, R.D.; Knupp, C.; Pinali, C.; Png, K.M.; Ralphs, J.R.; Bushby, A.J.; Starborg, T.; Kadler, K.E.; Quantock, A.J. Three-dimensional aspects of matrix assembly by cells in the developing cornea. Proc. Natl. Acad. Sci. USA 2014, 111, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.B.; Scott, A.; Zhu, M.; Munro, P.M.; Foss, A.J.; Hageman, G.S.; Gillies, M.C.; Fruttiger, M. Basement membrane changes in capillaries of the ageing human retina. Br. J. Ophthalmol. 2011, 95, 1316–1322. [Google Scholar] [CrossRef]
- Starborg, T.; Kalson, N.S.; Lu, Y.; Mironov, A.; Cootes, T.F.; Holmes, D.F.; Kadler, K.E. Using transmission electron microscopy and 3View to determine collagen fibril size and three-dimensional organization. Nat. Protoc. 2013, 8, 1433–1448. [Google Scholar] [CrossRef]
- Palaiologou, E.; Goggin, P.; Chatelet, D.S.; Ribeiro de Souza, R.; Chiu, W.; Ashley, B.; Lofthouse, E.M.; Sengers, B.G.; Torrens, C.; Page, A.M.; et al. Serial block-face scanning electron microscopy reveals novel intercellular connections in human term placental microvasculature. J. Anat. 2020. [Google Scholar] [CrossRef]
- Ach, T.; Huisingh, C.; McGwin, G., Jr.; Messinger, J.D.; Zhang, T.; Bentley, M.J.; Gutierrez, D.B.; Ablonczy, Z.; Smith, R.T.; Sloan, K.R.; et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4832–4841. [Google Scholar] [CrossRef]
- Ts’o, M.O.; Friedman, E. The retinal pigment epithelium. I. Comparative histology. Arch. Ophthalmol. (Chic.) 1967, 78, 641–649. [Google Scholar] [CrossRef]
- Chen, M.; Rajapakse, D.; Fraczek, M.; Luo, C.; Forrester, J.V.; Xu, H. Retinal pigment epithelial cell multinucleation in the aging eye—A mechanism to repair damage and maintain homoeostasis. Aging Cell 2016, 15, 436–445. [Google Scholar] [CrossRef]
- Del Priore, L.V.; Kuo, Y.H.; Tezel, T.H. Age-related changes in human RPE cell density and apoptosis proportion in situ. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3312–3318. [Google Scholar]
- Ding, J.D.; Johnson, L.V.; Herrmann, R.; Farsiu, S.; Smith, S.G.; Groelle, M.; Mace, B.E.; Sullivan, P.; Jamison, J.A.; Kelly, U.; et al. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, E279–E287. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.J.; Krebs, M.P.; Mao, H.; Jones, K.; Conners, M.; Lewin, A.S. Pathological consequences of long-term mitochondrial oxidative stress in the mouse retinal pigment epithelium. Exp. Eye Res. 2012, 101, 60–71. [Google Scholar] [CrossRef]
- Zhao, C.; Yasumura, D.; Li, X.; Matthes, M.; Lloyd, M.; Nielsen, G.; Ahern, K.; Snyder, M.; Bok, D.; Dunaief, J.L.; et al. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J. Clin. Investig. 2011, 121, 369–383. [Google Scholar] [CrossRef]
- Al-Hussaini, H.; Schneiders, M.; Lundh, P.; Jeffery, G. Drusen are associated with local and distant disruptions to human retinal pigment epithelium cells. Exp. Eye Res. 2009, 88, 610–612. [Google Scholar] [CrossRef]
- Kolosova, N.G.; Kozhevnikova, O.S.; Telegina, D.V.; Fursova, A.Z.; Stefanova, N.A.; Muraleva, N.A.; Venanzi, F.; Sherman, M.Y.; Kolesnikov, S.I.; Sufianov, A.A.; et al. p62 /SQSTM1 coding plasmid prevents age related macular degeneration in a rat model. Aging 2018, 10, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Young, R.W. The renewal of rod and cone outer segments in the rhesus monkey. J. Cell Biol. 1971, 49, 303–318. [Google Scholar] [CrossRef]
- Curcio, C.A.; Millican, C.L.; Allen, K.A.; Kalina, R.E. Aging of the human photoreceptor mosaic: Evidence for selective vulnerability of rods in central retina. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3278–3296. [Google Scholar]
- Curcio, C.A. Photoreceptor topography in ageing and age-related maculopathy. Eye (London) 2001, 15, 376–383. [Google Scholar] [CrossRef]
- Keeling, E.; Lotery, A.J.; Tumbarello, D.A.; Ratnayaka, J.A. Impaired Cargo Clearance in the Retinal Pigment Epithelium (RPE) Underlies Irreversible Blinding Diseases. Cells 2018, 7, 16. [Google Scholar] [CrossRef]
- Keeling, E.; Chatelet, D.S.; Johnston, D.A.; Page, A.; Tumbarello, D.A.; Lotery, A.J.; Ratnayaka, J.A. Oxidative Stress and Dysfunctional Intracellular Traffic Linked to an Unhealthy Diet Results in Impaired Cargo Transport in the Retinal Pigment Epithelium (RPE). Mol. Nutr. Food Res. 2019, e1800951. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Hyttinen, J.; Ryhanen, T.; Viiri, J.; Paimela, T.; Toropainen, E.; Sorri, I.; Salminen, A. Mechanisms of protein aggregation in the retinal pigment epithelial cells. Front. Biosci. (Elite Ed.) 2010, 2, 1374–1384. [Google Scholar] [CrossRef]
- Keeling, E.; Culling, A.J.; Johnston, D.A.; Chatelet, D.S.; Page, A.; Tumbarello, D.A.; Lotery, A.J.; Ratnayaka, J.A. An In-Vitro Cell Model of Intracellular Protein Aggregation Provides Insights into RPE Stress Associated with Retinopathy. Int. J. Mol. Sci. 2020, 21, 6647. [Google Scholar] [CrossRef]
- Feeney-Burns, L.; Hilderbrand, E.S.; Eldridge, S. Aging human RPE: Morphometric analysis of macular, equatorial, and peripheral cells. Investig. Ophthalmol. Vis. Sci. 1984, 25, 195–200. [Google Scholar]
- Sparrow, J.R.; Gregory-Roberts, E.; Yamamoto, K.; Blonska, A.; Ghosh, S.K.; Ueda, K.; Zhou, J. The bisretinoids of retinal pigment epithelium. Prog. Retin. Eye Res. 2012, 31, 121–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Cross, S.D.; Stanton, J.B.; Marmorstein, A.D.; Le, Y.Z.; Marmorstein, L.Y. Early AMD-like defects in the RPE and retinal degeneration in aged mice with RPE-specific deletion of Atg5 or Atg7. Mol. Vis. 2017, 23, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; Millican, C.L. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch. Ophthalmol. 1999, 117, 329–339. [Google Scholar] [CrossRef]
- Ramkumar, H.L.; Zhang, J.; Chan, C.C. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD). Prog. Retin. Eye Res. 2010, 29, 169–190. [Google Scholar] [CrossRef]
- Lynn, S.A.; Ward, G.; Keeling, E.; Scott, J.A.; Cree, A.J.; Johnston, D.A.; Page, A.; Cuan-Urquizo, E.; Bhaskar, A.; Grossel, M.C.; et al. Ex-vivo models of the Retinal Pigment Epithelium (RPE) in long-term culture faithfully recapitulate key structural and physiological features of native RPE. Tissue Cell 2017, 49, 447–460. [Google Scholar] [CrossRef]
- Park, S.W.; Im, S.; Jun, H.O.; Lee, K.; Park, Y.J.; Kim, J.H.; Park, W.J.; Lee, Y.H.; Kim, J.H. Dry age-related macular degeneration like pathology in aged 5XFAD mice: Ultrastructure and microarray analysis. Oncotarget 2017, 8, 40006–40018. [Google Scholar] [CrossRef]
- Curcio, C.A.; Messinger, J.D.; Sloan, K.R.; Mitra, A.; McGwin, G.; Spaide, R.F. Human chorioretinal layer thicknesses measured in macula-wide, high-resolution histologic sections. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3943–3954. [Google Scholar] [CrossRef]
- Gouras, P.; Ivert, L.; Neuringer, M.; Nagasaki, T. Mitochondrial elongation in the macular RPE of aging monkeys, evidence of metabolic stress. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 100858. [Google Scholar] [CrossRef] [PubMed]
- Woodell, A.; Coughlin, B.; Kunchithapautham, K.; Casey, S.; Williamson, T.; Ferrell, W.D.; Atkinson, C.; Jones, B.W.; Rohrer, B. Alternative complement pathway deficiency ameliorates chronic smoke-induced functional and morphological ocular injury. PLoS ONE 2013, 8, e67894. [Google Scholar] [CrossRef]
- Xiao, C.; Chen, X.; Li, W.; Li, L.; Wang, L.; Xie, Q.; Han, H. Automatic Mitochondria Segmentation for EM Data Using a 3D Supervised Convolutional Network. Front. Neuroanat. 2018, 12, 92. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age related macular degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef]
- Ratnayaka, J.A.; Lotery, A.J. Challenges in studying geographic atrophy (GA) age-related macular degeneration: The potential of a new mouse model with GA-like features. Neural Regen. Res. 2020, 15, 863–864. [Google Scholar] [CrossRef]
- Williams, A.M.; Stamer, W.D.; Allingham, R.R. Increasing the Availability and Quality of Donor Eyes for Research. JAMA Ophthalmol. 2016, 134, 351–352. [Google Scholar] [CrossRef][Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Arganda-Carreras, I.; Sorzano, C.O.S.; Marabini, R.; Carazo, J.M.; Ortiz-de-Solorzano, C.; Kybic, J. Consistent and Elastic Registration of Histological Sections Using Vector-Spline Regularization; Springer: Berlin/Heidelberg, Germany, 2006; pp. 85–95. [Google Scholar]
| Cell 1 | Cell 2 | Cell 3 | Cell 4 | Cell 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mono-Nucleate (Whole Cell) | Bi-Nucleate (Whole Cell) | Bi-Nucleate (Partial Cell) | Bi-Nucleate (Partial Cell) | Bi-Nucleate (Whole Cell) | ||||||
| Cell cytoplasm | Volume (µm3) | 2220.2 | 2360 | 1733 | 1803 | 2649 | ||||
| Surface area (µm2) | 1794.9 | 2104 | 1572 | 1754.7 | 2055.6 | |||||
| Microvilli | Volume (µm3) | 528.5 | 877 | 60.9 | 181 | 1158 | ||||
| Surface area (µm2) | 1556.4 | 2617.7 | 1814.5 | 3165.2 | 3146.3 | |||||
| Nuclei | Volume (µm3) | 141 | 146.8 | 144 | 139.5 | 126.5 | 128.2 | 138.1 | 123.6 | 131.6 |
| Surface area (µm2) | 172.1 | 187.3 | 187 | 171.5 | 158.1 | 164.2 | 169.2 | 156.3 | 156.7 | |
| Basal infolds (sub-RPE spaces) | Volume (µm3) | 40.9 | 164.1 | 92.4 | 116.7 | 172 | ||||
| Surface area (µm2) | 537.9 | 1637.1 | 1682.2 | 1884.3 | 2505 | |||||
| Surface of RPE microvilli in contact with photoreceptors (?m2) | 51,462 | 90,000 | 48,732 | 73,118 | 242,797 | |||||
| Number of photoreceptors supported per volume (?m3) of RPE cytoplasm | 0.041 | 0.059 | 0.059 | 0.12 | 0.041 | |||||
| Number of photoreceptors supported | 90 | 132 | 102 | 216 | 108 | |||||
| Mono-Nucleate RPE Cell | Bi-Nucleate RPE Cell | |
|---|---|---|
| (Cell 1) | (Cell 2) | |
| Number of mitochondria | 422 | 678 |
| Average mitochondrial volume (nm3) | 2.76 × 108 ± 4.38 × 108 SD | 3.04 × 108 ± 3.87 × 108 SD |
| Volume of the smallest mitochondria (nm3) | 2.35 × 104 | 2.69 × 104 |
| Total mitochondrial volume (nm3) | 1.17 × 1011 | 4.36 × 1011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keeling, E.; Chatelet, D.S.; Tan, N.Y.T.; Khan, F.; Richards, R.; Thisainathan, T.; Goggin, P.; Page, A.; Tumbarello, D.A.; Lotery, A.J.; et al. 3D-Reconstructed Retinal Pigment Epithelial Cells Provide Insights into the Anatomy of the Outer Retina. Int. J. Mol. Sci. 2020, 21, 8408. https://doi.org/10.3390/ijms21218408
Keeling E, Chatelet DS, Tan NYT, Khan F, Richards R, Thisainathan T, Goggin P, Page A, Tumbarello DA, Lotery AJ, et al. 3D-Reconstructed Retinal Pigment Epithelial Cells Provide Insights into the Anatomy of the Outer Retina. International Journal of Molecular Sciences. 2020; 21(21):8408. https://doi.org/10.3390/ijms21218408
Chicago/Turabian StyleKeeling, Eloise, David S. Chatelet, Nicole Y. T. Tan, Farihah Khan, Rhys Richards, Thibana Thisainathan, Patricia Goggin, Anton Page, David A. Tumbarello, Andrew J. Lotery, and et al. 2020. "3D-Reconstructed Retinal Pigment Epithelial Cells Provide Insights into the Anatomy of the Outer Retina" International Journal of Molecular Sciences 21, no. 21: 8408. https://doi.org/10.3390/ijms21218408
APA StyleKeeling, E., Chatelet, D. S., Tan, N. Y. T., Khan, F., Richards, R., Thisainathan, T., Goggin, P., Page, A., Tumbarello, D. A., Lotery, A. J., & Ratnayaka, J. A. (2020). 3D-Reconstructed Retinal Pigment Epithelial Cells Provide Insights into the Anatomy of the Outer Retina. International Journal of Molecular Sciences, 21(21), 8408. https://doi.org/10.3390/ijms21218408






