Why Vitamin C Could Be an Excellent Complementary Remedy to Conventional Therapies for Breast Cancer
Abstract
:1. Introduction
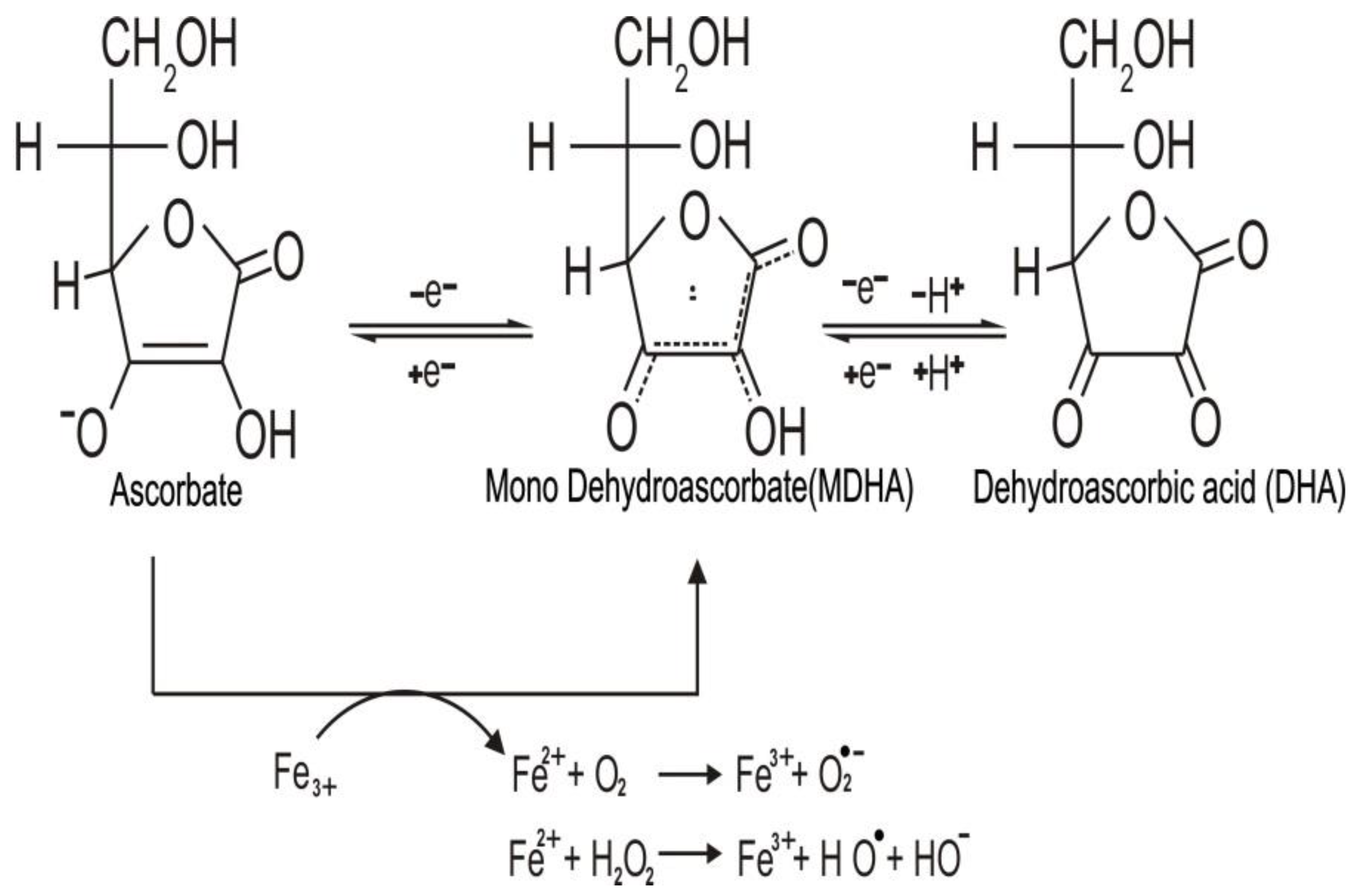
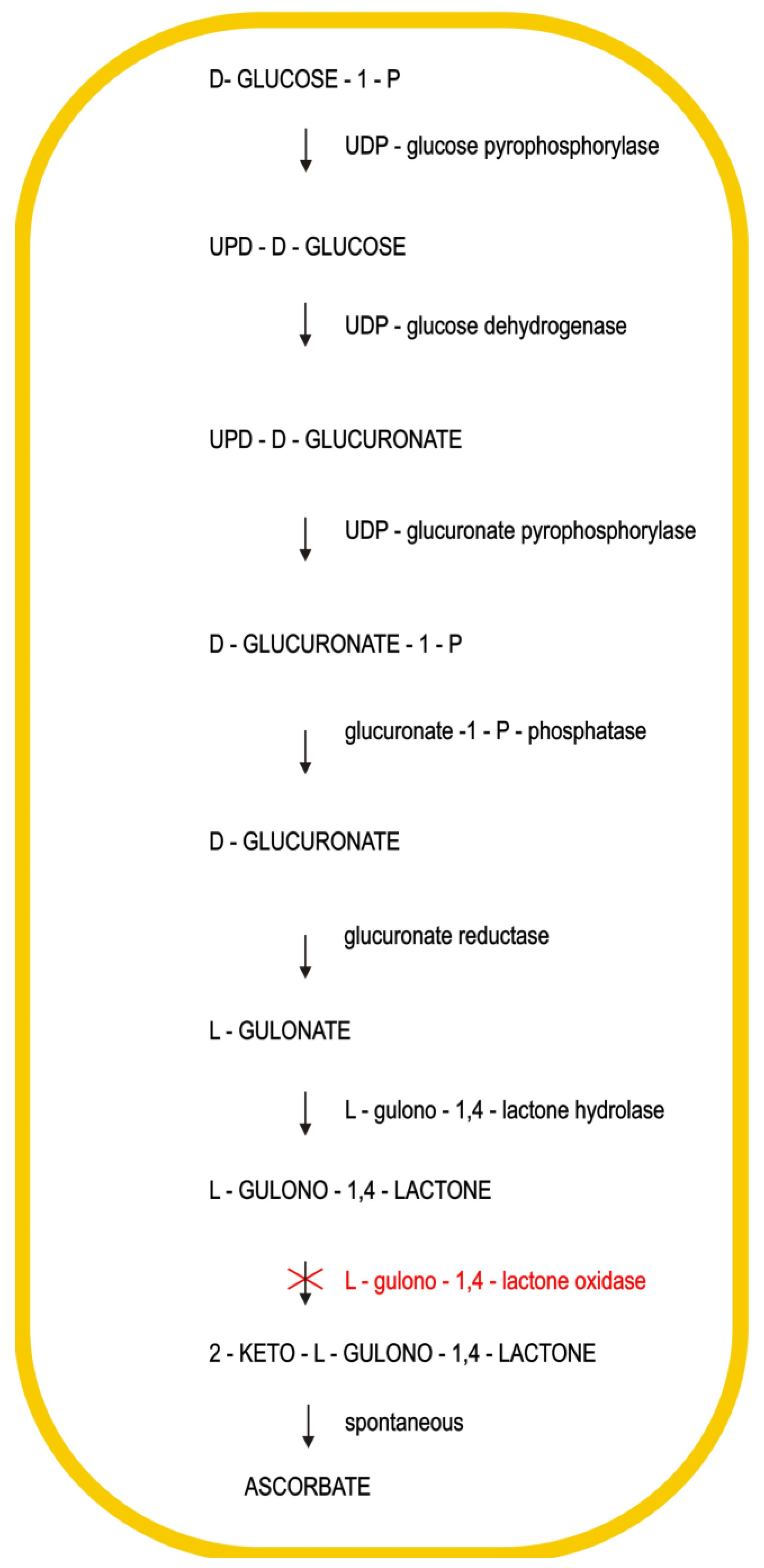
2. Chemistry and Biochemistry of Vitamin C
3. Anticancer Mechanisms of Vitamin C
4. Vitamin C Effects in Breast Cancer Cell Lines and Human Mammary Tumor Xenografts
5. Vitamin C Effects In Vivo Treatment of Cancer in Human
6. Why Should Vitamin C be Used in Breast Cancer Therapy?
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| αKGDDs | α-ketoglutarate-dependent dioxygenases |
| 5caC | 5-carboxyl-cytosine |
| 5fC | 5-formyl-cytosine |
| 5hmC | 5-hydroxy-methyl-cytosine |
| 5mC | 5-methyl-cytosine |
| COX-2 | Cyclooxygenase 2 |
| CSC | Cancer stem cell |
| 2-DG | 2-deoxy-D-glucose |
| DHA | Dehydroascorbate |
| DHA | Dehydroascorbic acid |
| d-TPP | Dodecyl-tri-phenyl-phosphonium |
| ER | Estrogen receptor |
| EU | European Union |
| GLUT | Glucose transporter |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| H2O2 | Hydrogen peroxide |
| HER2 | Human epidermal growth factor 2 |
| HIF | Hypoxia-inducible factor |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| HIF-1β | Hypoxia-inducible factor 1 beta |
| HO• | Hydroxyl radicals |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| IVC | Intravenous vitamin C |
| LTED | Long term estrogen deprived |
| MDHA | Monodehydroascorbate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK | Natural killer |
| O2•− | Peroxide ions |
| P53 | Protein 53 |
| PGE2 | Prostaglandin E2 |
| PR | Progesterone receptor |
| QoL | Quality of life |
| ROS | Reactive oxygen species |
| SVCT | Sodium dependent vitamin C transporters |
| TAM-R | Tamoxifen-resistant |
| TET | Ten eleven translocation proteins |
| TNBC | Triple negative breast cancer |
| TNF-α | Tumor necrosis factor alfa |
| WHO | World Health Organization |
References
- Carioli, G.; Malvezzi, M.; Rodriguez, T.; Bertuccio, P.; Negri, E.; La Vecchia, C. Trends and predictions to 2020 in breast cancer mortality in Europe. Breast 2017, 36, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, T.M.; Pandey, A.K.; Shyama, S.K. Breast cancer and etiology. Trends Med. 2017, 17, 1–7. [Google Scholar] [CrossRef]
- Kim, R.K.; Suh, Y.; Yoo, K.C.; Cui, Y.H.; Kim, H.; Kim, M.J.; Kim, I.G.; Lee, S.J. Activation of KRAS promotes the mesenchymal features of basal-type breast cancer. Exp. Mol. Med. 2015, 47, e137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, E.; Pauling, L.; Leibovitz, B. Ascorbic acid and cancer: A review. Cancer Res. 1979, 39, 663–681. [Google Scholar] [PubMed]
- Zaidi, S.; Hussain, S.; Verma, S.; Veqar, Z.; Khan, A.; Un Nazir, S.; Singh, N.; Ali Moiz, J.; Tanwar, P.; Srivastava, A.; et al. Efficacy of Complementary Therapies in the Quality of Life of Breast Cancer Survivors. Front. Oncol. 2017, 7, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyatt, G.; Sikorskii, A.; Wills, C.E.; Su, H. Complementary and alternative medicine use, spending, and quality of life in early stage breast cancer. Nurs Res. 2010, 59, 58–66. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and treatment of cancer. Biochim. Bio-Phys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Wohlrab, C.; Phillips, E.; Dachs, G.U. Vitamin C Transporters in Cancer: Current Understanding and Gaps in Knowledge. Front. Oncol. 2017, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Dewhirst, R.A.; Fry, S.C. The oxidation of dehydroascorbic acid and 2,3-diketogulonate by distinct reactive oxygen species. Biochem. J. 2018, 475, 3451–3470. [Google Scholar] [CrossRef] [Green Version]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, M.J.; Miranda-Massari, J.R.; Mora, E.M.; Guzmán, A.; Riordan, N.H.; Riordan, H.D.; Casciari, J.J.; Jackson, J.A.; Román-Franco, A. Orthomolecular Oncology Review: Ascorbic Acid and Cancer 25 Years Later. Integr. Cancer Ther. 2005, 4, 32–44. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant. Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Pal, S.; Jana, N.R. Pharmacologic Vitamin C Based Cell Therapy via Iron Oxide Nanoparticle-Induced Intracellular Fenton Reaction. Acs Appl. Nano Mater. 2020, 3, 1683–1692. [Google Scholar] [CrossRef]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1029. [Google Scholar]
- Rivas, C.I.; Zuniga, F.A.; Salas-Burgos, A.; Mardones, L.; Ormazabal, V.; Vera, J.C. Vitamin C transporters. J. Physiol. Biochem. 2008, 64, 357–375. [Google Scholar] [CrossRef]
- Gromova, O.A.; Torshin, I.Y.; Pronin, A.V.; Kilchevsky, M.A. Synergistic Application of Zinc and Vitamin C to Support Memory and Attention and to Decrease the Risk of Developing Nervous System Diseases. Neurosci. Behav. Physiol. 2019, 4, 357–364. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharm. 2018, 9, 1162. [Google Scholar] [CrossRef] [Green Version]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumour. Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef] [Green Version]
- Mc Cormick, W.J. Cancer: The preconditioning factor in pathogenesis. Arch. Pediat 1954, 71, 313–322. [Google Scholar]
- Cameron, E.; Rotman, D. Ascorbic acid, cell proliferation, and cancer. Lancet 1972, 1, 542. [Google Scholar] [CrossRef]
- El Attar, T.M.A.; Lin, H.S. Effect of vitamin C on prostaglandin synthesis by fibroblasts and squamous carcinoma cells. Prostaglandins Leukot. Essent. Fat. Acids 1992, 47, 253–257. [Google Scholar] [CrossRef]
- Wang, D.; Du Bais, R.N. Prostaglandin and cancer. Gut 2006, 55, 115–122. [Google Scholar] [CrossRef]
- Leekha, A.; Gurjar, B.S.; Tyagi, A.; Rizvi, M.A.; Verna, K. Vitamin C in synergism with cisplatin induces cell death in cervical cancer cells through altered redox cycling and p53 upregulation. J. Cancer Res. Clin. Oncol. 2016, 142, 2503–2514. [Google Scholar]
- Kim, J.; Lee, S.D.; Chang, B.; Jin, D.H.; Jung, S.I.; Park, M.Y.; Han, Y.; Kim, K.I.; Lim, J.S.; Kang, Y.S.; et al. Enhanced antitumor activity of vitamin C via p53 in cancer cells. Free Radic. Biol. Med. 2012, 53, 1607–1615. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Wagner, B.A.; Cramer-Morales, K.L.; Furqan, M. O 2- and H 2 O 2 -Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell 2017, 31, 487–500. [Google Scholar] [CrossRef] [Green Version]
- Olney, K.E.; Du, J.; van’t Erve, T.J.; Witmer, J.R.; Sibenaller, Z.A.; Wagner, B.A. Inhibitors of hydroperoxide metabolism enhance ascorbate-induced cytotoxicity. Free Radic. Res. 2013, 47, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.H.; Habib, H.M.; Kamal, H.; St Clair, D.K.; Chow, C.K. Mitochondrial superoxide mediates labile I on level: Evidence from Mn-SOD bgtransgenic mice and heterozygous knockout mice and isolated rat liver mitochondria. Free Radic. Biol. Med. 2013, 65, 143–149. [Google Scholar] [CrossRef]
- Lu, Y.X.; Wu, Q.N.; Chen, D.L.; Chen, L.Z.; Wang, Z.X.; Ren, C.; Mo, H.Y.; Chen, Y.; Sheng, H.; Wang, Y.N.; et al. Pharmacological ascorbate suppresses growth of gastric cancer cells with GLUT1 overexpression and enhances the efficacy of oxaliplatin through redox modulation. Theranostics 2018, 8, 1312–1326. [Google Scholar] [CrossRef] [Green Version]
- Ge, G.; Peng, D.; Ziying, X.; Guan, B.; Xin, Z.; He, Q.; Zhou, Y.; Li, X.; Zhou, L.; Ci, W. Restoration of 5-hydroxymethylcytosine by ascorbate blocks kidney tumour growth. EMBO Rep. 2018, 19, e45401. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Hore, T.A. Modulating epigenetic memory through vitamins and TET: Implications for regenerative medicine and cancer treatment. Epigenomics 2017, 9, 863–871. [Google Scholar] [CrossRef]
- Yun, J.; Johnson, J.L.; Hanigan, C.L.; Locasale, J.W. Interactions between epigenetics and metabolism in cancers. Front. Oncol. 2012, 15, 163. [Google Scholar] [CrossRef] [Green Version]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- and Antioxidant Effects of Vitamin C in Cancer in correspondence to Its Dietary and Pharmacological Concentrations. Oxidative Med. Cell. Longev. 2019, 2019, 7286737. [Google Scholar] [CrossRef] [Green Version]
- Mastrangelo, D.; Pelosi, E.; Castelli, G.; Lo-Coco, F.; Testa, U. Mechanisms of anti-cancer effects of ascorbate: Cytotoxic activity and epigenetic modulation. Blood Cells Mol. Dis. 2018, 69, 57–64. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of the breast cancer stem cell phenotype by hypoxia-inducible factors. Clin. Sci. 2015, 129, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Cook, J. Intravenous Vitamin C for Cancer Therapy—Identifying the Current Gaps in Our Knowledge. Front. Physiol. 2018, 9, 1182. [Google Scholar] [CrossRef]
- Gil, M.; Kim, K.E. Interleukin-18 Is a Prognostic Biomarker Correlated with CD8+ T Cell and Natural Killer Cell Infiltration in Skin Cutaneous Melanoma. J. Clin. Med. 2019, 8, 1993. [Google Scholar] [CrossRef] [Green Version]
- Rex, D.A.B.; Agarwal, N.; Prasad, T.S.K.; Kandasamy, R.K.; Subbannayya, Y.; Pinto, S.M. A comprehensive pathway map of IL-18-mediated signalling. J. Cell Commun. Signal. 2020, 14, 257–266. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Härtel, C.; Strunk, T.; Bucsky, P.; Schultz, C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokines 2004, 27, 101–106. [Google Scholar] [CrossRef]
- Bowie, A.G.; O’Neill, L.A.J. Vitamin C Inhibits NF-kB Activation by TNF Via the Activation of p38 Mitogen-Activated Protein Kinase. J. Immunol. 2000, 165, 7180–7188. [Google Scholar] [CrossRef] [Green Version]
- Munoz, E.; Blazquez, M.V.; Ortiz, C.; Gomez-Diaz, C.; Navas, P. Role of ascorbate in the activation of NF-kB by tumor necrosis factor-a in T-cells. Biochem. J. 1997, 325, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowie, A.G.; Carcamo, J.M.; Pedraza, A.; Borquez-Qjeda, O.; Golde, D.W. Vitamin C suppresses TNFa-induced NFkB activation by inhibiting IkαB phosphorilation. Biochemistry 2002, 41, 12995–13002. [Google Scholar] [CrossRef]
- Greten, F.R.; Grivennikow, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [Green Version]
- Kurbacher, C.M.; Wagner, U.; Kolster, B.; Andreotti, P.E.; Krebs, D.; Bruckner, H.W. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett. 1996, 103, 183–189. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, J.H.; Lee, I.H.; Lee, J.; Jung, J.H.; Park, H.Y.; Lee, D.H.; Chae, Y.S. Effect of High-dose Vitamin C Combined with Anti-cancer Treatment on Breast Cancer Cells. Anticancer Res. 2019, 39, 751–758. [Google Scholar] [CrossRef]
- Hatem, E.; Azzi, S.; El Banna, N.; He, T.; Heneman-Masurel, A.; Vernis, L.; Baılle, D.; Masson, V.; Dingli, F.; Loew, D.; et al. Auranofin/Vitamin C: A Novel Drug Combination Targeting Triple-Negative Breast Cancer. JNCI J. Natl. Cancer Inst. 2019, 111, 597–608. [Google Scholar] [CrossRef]
- Zeng, L.H.; Wang, Q.M.; Feng, L.Y.; Ke, Y.D.; Xu, Q.Z.; Wei, A.Y.; Zhang, C.; Ying, R.B. High-dose vitamin C suppresses the invasion and metastasis of breast cancer cells via inhibiting epithelial-mesenchymal transition. Oncotargets 2019, 12, 7405–7413. [Google Scholar] [CrossRef] [Green Version]
- Gan, L.; Camarena, V.; Mustafi, S.; Wang, G. Vitamin C Inhibits Triple-Negative Breast Cancer Metastasis by Affecting the Expression of YAP1 and Synaptopodin 2. Nutrients 2019, 11, 2997. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.H.; Peng, K.L.; Kang, M.L.; Chen, Y.R.; Yang, Y.C.; Tsai, C.H.; Chu, C.S.; Jeng, Y.M.; Chen, Y.T.; Lin, F.M.; et al. TET1 suppresses cancer invasion by activating the tissue inhibitors of metalloproteinases. Cell Rep. 2012, 2, 568–579. [Google Scholar] [CrossRef] [Green Version]
- Sant, D.W.; Mustafi, S.; Gustafson, C.B.; Chen, J.; Slingerland, J.M.; Wang, G. Vitamin C promotes apoptosis in breast cancer cells by increasing TRAIL expression. Sci. Rep. 2018, 8, 5306. [Google Scholar] [CrossRef]
- Cha, J.; Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int. J. Oncol. 2013, 42, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Yoon, S.Y.; Kim, K.E.; Lee, H.R.; Hur, D.Y.; Song, H.; Kim, D.; Bang, S.I.; Cho, D.H. Interleukin-18 induces transferrin expression in breast cancer cell line MCF-7. Cancer Lett. 2009, 286, 189–195. [Google Scholar] [CrossRef]
- De Francesco, E.M.; Ozsvari, B.; Sotgia, F.; Lisanti, M.P. Dodecyl-TPP Targets Mitochondria and Potently Eradicates Cancer Stem Cells (CSCs): Synergy With FDA-Approved Drugs and Natural Compounds (Vitamin C and Berberine). Front. Oncol. 2019, 9, 615. [Google Scholar] [CrossRef] [Green Version]
- Tsao, C.S. Inhibiting effect of ascorbic acid on the growth of human mammary tumor xenografts. Am. J. Clin. Nutr. 1991, 54, 1274S–1280S. [Google Scholar] [CrossRef]
- McCormick, W.J. Cancer: A collagen disease, secondary to nutrition deficiency. Arch. Pediat. 1959, 76, 166–171. [Google Scholar]
- Hoffman, F. Micronutrient requirements of cancer patients. Cancer 1985, 55, 14550. [Google Scholar] [CrossRef]
- van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.H.M.J.; Bos, G.M.J. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; McCall, C. The role of vitamin C in the treatment of pain: New insights. J. Transl. Med. 2017, 15, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeom, C.; Jung, G.; Song, K. Changes of terminal cancer patients health related quality of life after high dose vitamin C administration. Korean Med. Sci. 2007, 22, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Vollbracht, C.; Schneider, B.; Leendert, V.; Weiss, G.; Auerbach, L.; Beuth, J. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo 2011, 82, 983–990. [Google Scholar]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H., Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014, 6, 222ra18. [Google Scholar] [CrossRef]
- Riordan, H.D.; Casciari, J.J.; Gonzalez, M.J.; Riordan, N.H.; Miranda-Massari, J.R.; Taylor, P.; Jackson, J.A. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P. R. Health Sci. J. 2005, 24, 269–276. [Google Scholar]
- Hoffer, L.J.; Robitaille, L.; Zakarian, R.; Melnychuk, D.; Kavan, P.; Agulnik, J.; Cohen, V.; Small, D.; Miller, W.H., Jr. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: A phase I-II clinical trial. PLoS ONE 2015, 10, e0120228. [Google Scholar] [CrossRef]
- Agus, D.B.; Vera, J.C.; Golde, D.W. Stromal cell oxidation: A mechanism by which tumors obtain vitamin C. Cancer Res. 1999, 59, 4555–4558. [Google Scholar]
- Raloff, J. Antioxidants may help cancers thrive. Sci. News 2000, 157, 5. [Google Scholar] [CrossRef]
- Fromberg, A.; Gutsch, D.; Schulze, D.; Vollbracht, C.; Weiss, G.; Czubayko, F.; Aigner, A. Ascorbate exerts anti-proliferative effects through cell cycle inhibition and sensitizes tumor cells toward cytostatic drugs. Cancer Chemother. Pharm. 2011, 67, 1157–1166. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, K.; Hosokawa, Y.; Hazawa, M.; Kashiwakura, I.; Okumura, K.; Kaku, T.; Nakayama, E. Ascorbic acid enhances radiation-induced apoptosis in an HL60 human leukemia cell line. J. Ratiat. Res. 2011, 52, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Espey, M.; Chen, P.; Chalmers, B.; Drisko, J.; Sun, A.Y.; Levine, M.; Chen, Q. Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer. Free Radic. Biol. Med. 2011, 50, 1610–1619. [Google Scholar] [CrossRef] [Green Version]
- Simone, C.B.; Simone, N.L.; Simone, V.; Simone, C.B. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase survival, part 1. Atlern. Health Med. 2007, 13, 22–28. [Google Scholar]
- Block, K.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementaion on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.J.; Miranda—Massari, J.R. New Insights on Vitamin C and Cancer. In Springer Briefs in Cancer Research; Lau, A., Liu, Y., Tron, A., Inuzuka, H., Wei, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Khanzode, S.S.; Muddeshwar, M.G.; Khanzode, S.D.; Dakhale, G.N. Antioxidant enzymes and lipid peroxidation in different stages of breast cancer. Free Radic. Res. 2004, 38, 81–85. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Molina-Montes, E.; Salamanca-Fernández, E.; Garcia-Villanova, B.; Sánchez, M.J. The Impact of Plant-Based Dietary Patterns on Cancer-Related Outcomes: A Rapid Review and Meta-Analysis. Nutrients 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Harrison, H.; Pegg, H.J.; Thompson, J.; Bates, C.; Shore, P. HIF1-alpha expressing cells induce a hypoxic-like response in neighbouring cancer cells. BMC Cancer 2018, 18, 674. [Google Scholar] [CrossRef] [Green Version]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef] [Green Version]
- Knowles, H.J.; Raval, R.R.; Harris, A.L.; Ratcliffe, P.J. Effect of ascorbate on the activity of hypoxia-inducible factor in cancer cells. Cancer Res. 2003, 63, 1764–1768. [Google Scholar]
- Yang, H.; Liu, Y.; Bai, F.; Zhang, J.Y.; Ma, S.H.; Liu, J.; Xu, Z.D.; Zhu, H.G.; Ling, Z.Q.; Ye, D.; et al. Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation. Oncogene 2013, 32, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Zambrano, A.; Molt, M.; Uribe, E.; Salas, M. Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as a Potential Therapeutic Strategy. Int. J. Mol. Sci. 2019, 20, 3374. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Codini, M. Why Vitamin C Could Be an Excellent Complementary Remedy to Conventional Therapies for Breast Cancer. Int. J. Mol. Sci. 2020, 21, 8397. https://doi.org/10.3390/ijms21218397
Codini M. Why Vitamin C Could Be an Excellent Complementary Remedy to Conventional Therapies for Breast Cancer. International Journal of Molecular Sciences. 2020; 21(21):8397. https://doi.org/10.3390/ijms21218397
Chicago/Turabian StyleCodini, Michela. 2020. "Why Vitamin C Could Be an Excellent Complementary Remedy to Conventional Therapies for Breast Cancer" International Journal of Molecular Sciences 21, no. 21: 8397. https://doi.org/10.3390/ijms21218397
APA StyleCodini, M. (2020). Why Vitamin C Could Be an Excellent Complementary Remedy to Conventional Therapies for Breast Cancer. International Journal of Molecular Sciences, 21(21), 8397. https://doi.org/10.3390/ijms21218397




