Molecular and Cellular Mechanisms of Itch in Psoriasis
Abstract
1. Introduction
2. Clinical Characteristic of Itch in Psoriasis
3. Itch Transmission Pathway
4. Pathophysiology of Itch in Psoriasis
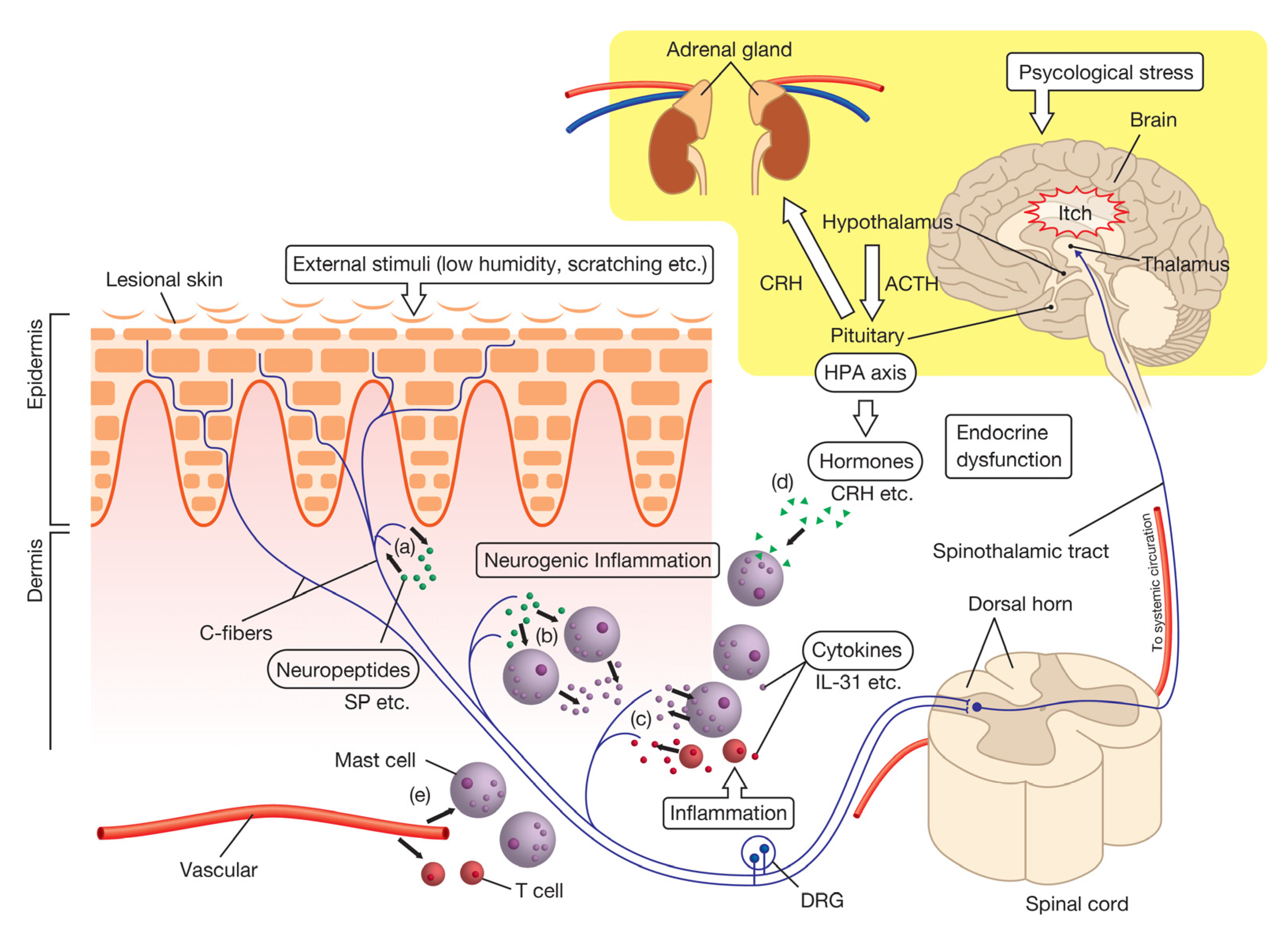
4.1. The Nervous System
4.1.1. Neuropeptides
- (1)
- Substance P (SP)
- (2)
- Calcitonin Gene-Related Peptide (CGRP)
- (3)
- Neuropeptide Y (NPY)
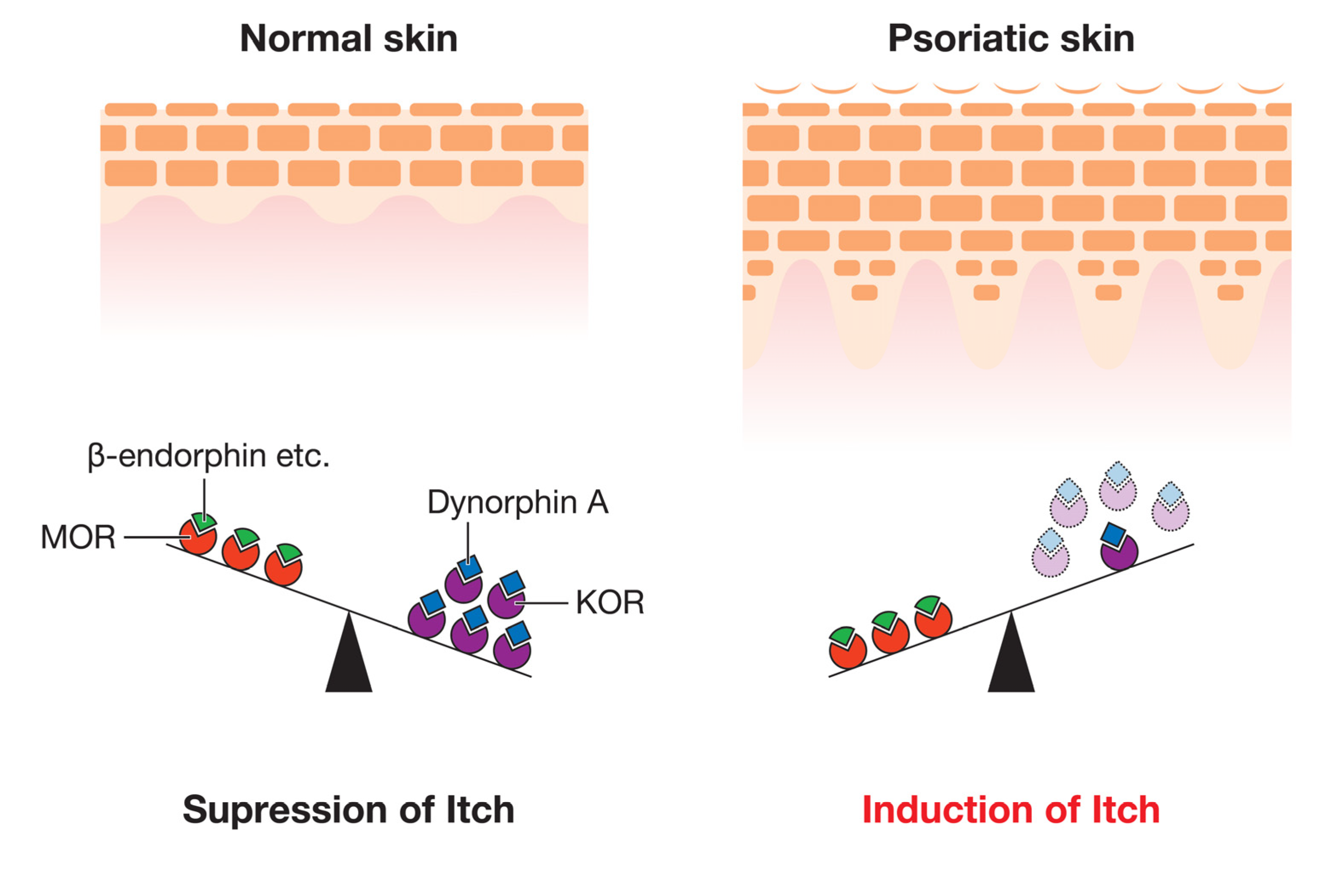
4.1.2. Opioid Ligands and Their Receptors
4.1.3. TRP Cation Channel Subfamily (TRP Channels)
4.1.4. Nerve Growth Factor (NGF)
4.1.5. Sensory Nerve Fiber Density
4.2. The Immune System
4.2.1. Cytokines
- (1)
- Itch-Mediating Cytokines
- (1.1)
- Interleukin-31 (IL-31)
- (1.2)
- Thymic Stromal Lymphopoietin (TSLP)
- (1.3)
- IL-2
- (2)
- Cytokines Involved in the Pathogenesis of Psoriasis
- (2.1)
- IL-17
- (2.2)
- IL-22
- (2.3)
- IL-23
- (2.4)
- IL-26
4.2.2. Mast Cells and Gamma-Amino Butyric Acid (GABA)-Expressing Inflammatory Cells
4.2.3. Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) Pathway
4.3. The Endocrine System
4.3.1. Corticotropin-Releasing Hormone (CRH)
4.3.2. α-Melanocyte-Stimulating Hormone (α-MSH)
4.4. The Vascular System
4.4.1. Vascular Endothelial Growth Factor (VEGF)
4.4.2. Prostaglandin E2 (PGE2)
4.4.3. Endothelin-1 (ET-1)
4.4.4. Cell Adhesion Molecules
4.5. Epidermal Keratinocytes
4.6. Others
4.6.1. Dipeptidyl Peptidase IV (DPPIV, CD26)
4.6.2. Lipocalin-2 (LCN2)
5. Ongoing and Future Trials
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α-MSH | α-melanocyte-stimulating hormone |
| CGRP | Calcitonin gene-related peptide |
| CRH | Corticotropin-releasing hormone |
| DPPIV | Dipeptidyl peptidase IV |
| DRG | Dorsal root ganglion |
| ET-1 | Endothelin-1 |
| GRP | Gastrin-releasing peptide |
| GABA | Gamma-amino butyric acid |
| HPA | Hypothalamic-pituitary-adrenal |
| IL | Interleukin |
| JAK | Janus kinase |
| LCN2 | Lipocalin-2 |
| KOR | κ-opioid receptor |
| MC1R | Melanocortin 1 receptor |
| MC5R | Melanocortin 5 receptor |
| MOR | μ-opioid receptor |
| Mrgpr | Mas-related G protein-coupled receptor |
| NGF | Nerve growth factor |
| NK-1R | Neurokinin-1 receptor |
| NPY | Neuropeptide Y |
| OSMR | Oncostatin M receptor |
| p75 | p75 neurotrophin receptor |
| PGA | Physician’s Global Assessment |
| PGE2 | Prostaglandin E2 |
| SP | Substance P |
| STAT | Signal transducer and activator of transcription |
| TrkA | Tropomyosin-receptor kinase A |
| TRP | Transient receptor potential |
| TRPA | Transient receptor potential ankyrin |
| TRPC | Transient receptor potential canonical |
| TRPM | Transient receptor potential melastatin |
| TRPV | Transient receptor potential vanilloid |
| TSLP | Thymic stromal lymphoprotein |
| Th | T helper type |
| VAP-1 | Vascular adhesion protein 1 |
| VAS | Visual Analog Scale |
| VEGF | Vascular endothelial growth factor |
References
- Szepietowski, J.C.; Reich, A. Pruritus in psoriasis: An update. Eur. J. Pain 2016, 20, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Fry, L. Psoriasis. Br. J. Dermatol. 1988, 119, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Szepietowski, J.C.; Reich, A. Itch in Psoriasis Management. Curr. Probl. Dermatol. 2016, 50, 102–110. [Google Scholar] [PubMed]
- Elewski, B.; Alexis, A.F.; Lebwohl, M.; Stein Gold, L.; Pariser, D.; Del Rosso, J.; Yosipovitch, G. Itch: An under-recognized problem in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.G.; Kavanaugh, A.; Armstrong, A.W.; Van Voorhees, A.S. US Perspectives in the Management of Psoriasis and Psoriatic Arthritis: Patient and Physician Results from the Population-Based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) Survey. Am. J. Clin. Dermatol. 2016, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Amatya, B.; Wennersten, G.; Nordlind, K. Patients’ perspective of pruritus in chronic plaque psoriasis: A questionnaire-based study. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 822–826. [Google Scholar] [CrossRef]
- Chang, S.E.; Han, S.S.; Jung, H.J.; Choi, J.H. Neuropeptides and their receptors in psoriatic skin in relation to pruritus. Br. J. Dermatol. 2007, 156, 1272–1277. [Google Scholar] [CrossRef]
- Stinco, G.; Trevisan, G.; Piccirillo, F.; Pezzetta, S.; Errichetti, E.; di Meo, N.; Valent, F.; Patrone, P. Pruritus in chronic plaque psoriasis: A questionnaire-based study of 230 Italian patients. Acta Dermatovenerol. Croat. 2014, 22, 122–128. [Google Scholar]
- Szepietowski, J.C.; Reich, A.; Wisnicka, B. Pruritus and psoriasis. Br. J. Dermatol. 2004, 151, 1284. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Goon, A.; Wee, J.; Chan, Y.H.; Goh, C.L. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br. J. Dermatol. 2000, 143, 969–973. [Google Scholar] [CrossRef]
- Shahwan, K.T.; Kimball, A.B. Itch intensity in moderate-to-severe plaque psoriasis versus atopic dermatitis: A meta-analysis. J. Am. Acad. Dermatol. 2017, 76, 1198–1200. [Google Scholar] [CrossRef] [PubMed]
- Sampogna, F.; Gisondi, P.; Melchi, C.F.; Amerio, P.; Girolomoni, G.; Abeni, D. Prevalence of symptoms experienced by patients with different clinical types of psoriasis. Br. J. Dermatol. 2004, 151, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.G.; Bachelez, H.; Barker, J.; Girolomoni, G.; Kavanaugh, A.; Langley, R.G.; Paul, C.F.; Puig, L.; Reich, K.; van de Kerkhof, P.C. Patient perspectives in the management of psoriasis: Results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J. Am. Acad. Dermatol. 2014, 70, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Welz-Kubiak, K.; Rams, L. Apprehension of the disease by patients suffering from psoriasis. Postepy Dermatol. Alergol. 2014, 31, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Mędrek, K.; Szepietowski, J.C. Interplay of Itch and Psyche in Psoriasis: An Update. Acta Derm. Venereol. 2016, 96, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.Q.; Blome, C.; Fritz, F.; Gerss, J.; Reich, A.; Ebata, T.; Augustin, M.; Szepietowski, J.C.; Ständer, S. Assessment of pruritus intensity: Prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm. Venereol. 2012, 92, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Szepietowski, J.C.; Wiśnicka, B.; Pacan, P. Does Stress Influence Itching in Psoriatic Patients? Dermatol. Psychosom. 2003, 4, 151–155. [Google Scholar] [CrossRef]
- Szepietowski, J.C.; Reich, A.; Wiśnicka, B. Itching in patients suffering from psoriasis. Acta Dermatovenerol. Croat. 2002, 10, 221–226. [Google Scholar]
- Kim, T.W.; Shim, W.H.; Kim, J.M.; Mun, J.H.; Song, M.; Kim, H.S.; Ko, H.C.; Kim, M.B.; Kim, B.S. Clinical characteristics of pruritus in patients with scalp psoriasis and their relation with intraepidermal nerve fiber density. Ann. Dermatol. 2014, 26, 727–732. [Google Scholar] [CrossRef]
- Korman, N.J.; Zhao, Y.; Li, Y.; Liao, M.; Tran, M.H. Clinical symptoms and self-reported disease severity among patients with psoriasis—Implications for psoriasis management. J. Dermatol. Treat. 2015, 26, 514–519. [Google Scholar] [CrossRef]
- Pithadia, D.J.; Reynolds, K.A.; Lee, E.B.; Wu, J.J. Psoriasis-associated cutaneous pain: Etiology, assessment, impact, and management. J. Dermatol. Treat. 2019, 30, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Roblin, D.; Wickramasinghe, R.; Yosipovitch, G. Pruritus severity in patients with psoriasis is not correlated with psoriasis disease severity. J. Am. Acad. Dermatol. 2014, 70, 390–391. [Google Scholar] [CrossRef] [PubMed]
- Sagi, L.; Trau, H. The Koebner phenomenon. Clin. Dermatol. 2011, 29, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.P.; Sonthalia, S. Koebner Phenomenon. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2020. [Google Scholar]
- Acton, D.; Ren, X.; Di Costanzo, S.; Dalet, A.; Bourane, S.; Bertocchi, I.; Eva, C.; Goulding, M. Spinal Neuropeptide Y1 Receptor-Expressing Neurons Form an Essential Excitatory Pathway for Mechanical Itch. Cell Rep. 2019, 28, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Ringkamp, M.; Schepers, R.J.; Shimada, S.G.; Johanek, L.M.; Hartke, T.V.; Borzan, J.; Shim, B.; LaMotte, R.H.; Meyer, R.A. A role for nociceptive, myelinated nerve fibers in itch sensation. J. Neurosci. 2011, 31, 14841–14849. [Google Scholar] [CrossRef]
- Lay, M.; Dong, X. Neural Mechanisms of Itch. Annu Rev. Neurosci. 2020, 43, 187–205. [Google Scholar] [CrossRef]
- Moniaga, C.S.; Tominaga, M.; Takamori, K. Mechanisms and Management of Itch in Dry Skin. Acta Derm. Venereol. 2020, 100, adv00024. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Tominaga, M.; Takamori, K. Itch and nerve fibers with special reference to atopic dermatitis: Therapeutic implications. J. Dermatol. 2014, 41, 205–212. [Google Scholar] [CrossRef]
- Ishiuji, Y. Addiction and the itch-scratch cycle. What do they have in common? Exp. Dermatol. 2019, 28, 1448–1454. [Google Scholar] [CrossRef]
- Domagała, A.; Szepietowski, J.; Reich, A. Antihistamines in the treatment of pruritus in psoriasis. Postepy Dermatol. Alergol. 2017, 34, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Prignano, F.; Ricceri, F.; Pescitelli, L.; Lotti, T. Itch in psoriasis: Epidemiology, clinical aspects and treatment options. Clin. Cosmet. Investig. Dermatol. 2009, 2, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Wiśnicka, B.; Szepietowski, J.C.; Reich, A.; Orda, A. Histamine, Substance P and Calcitonin Gene-Related Peptide Plasma Concentration and Pruritus in Patients Suffering from Psoriasis. Dermatol. Psychosom. 2004, 5, 73–78. [Google Scholar] [CrossRef]
- Nakamura, M.; Toyoda, M.; Morohashi, M. Pruritogenic mediators in psoriasis vulgaris: Comparative evaluation of itch-associated cutaneous factors. Br. J. Dermatol. 2003, 149, 718–730. [Google Scholar] [CrossRef]
- van Lingen, R.G.; van de Kerkhof, P.C.; Seyger, M.M.; de Jong, E.M.; van Rens, D.W.; Poll, M.K.; Zeeuwen, P.L.; van Erp, P.E. CD26/dipeptidyl-peptidase IV in psoriatic skin: Upregulation and topographical changes. Br. J. Dermatol. 2008, 158, 1264–1272. [Google Scholar] [CrossRef]
- Hägermark, O.; Hökfelt, T.; Pernow, B. Flare and itch induced by substance P in human skin. J. Investig. Dermatol. 1978, 71, 233–235. [Google Scholar] [CrossRef]
- Andoh, T.; Nagasawa, T.; Satoh, M.; Kuraishi, Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J. Pharmacol. Exp. Ther. 1998, 286, 1140–1145. [Google Scholar]
- McCoy, E.S.; Taylor-Blake, B.; Street, S.E.; Pribisko, A.L.; Zheng, J.; Zylka, M.J. Peptidergic CGRPα primary sensory neurons encode heat and itch and tonically suppress sensitivity to cold. Neuron 2013, 78, 138–151. [Google Scholar] [CrossRef]
- Reich, A.; Orda, A.; Wiśnicka, B.; Szepietowski, J.C. Plasma neuropeptides and perception of pruritus in psoriasis. Acta Derm. Venereol. 2007, 87, 299–304. [Google Scholar] [CrossRef]
- Taneda, K.; Tominaga, M.; Negi, O.; Tengara, S.; Kamo, A.; Ogawa, H.; Takamori, K. Evaluation of epidermal nerve density and opioid receptor levels in psoriatic itch. Br. J. Dermatol. 2011, 165, 277–284. [Google Scholar] [CrossRef]
- Glinski, W.; Brodecka, H.; Glinska-Ferenz, M.; Kowalski, D. Increased concentration of beta-endorphin in sera of patients with psoriasis and other inflammatory dermatoses. Br. J. Dermatol. 1994, 131, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.C. Neuraxial opioid-induced itch and its pharmacological antagonism. Handb. Exp. Pharmacol. 2015, 226, 315–335. [Google Scholar] [PubMed]
- Yamaguchi, J.; Aihara, M.; Kobayashi, Y.; Kambara, T.; Ikezawa, Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J. Dermatol. Sci. 2009, 53, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Rukwied, R.R.; Main, M.; Weinkauf, B.; Schmelz, M. NGF sensitizes nociceptors for cowhage- but not histamine-induced itch in human skin. J. Investig. Dermatol. 2013, 133, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Niyonsaba, F.; Ushio, H.; Hara, M.; Yokoi, H.; Tominaga, M.; Takamori, K.; Kajiwara, N.; Saito, H.; Nagaoka, I.; Ogawa, H.; et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J. Immunol. 2010, 184, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Narbutt, J.; Olejniczak, I.; Sobolewska-Sztychny, D.; Sysa-Jedrzejowska, A.; Słowik-Kwiatkowska, I.; Hawro, T.; Lesiak, A. Narrow band ultraviolet B irradiations cause alteration in interleukin-31 serum level in psoriatic patients. Arch. Dermatol. Res. 2013, 305, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.R.; Sprecher, C.; Hammond, A.; Bilsborough, J.; Rosenfeld-Franklin, M.; Presnell, S.R.; Haugen, H.S.; Maurer, M.; Harder, B.; Johnston, J.; et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat. Immunol. 2004, 5, 752–760. [Google Scholar] [CrossRef]
- Volpe, E.; Pattarini, L.; Martinez-Cingolani, C.; Meller, S.; Donnadieu, M.H.; Bogiatzi, S.I.; Fernandez, M.I.; Touzot, M.; Bichet, J.C.; Reyal, F.; et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J. Allergy Clin. Immunol. 2014, 134, 373–381. [Google Scholar] [CrossRef]
- Suwarsa, O.; Dharmadji, H.P.; Sutedja, E.; Herlina, L.; Sori, P.R.; Hindritiani, R.; Dwiyana, R.F.; Gunawan, H. Skin tissue expression and serum level of thymic stromal lymphopoietin in patients with psoriasis vulgaris. Dermatol. Rep. 2019, 11, 8006. [Google Scholar] [CrossRef]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef]
- Martin, H.A.; Murphy, P.R. Interleukin-2 activates a sub-population of cutaneous C-fibre polymodal nociceptors in the rat hairy skin. Arch. Physiol. Biochem. 1995, 103, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Darsow, U.; Scharein, E.; Bromm, B.; Ring, J. Skin testing of the pruritogenic activity of histamine and cytokines (interleukin-2 and tumour necrosis factor-alpha) at the dermal-epidermal junction. Br. J. Dermatol 1997, 137, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Wahlgren, C.F.; Tengvall Linder, M.; Hägermark, O.; Scheynius, A. Itch and inflammation induced by intradermally injected interleukin-2 in atopic dermatitis patients and healthy subjects. Arch. Dermatol. Res. 1995, 287, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Moynes, D.M.; Vanner, S.J.; Lomax, A.E. Participation of interleukin 17A in neuroimmune interactions. Brain Behav. Immun. 2014, 41, 1–9. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Tampa, M.; Caruntu, C.; Sarbu, M.I.; Mitran, C.I.; Mitran, M.I.; Matei, C.; Constantin, C.; Neagu, M. Advances in Understanding the Immunological Pathways in Psoriasis. Int. J. Mol. Sci. 2019, 20, 739. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Lu, J.; Choi, E.B.; Oh, M.H.; Jeong, M.; Barmettler, S.; Zhu, Z.; Zheng, T. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. J. Immunol. 2017, 198, 2543–2555. [Google Scholar] [CrossRef]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef]
- Riol-Blanco, L.; Ordovas-Montanes, J.; Perro, M.; Naval, E.; Thiriot, A.; Alvarez, D.; Paust, S.; Wood, J.N.; von Andrian, U.H. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 2014, 510, 157–161. [Google Scholar] [CrossRef]
- Itoh, T.; Hatano, R.; Komiya, E.; Otsuka, H.; Narita, Y.; Aune, T.M.; Dang, N.H.; Matsuoka, S.; Naito, H.; Tominaga, M.; et al. Biological Effects of IL-26 on T Cell-Mediated Skin Inflammation, Including Psoriasis. J. Investig. Dermatol. 2019, 139, 878–889. [Google Scholar] [CrossRef]
- Hadjab, S.; Franck, M.C.; Wang, Y.; Sterzenbach, U.; Sharma, A.; Ernfors, P.; Lallemend, F. A local source of FGF initiates development of the unmyelinated lineage of sensory neurons. J. Neurosci. 2013, 33, 17656–17666. [Google Scholar] [CrossRef]
- Kim, J.E.; Cho, D.H.; Kim, H.S.; Kim, H.J.; Lee, J.Y.; Cho, B.K.; Park, H.J. Expression of the corticotropin-releasing hormone-proopiomelanocortin axis in the various clinical types of psoriasis. Exp. Dermatol. 2007, 16, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Siiskonen, H.; Harvima, I. Mast Cells and Sensory Nerves Contribute to Neurogenic Inflammation and Pruritus in Chronic Skin Inflammation. Front. Cell Neurosci. 2019, 13, 422. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Singh, L.K.; Boucher, W.; Pang, X.; Letourneau, R.; Webster, E.; Chrousos, G. Corticotropin-releasing hormone induces skin mast cell degranulation and increased vascular permeability, a possible explanation for its proinflammatory effects. Endocrinology 1998, 139, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Andoh, T.; Yoshihisa, Y.; Shimizu, T. Histamine released from epidermal keratinocytes plays a role in α-melanocyte-stimulating hormone-induced itching in mice. Am. J. Pathol. 2015, 185, 3003–3010. [Google Scholar] [CrossRef]
- Andoh, T.; Akasaka, C.; Shimizu, K.; Lee, J.B.; Yoshihisa, Y.; Shimizu, T. Involvement of α-Melanocyte-Stimulating Hormone-Thromboxane A(2) System on Itching in Atopic Dermatitis. Am. J. Pathol. 2019, 189, 1775–1785. [Google Scholar] [CrossRef]
- Wong, L.S.; Otsuka, A.; Yamamoto, Y.; Nonomura, Y.; Nakashima, C.; Honda, T.; Dainichi, T.; Kitoh, A.; Nakajima, S.; Hirakawa, S.; et al. Vascular endothelial growth factor partially induces pruritus via epidermal hyperinnervation in imiquimod-induced psoriasiform dermatitis in mice. J. Dermatol. Sci. 2016, 83, 148–151. [Google Scholar] [CrossRef]
- Shimauchi, T.; Hirakawa, S.; Suzuki, T.; Yasuma, A.; Majima, Y.; Tatsuno, K.; Yagi, H.; Ito, T.; Tokura, Y. Serum interleukin-22 and vascular endothelial growth factor serve as sensitive biomarkers but not as predictors of therapeutic response to biologics in patients with psoriasis. J. Dermatol. 2013, 40, 805–812. [Google Scholar] [CrossRef]
- Hammarström, S.; Hamberg, M.; Samuelsson, B.; Duell, E.A.; Stawiski, M.; Voorhees, J.J. Increased concentrations of nonesterified arachidonic acid, 12L-hydroxy-5,8,10,14-eicosatetraenoic acid, prostaglandin E2, and prostaglandin F2alpha in epidermis of psoriasis. Proc. Natl. Acad. Sci. USA 1975, 72, 5130–5134. [Google Scholar] [CrossRef]
- Fjellner, B.; Hägermark, O. Pruritus in polycythemia vera: Treatment with aspirin and possibility of platelet involvement. Acta Derm. Venereol. 1979, 59, 505–512. [Google Scholar]
- Hägermark, O.; Strandberg, K. Pruritogenic activity of prostaglandin E2. Acta Derm. Venereol. 1977, 57, 37–43. [Google Scholar]
- Nakahara, T.; Kido-Nakahara, M.; Ohno, F.; Ulzii, D.; Chiba, T.; Tsuji, G.; Furue, M. The pruritogenic mediator endothelin-1 shifts the dendritic cell-T-cell response toward Th17/Th1 polarization. Allergy 2018, 73, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Katugampola, R.; Church, M.K.; Clough, G.F. The neurogenic vasodilator response to endothelin-1: A study in human skin in vivo. Exp. Physiol. 2000, 85, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Madej, A.; Reich, A.; Orda, A.; Szepietowski, J.C. Vascular adhesion protein-1 (VAP-1) is overexpressed in psoriatic patients. J. Eur. Acad. Dermatol. Venereol. 2007, 21, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Komiya, E.; Hatano, R.; Otsuka, H.; Itoh, T.; Yamazaki, H.; Yamada, T.; Dang, N.H.; Tominaga, M.; Suga, Y.; Kimura, U.; et al. A possible role for CD26/DPPIV enzyme activity in the regulation of psoriatic pruritus. J. Dermatol. Sci. 2017, 86, 212–221. [Google Scholar] [CrossRef]
- Aizawa, N.; Ishiuji, Y.; Tominaga, M.; Sakata, S.; Takahashi, N.; Yanaba, K.; Umezawa, Y.; Asahina, A.; Kimura, U.; Suga, Y.; et al. Relationship between the Degrees of Itch and Serum Lipocalin-2 Levels in Patients with Psoriasis. J. Immunol. Res. 2019, 2019, 8171373. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Koga, K.; Tozaki-Saitoh, H.; Kohro, Y.; Toyonaga, H.; Yamaguchi, C.; Hasegawa, A.; Nakahara, T.; Hachisuka, J.; Akira, S.; et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat. Med. 2015, 21, 927–931. [Google Scholar] [CrossRef]
- van den Pol, A.N. Neuropeptide transmission in brain circuits. Neuron 2012, 76, 98–115. [Google Scholar] [CrossRef]
- Geppetti, P.; Nassini, R.; Materazzi, S.; Benemei, S. The concept of neurogenic inflammation. BJU Int. 2008, 101, 2–6. [Google Scholar] [CrossRef]
- Saraceno, R.; Kleyn, C.E.; Terenghi, G.; Griffiths, C.E. The role of neuropeptides in psoriasis. Br. J. Dermatol. 2006, 155, 876–882. [Google Scholar] [CrossRef]
- Amatya, B.; El-Nour, H.; Holst, M.; Theodorsson, E.; Nordlind, K. Expression of tachykinins and their receptors in plaque psoriasis with pruritus. Br. J. Dermatol. 2011, 164, 1023–1029. [Google Scholar] [CrossRef]
- Han, L.; Dong, X. Itch mechanisms and circuits. Annu. Rev. Biophys. 2014, 43, 331–355. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Ständer, S.; Kerby, M.B.; Larrick, J.W.; Perlman, A.J.; Schnipper, E.F.; Zhang, X.; Tang, J.Y.; Luger, T.; Steinhoff, M. Serlopitant for the treatment of chronic pruritus: Results of a randomized, multicenter, placebo-controlled phase 2 clinical trial. J. Am. Acad. Dermatol. 2018, 78, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Azimi, E.; Reddy, V.B.; Shade, K.C.; Anthony, R.M.; Talbot, S.; Pereira, P.J.S.; Lerner, E.A. Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight 2016, 1, e89362. [Google Scholar] [CrossRef] [PubMed]
- Azimi, E.; Reddy, V.B.; Pereira, P.J.S.; Talbot, S.; Woolf, C.J.; Lerner, E.A. Substance P activates Mas-related G protein-coupled receptors to induce itch. J. Allergy Clin. Immunol. 2017, 140, 447–453. [Google Scholar] [CrossRef]
- Heymann, E.; Mentlein, R. Liver dipeptidyl aminopeptidase IV hydrolyzes substance P. FEBS Lett. 1978, 91, 360–364. [Google Scholar] [CrossRef]
- Mogil, J.S.; Miermeister, F.; Seifert, F.; Strasburg, K.; Zimmermann, K.; Reinold, H.; Austin, J.S.; Bernardini, N.; Chesler, E.J.; Hofmann, H.A.; et al. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc. Natl. Acad. Sci. USA 2005, 102, 12938–12943. [Google Scholar] [CrossRef]
- Roosterman, D.; Goerge, T.; Schneider, S.W.; Bunnett, N.W.; Steinhoff, M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol. Rev. 2006, 86, 1309–1379. [Google Scholar] [CrossRef]
- Takahashi, N.; Tominaga, M.; Kosaka, R.; Kamata, Y.; Umehara, Y.; Matsuda, H.; Sakaguchi, A.; Ogawa, H.; Takamori, K. Involvement of µ-opioid Receptors and κ-opioid Receptors in Itch-related Scratching Behaviour of Imiquimod-induced Psoriasis-like Dermatitis in Mice. Acta Derm. Venereol. 2017, 97, 928–933. [Google Scholar] [CrossRef]
- Benarroch, E.E. Endogenous opioid systems: Current concepts and clinical correlations. Neurology 2012, 79, 807–814. [Google Scholar] [CrossRef]
- Kupczyk, P.; Reich, A.; Hołysz, M.; Gajda, M.; Wysokińska, E.; Kobuszewska, A.; Nevozhay, D.; Nowakowska, B.; Strzadała, L.; Jagodziński, P.P.; et al. Opioid Receptors in Psoriatic Skin: Relationship with Itch. Acta Derm. Venereol. 2017, 97, 564–570. [Google Scholar] [CrossRef]
- Kittaka, H.; Tominaga, M. The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergol. Int. 2017, 66, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Dong, X. Trp channels and itch. Semin. Immunopathol. 2016, 38, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Tonello, R.; Choi, Y.; Jung, S.J.; Berta, T. Sensory Neuron-Expressed TRPC4 Is a Target for the Relief of Psoriasiform Itch and Skin Inflammation in Mice. J. Investig. Dermatol. 2020, 140, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.N.; Berberian, B.; Sulica, V.I.; Dodd, W.A.; Jarratt, M.T.; Katz, H.I.; Prawer, S.; Krueger, G.; Rex, I.H., Jr.; Wolf, J.E. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J. Am. Acad. Dermatol. 1993, 29, 438–442. [Google Scholar] [CrossRef]
- Levi-Montalcini, R.; Angeletti, P.U. Essential role of the nerve growth factor in the survival and maintenance of dissociated sensory and sympathetic embryonic nerve cells in vitro. Dev. Biol. 1963, 6, 653–659. [Google Scholar] [CrossRef]
- Bibel, M.; Barde, Y.A. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000, 14, 2919–2937. [Google Scholar] [CrossRef]
- Roblin, D.; Yosipovitch, G.; Boyce, B.; Robinson, J.; Sandy, J.; Mainero, V.; Wickramasinghe, R.; Anand, U.; Anand, P. Topical TrkA Kinase Inhibitor CT327 is an Effective, Novel Therapy for the Treatment of Pruritus due to Psoriasis: Results from Experimental Studies, and Efficacy and Safety of CT327 in a Phase 2b Clinical Trial in Patients with Psoriasis. Acta Derm. Venereol. 2015, 95, 542–548. [Google Scholar] [CrossRef]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508. [Google Scholar] [CrossRef]
- Pergolizzi, S.; Vaccaro, M.; Magaudda, L.; Mondello, M.R.; Arco, A.; Bramanti, P.; Cannavò, S.P.; Guarneri, B. Immunohistochemical study of epidermal nerve fibres in involved and uninvolved psoriatic skin using confocal laser scanning microscopy. Arch. Dermatol. Res. 1998, 290, 483–489. [Google Scholar] [CrossRef]
- Sakai, K.; Sanders, K.M.; Youssef, M.R.; Yanushefski, K.M.; Jensen, L.E.; Yosipovitch, G.; Akiyama, T. Role of neurturin in spontaneous itch and increased nonpeptidergic intraepidermal fiber density in a mouse model of psoriasis. Pain 2017, 158, 2196–2202. [Google Scholar] [CrossRef]
- Leon, A.; Rosen, J.D.; Hashimoto, T.; Fostini, A.C.; Paus, R.; Yosipovitch, G. Itching for an answer: A review of potential mechanisms of scalp itch in psoriasis. Exp. Dermatol. 2019, 28, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Kasutani, K.; Fujii, E.; Ohyama, S.; Adachi, H.; Hasegawa, M.; Kitamura, H.; Yamashita, N. Anti-IL-31 receptor antibody is shown to be a potential therapeutic option for treating itch and dermatitis in mice. Br. J. Pharmacol. 2014, 171, 5049–5058. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Operacz, M.; Polańska, A.; Klimańska, M.; Teresiak-Mikołajczak, E.; Molińska-Glura, M.; Adamski, Z.; Jenerowicz, D. Itching sensation in psoriatic patients and its relation to body mass index and IL-17 and IL-31 concentrations. Postepy Dermatol. Alergol. 2015, 32, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, B.F.; Patsinakidis, N.; Raap, U. Role of the Pruritic Cytokine IL-31 in Autoimmune Skin Diseases. Front. Immunol. 2019, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Gago-Lopez, N.; Mellor, L.F.; Megías, D.; Martín-Serrano, G.; Izeta, A.; Jimenez, F.; Wagner, E.F. Role of bulge epidermal stem cells and TSLP signaling in psoriasis. EMBO Mol. Med. 2019, 11, e10697. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E.; Gaspari, A.A.; Lotze, M.T.; Chang, A.E.; Rosenberg, S.A. Interleukin 2 and psoriasis. Arch. Dermatol. 1988, 124, 1811–1815. [Google Scholar] [CrossRef]
- Kimball, A.B.; Luger, T.; Gottlieb, A.; Puig, L.; Kaufmann, R.; Burge, R.; Lin, C.Y.; Yosipovitch, G. Long-term Impact of Ixekizumab on Psoriasis Itch Severity: Results from a Phase III Clinical Trial and Long-term Extension. Acta Derm. Venereol. 2018, 98, 98–102. [Google Scholar] [CrossRef]
- Kimball, A.B.; Luger, T.; Gottlieb, A.; Puig, L.; Kaufmann, R.; Nikaï, E.; Zhu, B.; Edson-Heredia, E.; Carlier, H.; Lin, C.Y.; et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: Results from 3 phase III psoriasis clinical trials. J. Am. Acad. Dermatol. 2016, 75, 1156–1161. [Google Scholar] [CrossRef]
- Strober, B.; Sigurgeirsson, B.; Popp, G.; Sinclair, R.; Krell, J.; Stonkus, S.; Septe, M.; Elewski, B.E.; Gottlieb, A.B.; Zhao, Y.; et al. Secukinumab improves patient-reported psoriasis symptoms of itching, pain, and scaling: Results of two phase 3, randomized, placebo-controlled clinical trials. Int. J. Dermatol. 2016, 55, 401–407. [Google Scholar] [CrossRef]
- Wolk, K.; Witte, E.; Wallace, E.; Döcke, W.D.; Kunz, S.; Asadullah, K.; Volk, H.D.; Sterry, W.; Sabat, R. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: A potential role in psoriasis. Eur. J. Immunol. 2006, 36, 1309–1323. [Google Scholar] [CrossRef]
- Ruano, J.; Suárez-Fariñas, M.; Shemer, A.; Oliva, M.; Guttman-Yassky, E.; Krueger, J.G. Molecular and Cellular Profiling of Scalp Psoriasis Reveals Differences and Similarities Compared to Skin Psoriasis. PLoS ONE 2016, 11, e0148450. [Google Scholar] [CrossRef] [PubMed]
- Nattkemper, L.A.; Zhao, Z.Q.; Nichols, A.J.; Papoiu, A.D.P.; Shively, C.A.; Chen, Z.F.; Yosipovitch, G. Overexpression of the gastrin-releasing peptide in cutaneous nerve fibers and its receptor in the spinal cord in primates with chronic itch. J. Investig. Dermatol. 2013, 133, 2489–2492. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.M.; Liu, X.T.; Liu, B.; Liu, X.Y.; Gao, F.; Zeng, X.; Liu, J.; Yang, Q.; Wilhelm, S.; Yin, J.; et al. Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors. Nat. Commun. 2020, 11, 1397. [Google Scholar] [CrossRef]
- Eberle, F.C.; Brück, J.; Holstein, J.; Hirahara, K.; Ghoreschi, K. Recent advances in understanding psoriasis. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Reich, K.; Tsai, T.F.; Tyring, S.; Vanaclocha, F.; Kingo, K.; Ziv, M.; Pinter, A.; Vender, R.; Hugot, S.; et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study. J. Am. Acad. Dermatol. 2017, 76, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Sheikh, F.; Dickensheets, H.; Savan, R.; Young, H.A.; Walter, M.R. Interleukin-26: An IL-10-related cytokine produced by Th17 cells. Cytokine Growth Factor Rev. 2010, 21, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V. The family of IL-10-related cytokines and their receptors: Related, but to what extent? Cytokine Growth Factor Rev. 2002, 13, 223–240. [Google Scholar] [CrossRef]
- Schoenborn, J.R.; Dorschner, M.O.; Sekimata, M.; Santer, D.M.; Shnyreva, M.; Fitzpatrick, D.R.; Stamatoyannopoulos, J.A.; Wilson, C.B. Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-gamma. Nat. Immunol. 2007, 8, 732–742. [Google Scholar] [CrossRef]
- Hatano, R.; Itoh, T.; Otsuka, H.; Okamoto, S.; Komiya, E.; Iwata, S.; Aune, T.M.; Dang, N.H.; Kuwahara-Arai, K.; Ohnuma, K.; et al. Characterization of novel anti-IL-26 neutralizing monoclonal antibodies for the treatment of inflammatory diseases including psoriasis. MAbs 2019, 11, 1428–1442. [Google Scholar] [CrossRef]
- Harvima, I.T.; Nilsson, G.; Suttle, M.M.; Naukkarinen, A. Is there a role for mast cells in psoriasis? Arch. Dermatol. Res. 2008, 300, 461–478. [Google Scholar] [CrossRef]
- Nigam, R.; El-Nour, H.; Amatya, B.; Nordlind, K. GABA and GABA(A) receptor expression on immune cells in psoriasis: A pathophysiological role. Arch. Dermatol. Res. 2010, 302, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Meier, K.; Ghoreschi, K. Emerging Topical and Systemic JAK Inhibitors in Dermatology. Front. Immunol. 2019, 10, 2847. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Selectivity and therapeutic inhibition of kinases: To be or not to be? Nat. Immunol. 2009, 10, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, C.; Lüscher-Firzlaff, J.; Baron, J.M.; Lüscher, B. Signaling by IL-31 and functional consequences. Eur. J. Cell Biol. 2012, 91, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A.; Marone, G. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lin, J.X.; Leonard, W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Teng, M.W.; Bowman, E.P.; McElwee, J.J.; Smyth, M.J.; Casanova, J.L.; Cooper, A.M.; Cua, D.J. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015, 21, 719–729. [Google Scholar] [CrossRef]
- Larochette, V.; Miot, C.; Poli, C.; Beaumont, E.; Roingeard, P.; Fickenscher, H.; Jeannin, P.; Delneste, Y. IL-26, a Cytokine With Roles in Extracellular DNA-Induced Inflammation and Microbial Defense. Front. Immunol. 2019, 10, 204. [Google Scholar] [CrossRef]
- Hald, A.; Andrés, R.M.; Salskov-Iversen, M.L.; Kjellerup, R.B.; Iversen, L.; Johansen, C. STAT1 expression and activation is increased in lesional psoriatic skin. Br. J. Dermatol. 2013, 168, 302–310. [Google Scholar] [CrossRef]
- Andrés, R.M.; Hald, A.; Johansen, C.; Kragballe, K.; Iversen, L. Studies of Jak/STAT3 expression and signalling in psoriasis identifies STAT3-Ser727 phosphorylation as a modulator of transcriptional activity. Exp. Dermatol. 2013, 22, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bushmakin, A.G.; Mamolo, C.; Cappelleri, J.C.; Stewart, M. The relationship between pruritus and the clinical signs of psoriasis in patients receiving tofacitinib. J. Dermatol. Treat. 2015, 26, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Harvima, I.T.; Nilsson, G. Stress, the neuroendocrine system and mast cells: Current understanding of their role in psoriasis. Exp. Rev. Clin. Immunol. 2012, 8, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ayasse, M.T.; Buddenkotte, J.; Alam, M.; Steinhoff, M. Role of neuroimmune circuits and pruritus in psoriasis. Exp. Dermatol. 2020, 29, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.K.; Pang, X.; Alexacos, N.; Letourneau, R.; Theoharides, T.C. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P: A link to neurogenic skin disorders. Brain Behav. Immun. 1999, 13, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, T.; Liang, Z.; Suzuki, H.; Kawana, S. Inhibitory effects of antipsychotic and anxiolytic agents on stress-induced degranulation of mouse dermal mast cells. Clin. Exp. Dermatol. 2010, 35, 531–536. [Google Scholar] [CrossRef]
- Webster, E.L.; Torpy, D.J.; Elenkov, I.J.; Chrousos, G.P. Corticotropin-releasing hormone and inflammation. Ann. N. Y. Acad. Sci. 1998, 840, 21–32. [Google Scholar] [CrossRef]
- Paus, R.; Theoharides, T.C.; Arck, P.C. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006, 27, 32–39. [Google Scholar] [CrossRef]
- Malecic, N.; Young, H.S. Novel investigational vascular endothelial growth factor (VEGF) receptor antagonists for psoriasis. Exp. Opin. Investig. Drugs 2016, 25, 455–462. [Google Scholar] [CrossRef]
- Sakamoto, M.; Miyagaki, T.; Kamijo, H.; Oka, T.; Takahashi, N.; Suga, H.; Yoshizaki, A.; Asano, Y.; Sugaya, M.; Sato, S. Serum vascular endothelial growth factor A levels reflect itch severity in mycosis fungoides and Sézary syndrome. J. Dermatol. 2018, 45, 95–99. [Google Scholar] [CrossRef]
- Williams, C.S.; DuBois, R.N. Prostaglandin endoperoxide synthase: Why two isoforms? Am. J. Physiol. 1996, 270, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Kuraishi, Y. Frontiers in Neuroscience Lipid Mediators and Itch. In Itch: Mechanisms and Treatment; Carstens, E., Akiyama, T., Eds.; CRC Press/Taylor & Francis LLC.: Boca Raton, FL, USA, 2014. [Google Scholar]
- Andoh, T.; Kuraishi, Y. Intradermal leukotriene B4, but not prostaglandin E2, induces itch-associated responses in mice. Eur. J. Pharmacol. 1998, 353, 93–96. [Google Scholar] [CrossRef]
- Arai, I.; Takano, N.; Hashimoto, Y.; Futaki, N.; Sugimoto, M.; Takahashi, N.; Inoue, T.; Nakaike, S. Prostanoid DP1 receptor agonist inhibits the pruritic activity in NC/Nga mice with atopic dermatitis. Eur. J. Pharmacol. 2004, 505, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Kido-Nakahara, M.; Ulzii, D.; Miake, S.; Fujishima, K.; Sakai, S.; Chiba, T.; Tsuji, G.; Furue, M. Topical application of endothelin receptor a antagonist attenuates imiquimod-induced psoriasiform skin inflammation. Sci. Rep. 2020, 10, 9510. [Google Scholar] [CrossRef] [PubMed]
- Trentin, P.G.; Fernandes, M.B.; D’Orléans-Juste, P.; Rae, G.A. Endothelin-1 causes pruritus in mice. Exp. Biol. Med. (Maywood) 2006, 231, 1146–1151. [Google Scholar]
- McQueen, D.S.; Noble, M.A.; Bond, S.M. Endothelin-1 activates ETA receptors to cause reflex scratching in BALB/c mice. Br. J. Pharmacol. 2007, 151, 278–284. [Google Scholar] [CrossRef]
- Silva, M.; Videira, P.A.; Sackstein, R. E-Selectin Ligands in the Human Mononuclear Phagocyte System: Implications for Infection, Inflammation, and Immunotherapy. Front. Immunol. 2017, 8, 1878. [Google Scholar] [CrossRef]
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef]
- Pincelli, C. Nerve growth factor and keratinocytes: A role in psoriasis. Eur. J. Dermatol. 2000, 10, 85–90. [Google Scholar]
- Morimoto, C.; Schlossman, S.F. The structure and function of CD26 in the T-cell immune response. Immunol. Rev. 1998, 161, 55–70. [Google Scholar] [CrossRef]
- Ohnuma, K.; Dang, N.H.; Morimoto, C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008, 29, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ohnuma, K.; Hatano, R.; Komiya, E.; Otsuka, H.; Itoh, T.; Iwao, N.; Kaneko, Y.; Yamada, T.; Dang, N.H.; Morimoto, C. A novel role for CD26/dipeptidyl peptidase IV as a therapeutic target. Front. Biosci. (Landmark Ed.) 2018, 23, 1754–1779. [Google Scholar] [CrossRef]
- Ohnuma, K.; Hosono, O.; Dang, N.H.; Morimoto, C. Dipeptidyl peptidase in autoimmune pathophysiology. Adv. Clin. Chem. 2011, 53, 51–84. [Google Scholar] [PubMed]
- Kjeldsen, L.; Bainton, D.F.; Sengeløv, H.; Borregaard, N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 1994, 83, 799–807. [Google Scholar] [CrossRef]
- Xiao, X.; Yeoh, B.S.; Vijay-Kumar, M. Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation. Annu. Rev. Nutr. 2017, 37, 103–130. [Google Scholar] [CrossRef] [PubMed]
- Pariser, D.M.; Bagel, J.; Lebwohl, M.; Yosipovitch, G.; Chien, E.; Spellman, M.C. Serlopitant for psoriatic pruritus: A phase 2 randomized, double-blind, placebo-controlled clinical trial. J. Am. Acad. Dermatol. 2020, 82, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.; Yosipovitch, G. A New Generation of Treatments for Itch. Acta Derm. Venereol. 2020, 100, adv00027. [Google Scholar] [CrossRef]
- Keating, G.M. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs 2017, 77, 459–472. [Google Scholar] [CrossRef]
- Schafer, P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem. Pharmacol. 2012, 83, 1583–1590. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Soung, J.; Weiss, J.; Muscianisi, E.; Meng, X.; Gilloteau, I.; Elewski, B.E. Secukinumab Provides Rapid Relief From Itching and Pain in Patients with Moderate-to-Severe Psoriasis: Patient Symptom Diary Data from Two Phase 3, Randomized, Placebo-controlled Clinical Trials. Acta Derm. Venereol. 2019, 99, 820–821. [Google Scholar] [CrossRef]
- Griffiths, C.E.; Reich, K.; Lebwohl, M.; van de Kerkhof, P.; Paul, C.; Menter, A.; Cameron, G.S.; Erickson, J.; Zhang, L.; Secrest, R.J.; et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): Results from two phase 3 randomised trials. Lancet 2015, 386, 541–551. [Google Scholar] [CrossRef]
- Feldman, S.R.; Thaçi, D.; Gooderham, M.; Augustin, M.; de la Cruz, C.; Mallbris, L.; Buonanno, M.; Tatulych, S.; Kaur, M.; Lan, S.; et al. Tofacitinib improves pruritus and health-related quality of life up to 52 weeks: Results from 2 randomized phase III trials in patients with moderate to severe plaque psoriasis. J. Am. Acad. Dermatol. 2016, 75, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, N.; Bastan, R.; Peachell, P.T. Regulation of human skin mast cell histamine release by PDE inhibitors. Allergol. Immunopathol. (Madr.) 2015, 43, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Yoshida, T.; Kuraishi, Y. Topical E6005, a novel phosphodiesterase 4 inhibitor, attenuates spontaneous itch-related responses in mice with chronic atopy-like dermatitis. Exp. Dermatol. 2014, 23, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Wakita, H.; Ohkuro, M.; Ishii, N.; Hishinuma, I.; Shirato, M. A putative antipruritic mechanism of the phosphodiesterase-4 inhibitor E6005 by attenuating capsaicin-induced depolarization of C-fibre nerves. Exp. Dermatol. 2015, 24, 215–216. [Google Scholar] [CrossRef]
- Hashimoto, T.; Sakai, K.; Sanders, K.M.; Yosipovitch, G.; Akiyama, T. Antipruritic Effects of Janus Kinase Inhibitor Tofacitinib in a Mouse Model of Psoriasis. Acta Derm. Venereol. 2019, 99, 298–303. [Google Scholar] [CrossRef]
| System | Category | Mediator | Expression Changes in the Mediator | Mechanisms (Such as Receptors) | Expression Changes in Receptors of the Mediator | Predictive Effects on Itch |
|---|---|---|---|---|---|---|
| Nervous | Neuropeptides | SP | ↑ (L [35], B [36]) | NK-1R | ↑ (L [7]) | Induction of itch [37,38] |
| MrgprX2(Hu) | - | |||||
| MrgprB2(Ms) | - | |||||
| MrgprA1(Ms) | - | |||||
| CGRP | ↑ (B [34]) | CGRPR | ↑ (B [7]) | Aggravation of itch? [39] | ||
| NPY | ↓ (B [40]) | NPY1R | - | Suppression of mechanical itch [25] | ||
| Opioids | β-endorphin | UC [41]/↑ (L [42]) | MOR | UC [41] | Induction of itch [43] | |
| Dynorphin A | ↓ (L [41]) | KOR | ↓ (L [41]) | Suppression of itch [42,43] | ||
| Neurotrophins | NGF | ↑ (L [35,44]) | TrkA | ↑ (L [35]) | NGF-TrkA axis: aggravation of histamine-independent itch [45] | |
| P75 | - | |||||
| Immune | Cytokines | IL-31 | ↑ (L [46], B [47]) | IL-31RA | - | Induction of itch [48] |
| OSMRβ | - | |||||
| TSLP | ↑ (L [49], B [50]) | TSLPR, | - | Induction of itch [51] | ||
| IL-7Rα | - | |||||
| IL-2 | ↑ (L [35]) | IL-2Rα | - | Induction of itch [52,53,54] | ||
| IL-2Rβ | - | |||||
| IL-2Rγ | - | |||||
| IL-17 | ↑ (L [21]) | IL-17Rs | - | Enhancement of itch by altering perception? [55] | ||
| IL-22 | ↑ (L [56]) | IL-22R1 | - | Enhancement of itch by activation of the GRP-GRPR signal? [57] | ||
| IL-10R2 | - | |||||
| IL-23 | ↑ (L [56,58]) | IL-23R | - | Enhancement of itch though the aggravation of inflammation? [59] | ||
| IL-12Rβ1 | - | |||||
| IL-26 | ↑ (L [60]) | Il-20R1 | - | Enhancement of itch by promoting the sensory neuronal development? [61] | ||
| IL-10R2 | - | |||||
| Endocrine | HPA axis | CRH | ↑ (L [62]) | CRHR1 | - | Induction/aggravation of itch by mast cell degranulation [63,64] |
| α-MSH | ↑ (L [62]) | MC1R | - | Induction of itch [65,66] | ||
| MC5R | ||||||
| Vascular | Growth factors | VEGF | ↑ (L [67], B [68]) | VEGFRs | - | Aggravation of itch? [67] |
| Prostanoids | PGE2 | ↑ (L [69]) | cAMP | - | Induction of weak itch and enhancement of histamine-/serotonin- induced itch [70,71] | |
| Autacoids | ET-1 | ↑ (L [72]) | ET-A/ET-B | - | Induction of itch [73] | |
| Cell adhesion molecules | E-selectin | ↑ (L [35]) | - | - | Aggravation of itch? | |
| VAP-1 | ↑ (B [74]) | - | - | Aggravation of itch? | ||
| Others | Peptidases | DPPIV | ↑ (L [36], B [75]) | SP (cleavage) | ↑ (L [35], B [36]) | Aggravation of itch [39,75] |
| Lipocalins | LCN2 | ↑ (B [76]) | GRP (production) | - | Aggravation of itch [77] |
| Category | Drug Name | Target Interaction | Phase | Administration Type | Significant Findings | NCT# | References |
|---|---|---|---|---|---|---|---|
| NK-1R inhibitor | Serlopitant | NK-1R | 2 | Oral | Yes | NCT03343639 | [158] |
| TrkA inhibitor | CT327 | TrkA | 2b | Topical | Yes | NCT01465282 | [98] |
| SNA-120 | 2b | Topical | No * | NCT03322137 | [159] | ||
| PDE4 inhibitor | Apremilast | PDE4 | Scalp: 4 | Oral | - | NCT03553433 | [159,160] |
| Plaque: 3 | Oral | Yes | NCT03721172 | ||||
| OSMRβ moAb | KPL-716 | OSMRβ | Plaque: 2 (pilot study) | Injection | - | NCT03858634 | [161] |
| IL-17 moAb | Secukinumab | IL-17A | 3 | SC | Yes | NCT01365455 | [110] |
| 3 | SC | Yes | NCT01358578 | [162] | |||
| Ixekizumab | IL-17A | 3 | SC | Yes | NCT01597245 | [163] | |
| 3 (long-term test) | SC | Yes | NCT01474512 | [108] | |||
| JAK inhibitor | Tofacitinib | JAK-STAT pathway | 3 | Oral | Yes | NCT01276639 | [164] |
| Yes | NCT01309737 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komiya, E.; Tominaga, M.; Kamata, Y.; Suga, Y.; Takamori, K. Molecular and Cellular Mechanisms of Itch in Psoriasis. Int. J. Mol. Sci. 2020, 21, 8406. https://doi.org/10.3390/ijms21218406
Komiya E, Tominaga M, Kamata Y, Suga Y, Takamori K. Molecular and Cellular Mechanisms of Itch in Psoriasis. International Journal of Molecular Sciences. 2020; 21(21):8406. https://doi.org/10.3390/ijms21218406
Chicago/Turabian StyleKomiya, Eriko, Mitsutoshi Tominaga, Yayoi Kamata, Yasushi Suga, and Kenji Takamori. 2020. "Molecular and Cellular Mechanisms of Itch in Psoriasis" International Journal of Molecular Sciences 21, no. 21: 8406. https://doi.org/10.3390/ijms21218406
APA StyleKomiya, E., Tominaga, M., Kamata, Y., Suga, Y., & Takamori, K. (2020). Molecular and Cellular Mechanisms of Itch in Psoriasis. International Journal of Molecular Sciences, 21(21), 8406. https://doi.org/10.3390/ijms21218406





