Antifibrotic and Regenerative Effects of Treamid in Pulmonary Fibrosis
Abstract
:1. Introduction
2. Results
2.1. The Effect of Treamid on Lungs Damaged by Bleomycin
2.1.1. Effects of Treamid on Tissue Morphology
2.1.2. Evaluation of Collagen Fibers
2.1.3. Mean Linear Intercept (Lm)
2.1.4. Destructive Index (DI)
2.1.5. Capillaries of the Lungs
2.2. Effect of Treamid on the Molecules of Fibroblastic Process Expression in Bleomycin Damaged Lungs
2.3. Effects of Treamid on IL-13 in Bleomycin Damaged Lungs
2.4. Effects of Treamid on Lung Endothelial Progenitor Cells In Vivo
2.5. The Effect of Treamid on CD31+ Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Modeling of Experimental Pulmonary Fibrosis
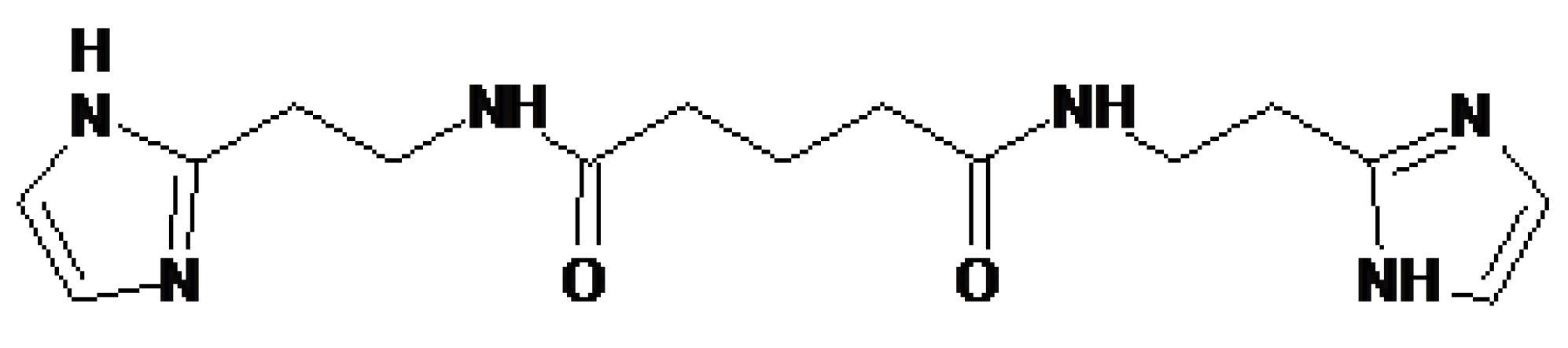
4.3. Treamid
4.4. Experimental Groups
4.5. Enzyme-Linked Immunosorbent Assay
4.5.1. Hydroxyproline, Collagen Type I, Fibronectin, and Connective Tissue Growth Factor Measurements
4.5.2. Total Soluble Collagen Assay
4.5.3. Interleukin 13 Measurements
4.6. Histological Examination of Lung Tissue
4.7. Flow Cytometric Analysis
4.8. Lung Tissue Dissociation, Isolation and Magnetic Separation of CD31+ Lung Endothelial Cells
4.9. Cultivation of CD31+ Lung Endothelial Cells with Treamid
4.10. Lung Tissue Dissociation, Isolation and Magnetic Separation of CD326+ Lung Epithelial Cells
4.11. Cultivation of CD326+ Lung Epithelial Cells
4.12. Cellular Imaging
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IPF | Idiopathic pulmonary fibrosis |
| MSC | Mesenchymal stem cells |
| BDDA | Bisamide derivative of dicarboxylic acid or Treamid |
| BLM | Bleomycin |
| Lm | Mean linear intercept |
| DI | Destructive index |
| PBS | Phosphate buffered saline |
| FBS | Fetal bovine serum |
| IL 13 | Interleukin 13 |
| CFSE | Carboxyfluorescein succinimidyl ester |
References
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care. Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Wilson, K.C.; Raghu, G. Diagnosing idiopathic pulmonary fibrosis in 2018: Bridging recommendations made by experts serving different societies. Eur. Respir. J. 2018, 52, 1801485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgalla, G.; Franciosa, C.; Simonetti, J.; Richeldi, L. Pamrevlumab for the treatment of idiopathic pulmonary fibrosis. Expert Opin. Investig. Drugs 2020. published online ahead of print, 23 May 2020. [Google Scholar] [CrossRef] [PubMed]
- Kishaba, T. Evaluation and management of Idiopathic Pulmonary Fibrosis. Respir. Investig. 2019, 57, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Luckhardt, T.R. Updated Evaluation of the Safety, Efficacy and Tolerability of Pirfenidone in the Treatment of Idiopathic Pulmonary Fibrosis. Drug Healthc. Patient. Saf. 2020, 12, 85–94. [Google Scholar] [CrossRef]
- Richeldi, L.; Kolb, M.; Jouneau, S.; Wuyts, W.A.; Schinzel, B.; Stowasser, S.; Quaresma, M.; Raghu, G. Efficacy and safety of nintedanib in patients with advanced idiopathic pulmonary fibrosis. BMC Pulm. Med. 2020, 20, 3. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Ye, T.; Sun, P.; Gui, S.; Liang, B.; Li, L.; Zheng, D.; Wang, J.; Hesketh, R.L.; Yang, L.; et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020, 295, 715–721. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, B.J.; Yang, J.C.; Wang, M.Y.; Chen, C.; Luo, G.X.; He, W.F. Advances in the research of mechanism of pulmonary fibrosis induced by corona virus disease 2019 and the corresponding therapeutic measures. Zhonghua Shao Shang Za Zhi 2020, 36, E006. [Google Scholar] [CrossRef]
- Malli, F.; Koutsokera, A.; Paraskeva, E.; Zakynthinos, E.; Papagianni, M.; Makris, D.; Tsilioni, I.; Molyvdas, P.A.; Gourgoulianis, K.I.; Daniil, Z. Endothelial progenitor cells in the pathogenesis of idiopathic pulmonary fibrosis: An evolving concept. PloS ONE 2013, 8, e53658. [Google Scholar] [CrossRef] [Green Version]
- Fadini, G.P.; Losordo, D.; Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circulation Res. 2012, 110, 624–637. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Ogawa, R. The Vascular Involvement in Soft Tissue Fibrosis-Lessons Learned from Pathological Scarring. Int. J. Mol. Sci. 2020, 21, 2542. [Google Scholar] [CrossRef] [Green Version]
- Cooper, G.J.; Phillips, A.R.; Choong, S.Y.; Leonard, B.L.; Crossman, D.J.; Brunton, D.H.; Saafi, E.L.; Dissanayake, A.M.; Cowan, B.R.; Young, A.A. Regeneration of the heart in diabetes by selective copper chelation. Diabetes 2004, 53, 2501–2508. [Google Scholar] [CrossRef] [Green Version]
- Cooper, G.J. Therapeutic potential of copper chelation with triethylenetetramine in managing diabetes mellitus and Alzheimer’s disease. Drugs 2011, 71, 1281–1320. [Google Scholar] [CrossRef]
- Cooper, G.J. Selective divalent copper chelation for the treatment of diabetes mellitus. Curr Med Chem. 2012, 19, 2828–2860. [Google Scholar] [CrossRef]
- Lu, J.; Pontré, B.; Pickup, S.; Choong, S.Y.; Li, M.; Xu, H.; Gamble, G.D.; Phillips, A.R.; Cowan, B.R.; Young, A.A.; et al. Treatment with a copper-selective chelator causes substantive improvement in cardiac function of diabetic rats with left-ventricular impairment. Cardiovasc. Diabetol. 2013, 12, 28. [Google Scholar] [CrossRef] [Green Version]
- Nebolsin, V.E.; Rydlovskaya, A.V.; Dygai, A.M.; Borovskaya, T.G.; Skurikhin, E.G.; Obschestvo S Ogranichennoi Otvetstvennostiyu. Bisamide Derivative of Dicarboxylic Acid as an Agent for Stimulating Tissue Regeneration and Recovery of Diminished Tissue Function. U.S. Patent WO 2016190785, 1 December 2016. [Google Scholar]
- Nebolsin, V.E.; Rydlovskaya, A.V.; Dygai, A.M.; Borovskaya, T.G.; Skurikhin, E.G. Bisamide Derivative Of Dicarboxylic Acid As An Agent For Stimulating Tissue Regeneration And Recovery Of Diminished Tissue Function. U.S. Patent 10,076,511, 18 September 2018. [Google Scholar]
- Pakhomova, A.V.; Nebolsin, V.E.; Pershina, O.V.; Krupin, V.A.; Sandrikina, L.A.; Alexandrovna Sandrikina, L.; Sergeevich Pan, E.; Nicolaevna Ermakova, N.; Evgenevna Vaizova, O.; Widera, D. Antidiabetic Effects of Bisamide Derivative of Dicarboxylic Acid in Metabolic Disorders. Int. J. Mol. Sci. 2020, 21, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, G.; Kwon, S.; Caraher, E.J.; Haider, S.H.; Lam, R.; Batra, P.; Melles, D.; Liu, M.; Nolan, A. Quantitative lung morphology: Semi-automated measurement of mean linear intercept. BMC Pulm. Med. 2019, 19, 206. [Google Scholar] [CrossRef]
- Knudsen, L.; Weibel, E.R.; Gundersen, H.J.; Weinstein, F.V.; Ochs, M. Assessment of air space size characteristics by intercept (chord) measurement: An accurate and efficient stereological approach. J. Appl. Physiol. 2010, 10, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, T.R.; Gieseck, R.L.; Acciani, T.H.; Hart, K.M.; Cheever, A.W.; Mentink-Kane, M.M.; Vannella, K.M.; Wynn, T.A. Enhanced protection from fibrosis and inflammation in the combined absence of IL-13 and IFN-γ. J. Pathol. 2016, 239, 344–354. [Google Scholar] [CrossRef] [Green Version]
- Costabel, U.; Albera, C.; Lancaster, L.H.; Lin, C.Y.; Hormel, P.; Hulter, H.N.; Noble, P.W. An Open-Label Study of the Long-Term Safety of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis (RECAP). Respiration 2017, 94, 408–415. [Google Scholar] [CrossRef]
- Meyer, K.C. Pulmonary fibrosis, part I: Epidemiology, pathogenesis, and diagnosis. Expert. Rev. Respir. Med. 2017, 11, 343–359. [Google Scholar] [CrossRef]
- Adamali, H.I.; Maher, T.M. Current and novel drug therapies for idiopathic pulmonary fibrosis. Drug Des. Devel. Ther. 2012, 6, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Richeldi, L.; du Bois, R.M.; Raghu, G.; Azuma, A.; Brown, K.K.; Costabel, U.; Cottin, V.; Flaherty, K.R.; Hansell, D.M.; Inoue, Y.; et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 2014, 370, 2071–2082. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Goto, T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int. J. Mol. Sci. 2019, 20, 1461. [Google Scholar] [CrossRef] [Green Version]
- Misharin, A.V.; Morales-Nebreda, L.; Mutlu, G.M.; Budinger, G.R.; Perlman, H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell. Mol. Biol. 2013, 49, 503–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wick, G.; Grundtman, C.; Mayerl, C.; Wimpissinger, T.F.; Feichtinger, J.; Zelger, B.; Sgonc, R.; Wolfram, D. The immunology of fibrosis. Annu Rev Immunol. 2013, 31, 107–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dakhlallah, D.; Wang, Y.; Bobo, T.A.; Ellis, E.; Mo, X.; Piper, M.G.; Eubank, T.D.; Marsh, C.B. Constitutive AKT Activity Predisposes Lung Fibrosis by Regulating Macrophage, Myofibroblast and Fibrocyte Recruitment and Changes in Autophagy. Adv. Biosci. Biotechnol. 2019, 10, 346–373. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Wu, Z.; Bai, D.; Liu, T.; Ullenbruch, M.R.; Phan, S.H. Ullenbruch, and Sem H. Phan. Mesenchymal Deficiency of Notch1 Attenuates Bleomycin-Induced Pulmonary Fibrosis. Am. J. Pathol. 2015, 185, 3066–3075. [Google Scholar] [CrossRef] [Green Version]
- Smadja, D.M.; Mauge, L.; Nunes, H.; d’Audigier, C.; Juvin, K.; Borie, R.; Carton, Z.; Bertil, S.; Blanchard, A.; Crestani, B.; et al. Imbalance of circulating endothelial cells and progenitors in idiopathic pulmonary fibrosis. Angiogenesis 2013, 16, 147–157. [Google Scholar] [CrossRef]
- De Biasi, S.; Cerri, S.; Bianchini, E.; Gibellini, L.; Persiani, E.; Montanari, G.; Luppi, F.; Carbonelli, C.M.; Zucchi, L.; Bocchino, M.; et al. Levels of circulating endothelial cells are low in idiopathic pulmonary fibrosis and are further reduced by anti-fibrotic treatments. BMC Med. 2015, 13, 277. [Google Scholar] [CrossRef] [Green Version]
- Fichtner-Feigl, S.; Strober, W.; Kawakami, K.; Puri, R.K.; Kitani, A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 2006, 12, 99–106. [Google Scholar] [CrossRef]
- Wilson, M.S.; Madala, S.K.; Ramalingam, T.R.; Gochuico, B.R.; Rosas, I.O.; Cheever, A.W.; Wynn, T.A. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J. Exp. Med. 2010, 207, 535–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz, L.A.; Gambelli, F.; McBride, C.; Gaupp, D.; Baddoo, M.; Kaminski, N.; Phinney, D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl. Acad. Sci. USA 2003, 100, 8407–8411. [Google Scholar] [CrossRef] [Green Version]
- Skurikhin, E.G.; Pershina, O.V.; Pakhomova, A.V.; Pan, E.S.; Krupin, V.A.; Ermakova, N.N.; Vaizova, O.E.; Pozdeeva, A.S.; Zhukova, M.A.; Skurikhina, V.E.; et al. Endothelial Progenitor Cells as Pathogenetic and Diagnostic Factors, and Potential Targets for GLP-1 in Combination with Metabolic Syndrome and Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2019, 20, 1105. [Google Scholar] [CrossRef] [Green Version]
- Parameswaran, H.; Majumdar, A.; Ito, S.; Alencar, A.M.; Suki, B. Quantitative characterization of airspace enlargement in emphysema. J. Appl. Physiol. 2006, 100, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Barrutia, A.; Ceresa, M.; Artaechevarria, X.; Montuenga, L.M.; Ortiz-de-Solorzano, C. Quantification of lung damage in an elastase-induced mouse model of emphysema. Int. J. Biomed. Imaging 2012, 2012, 734734. [Google Scholar] [CrossRef]
- Sato, S.; Bartolák-Suki, E.; Parameswaran, H.; Hamakawa, H.; Suki, B. Scale dependence of structure-function relationship in the emphysematous mouse lung. Front. Physiol. 2015, 6, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thurlbeck, W.M. Measurement of pulmonary emphysema. Am. Rev. Respir. Dis. 1967, 95, 752–764. [Google Scholar] [CrossRef]
- Bracke, K.R.; D’hulst, A.I.; Maes, T.; Moerloose, K.B.; Demedts, I.K.; Lebecque, S.; Joos, G.F.; Brusselle, G.G. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J. Immunol. 2006, 177, 4350–4359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, J.; Tian, J.; Zhou, L.; Le, Y.; Sun, Y. Interleukin-17A Deficiency Attenuated Emphysema and Bone Loss in Mice Exposed to Cigarette Smoke. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Saetta, M.; Shiner, R.J.; Angus, G.E.; Kim, W.D.; Wang, N.S.; King, M.; Ghezzo, H.; Cosio, M.G. Destructive index: A measurement of lung parenchymal destruction in smokers. Am. Rev. Respir. Dis. 1985, 131, 764–769. [Google Scholar] [CrossRef]
- Tilton, R.G.; Miller, E.J.; Kilo, C.; Williamson, J.R. Pericyte form and distribution in rat retinal and uveal capillaries. Invest. Ophthalmol. Vis. Sci. 1985, 26, 68–73. [Google Scholar]
- Prentø, P. Van Gieson’s picrofuchsin. The staining mechanisms for collagen and cytoplasm, and an examination of the dye diffusion rate model of differential staining. Histochemistry 1993, 99, 163–174. [Google Scholar] [CrossRef]
- Singh, M.; Chaudhary, A.K.; Pandya, S.; Debnath, S.; Singh, M.; Singh, P.A.; Mehrotra, R. Morphometric analysis in potentially malignant head and neck lesions: Oral submucous fibrosis. Asian Pac. J. Cancer Prev. 2010, 11, 257–260. [Google Scholar]
- Skurikhin, E.G.; Pershina, O.V.; Reztsova, A.M.; Ermakova, N.N.; Khmelevskaya, E.S.; Krupin, V.A.; Stepanova, I.E.; Artamonov, A.V.; Bekarev, A.A.; Madonov, P.G.; et al. Modulation of bleomycin-induced lung fibrosis by pegylated hyaluronidase and dopamine receptor antagonist in mice. PLoS One 2015, 10, e0125065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skurikhin, E.G.; Krupin, V.A.; Pershina, O.V.; Pan, E.S.; Ermolaeva, L.A.; Pakhomova, A.V.; Rybalkina, O.Y.; Ermakova, N.N.; Khmelevskaya, E.S.; Vaizova, O.E.; et al. Endothelial Progenitor Cells and Notch-1 Signaling as Markers of Alveolar Endothelium Regeneration in Pulmonary Emphysema. Bull. Exp. Biol. Med. 2018, 166, 201–206. [Google Scholar] [CrossRef]
- Fehrenbach, M.L.; Cao, G.; Williams, J.T.; Finklestein, J.M.; Delisser, H.M. Isolation of murine lung endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 296, L1096–L1103. [Google Scholar] [CrossRef]
- Lim, Y.C.; Garcia-Cardena, G.; Allport, J.R.; Zervoglos, M.; Connolly, A.J.; Gimbrone, M.A., Jr.; Luscinskas, F.W. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am. J. Pathol. 2003, 162, 1591–1601. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.C.; Luscinskas, F.W. Isolation and culture of murine heart and lung endothelial cells for in vitro model systems. Methods Mol. Biol. 2006, 341, 141–154. [Google Scholar] [CrossRef]
- McQualter, J.L.; Yuen, K.; Williams, B.; Bertoncello, I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc. Natl. Acad. Sci. USA 2010, 107, 1414–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skurikhin, E.; Nebolsin, V.; Widera, D.; Ermakova, N.; Pershina, O.; Pakhomova, A.; Krupin, V.; Pan, E.; Zhukova, M.; Novikov, F.; et al. Antifibrotic and Regenerative Effects of Treamid in Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 8380. https://doi.org/10.3390/ijms21218380
Skurikhin E, Nebolsin V, Widera D, Ermakova N, Pershina O, Pakhomova A, Krupin V, Pan E, Zhukova M, Novikov F, et al. Antifibrotic and Regenerative Effects of Treamid in Pulmonary Fibrosis. International Journal of Molecular Sciences. 2020; 21(21):8380. https://doi.org/10.3390/ijms21218380
Chicago/Turabian StyleSkurikhin, Evgenii, Vladimir Nebolsin, Darius Widera, Natalia Ermakova, Olga Pershina, Angelina Pakhomova, Vyacheslav Krupin, Edgar Pan, Mariia Zhukova, Fedor Novikov, and et al. 2020. "Antifibrotic and Regenerative Effects of Treamid in Pulmonary Fibrosis" International Journal of Molecular Sciences 21, no. 21: 8380. https://doi.org/10.3390/ijms21218380
APA StyleSkurikhin, E., Nebolsin, V., Widera, D., Ermakova, N., Pershina, O., Pakhomova, A., Krupin, V., Pan, E., Zhukova, M., Novikov, F., Sandrikina, L., Morozov, S., Kubatiev, A., & Dygai, A. (2020). Antifibrotic and Regenerative Effects of Treamid in Pulmonary Fibrosis. International Journal of Molecular Sciences, 21(21), 8380. https://doi.org/10.3390/ijms21218380






