Characterization of PC12 Cell Subclones with Different Sensitivities to Programmed Thermal Stimulation
Abstract
:1. Introduction
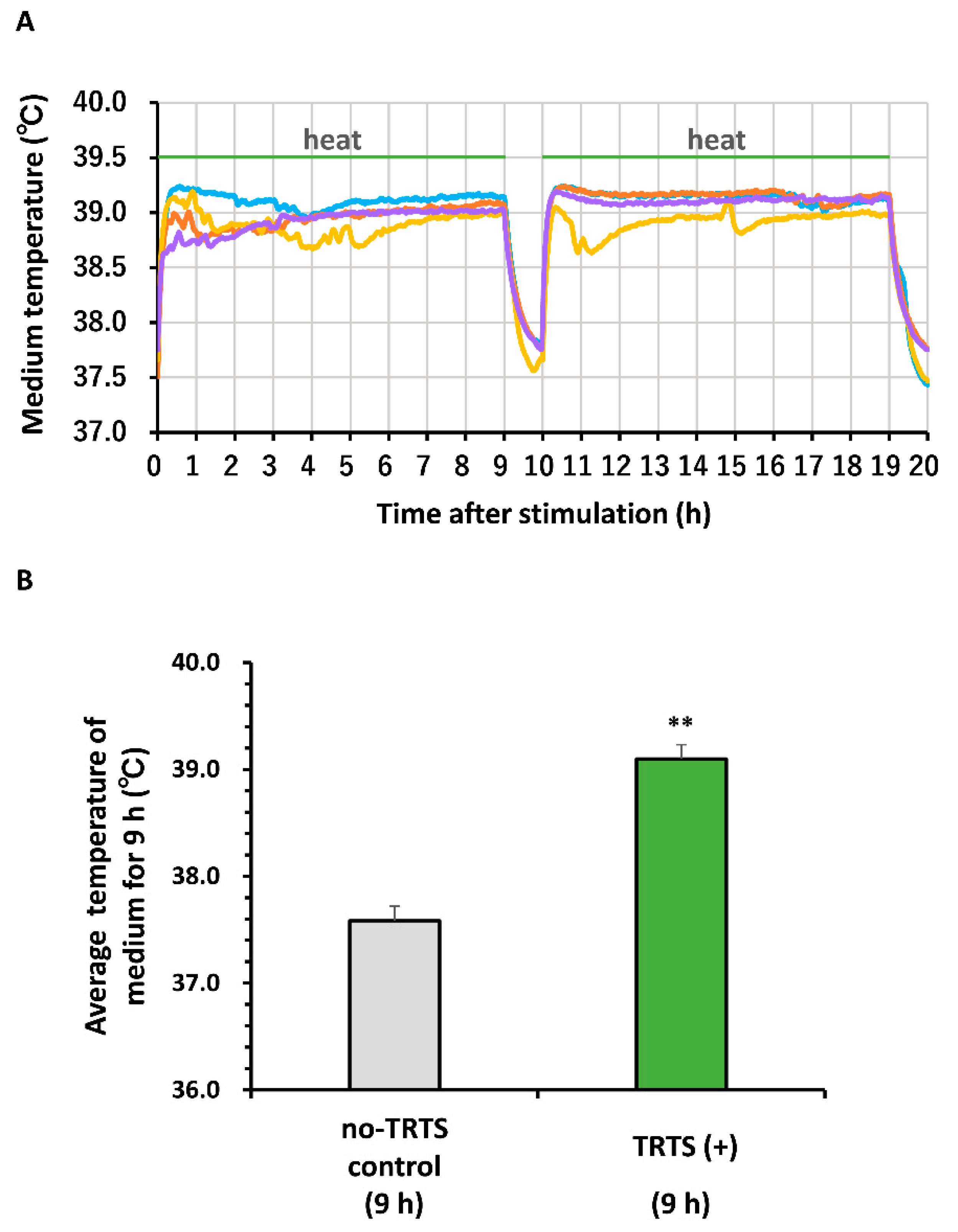
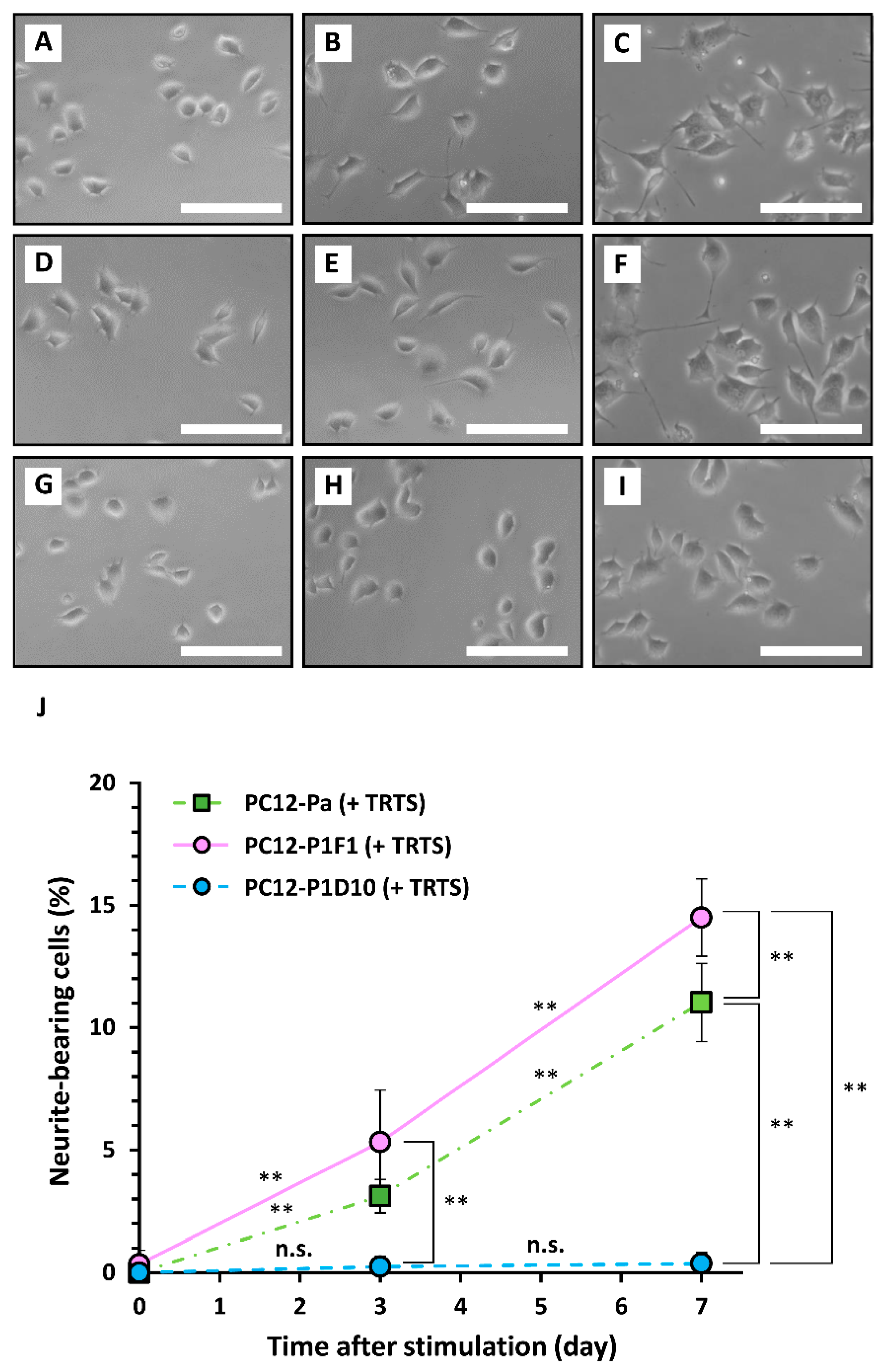
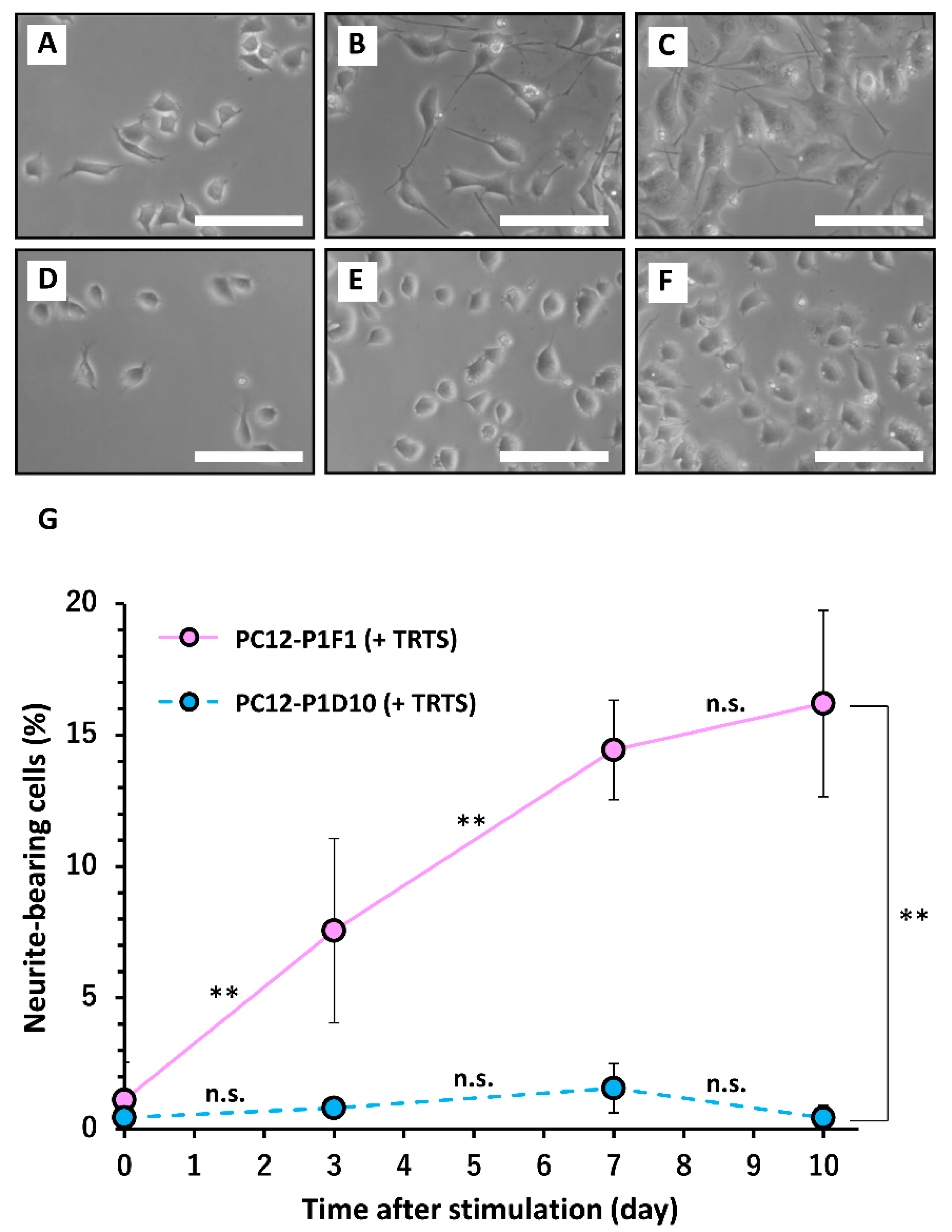
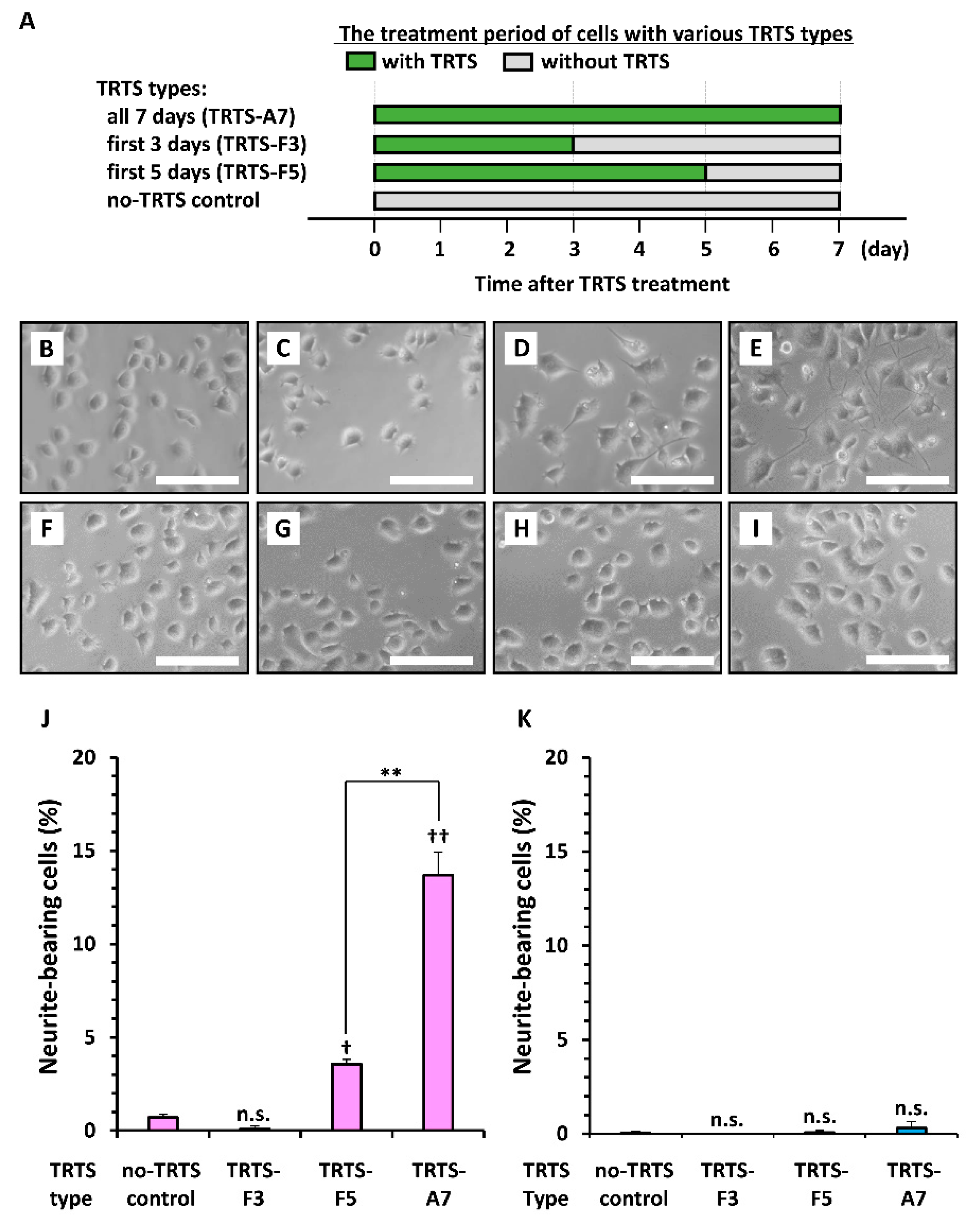
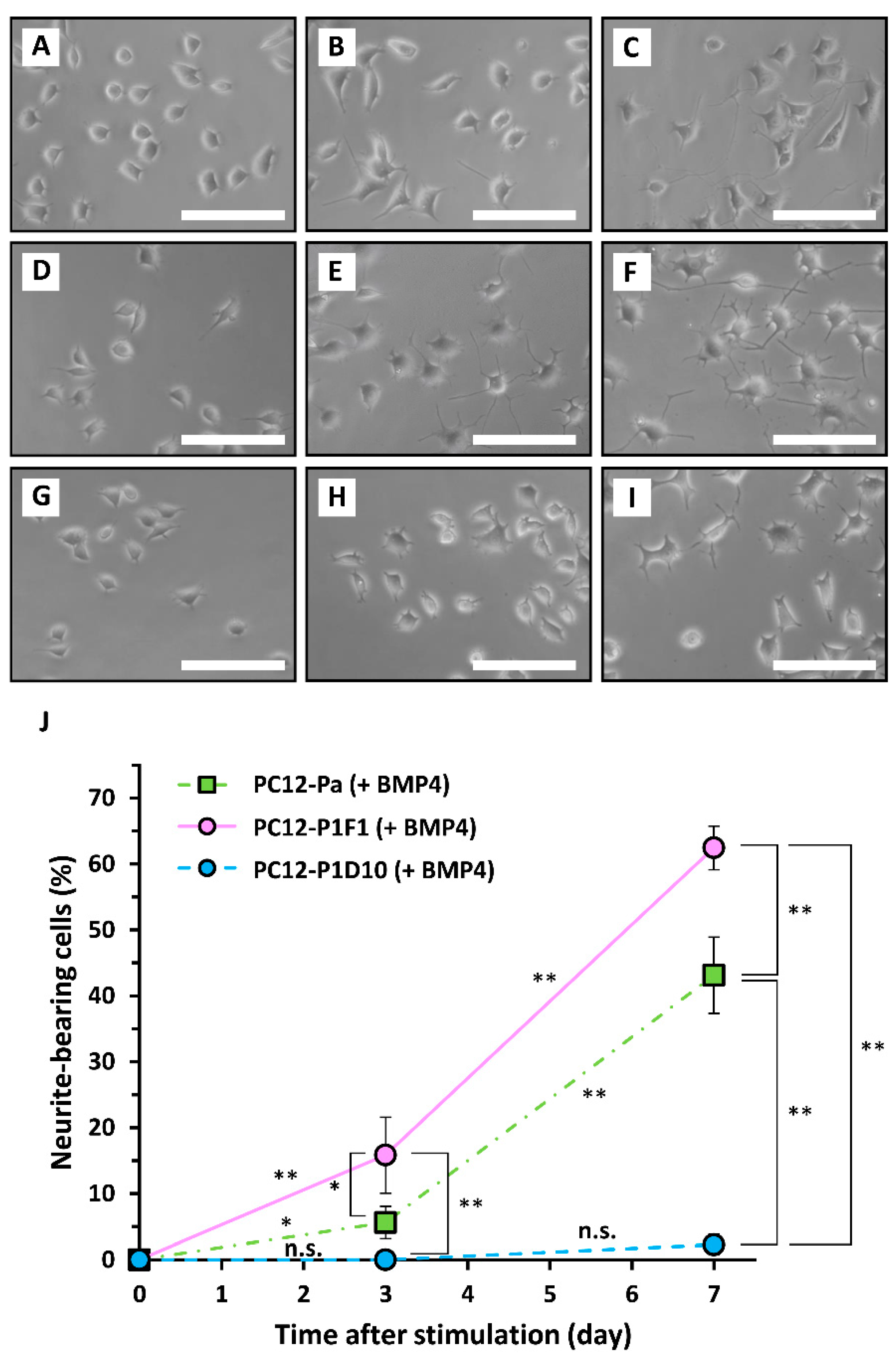
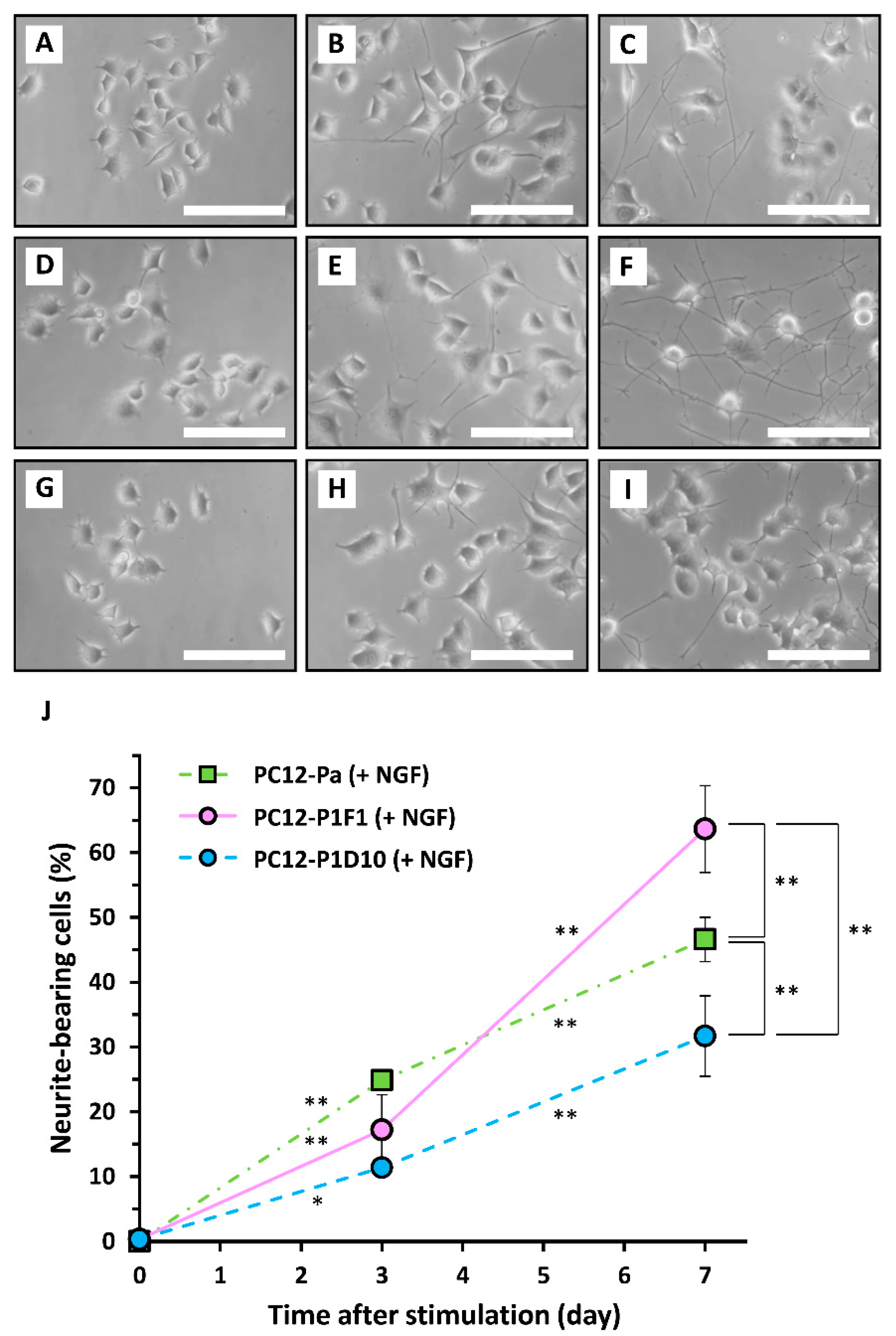
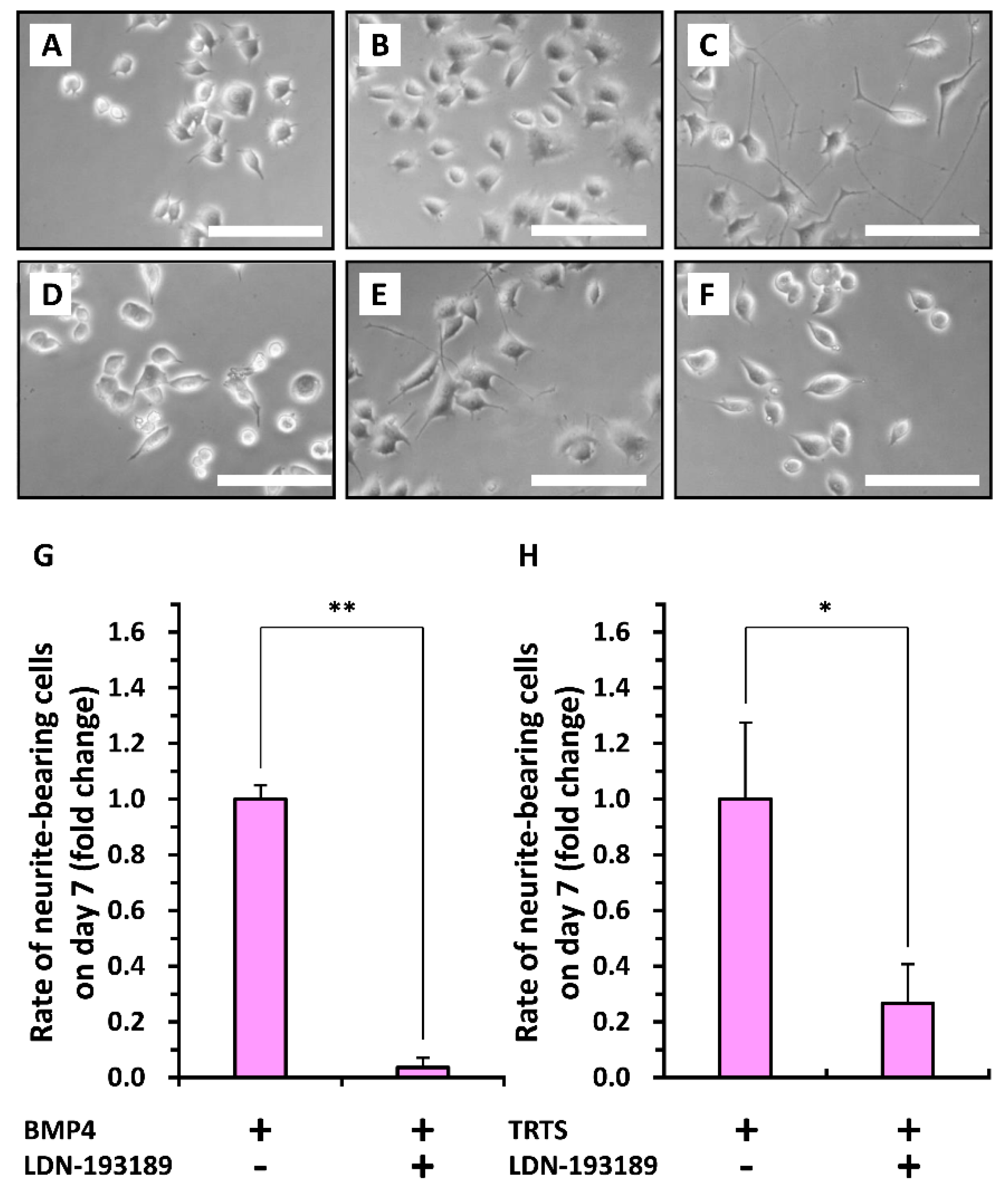
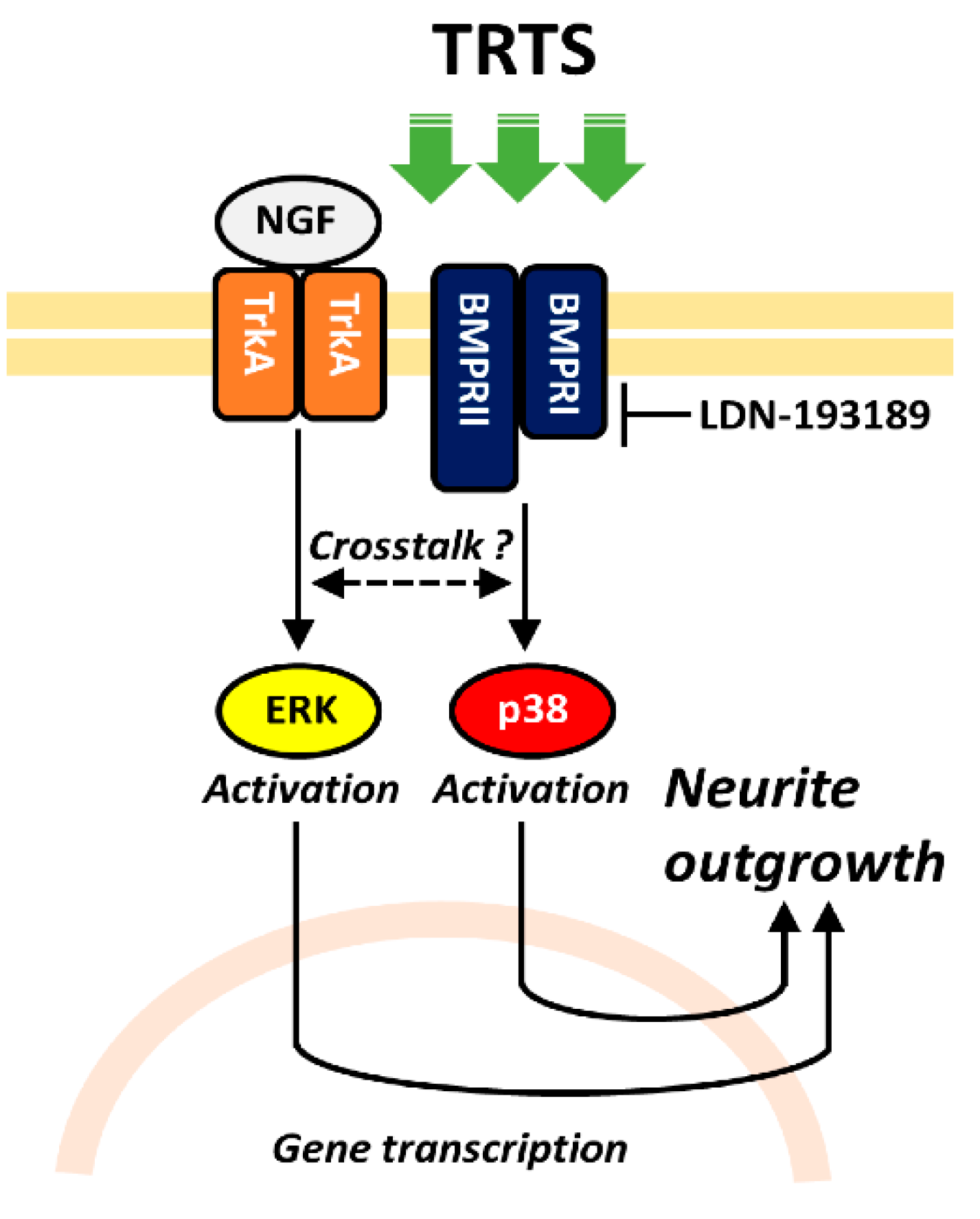
2. Results and Discussion
3. Materials and Methods
3.1. Cells and Reagents
3.2. Cell Culture and the Induction of Neurite Outgrowth
3.3. Generation and Selection of PC12 Subclones
3.4. Thermal Evaluation of the Medium
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muramatsu, R.; Ueno, M.; Yamashita, T. Intrinsic regenerative mechanisms of central nervous system neurons. Biosci. Trends 2009, 3, 179–183. [Google Scholar]
- Teng, K.K.; Georgieff, I.S.; Aletta, J.M.; Nunez, J.; Shelanski, M.L.; Greene, L.A. Characterization of a PC12 cell sub-clone (PC12-C41) with enhanced neurite outgrowth capacity: Implications for a modulatory role of high molecular weight tau in neuritogenesis. J. Cell Sci. 1993, 106, 611–626. [Google Scholar] [PubMed]
- Aguayo, A.J.; David, S.; Bray, G.M. Influences of the glial environment on the elongation of axons after injury: Transplantation studies in adult rodents. J. Exp. Biol. 1981, 95, 231–240. [Google Scholar] [PubMed]
- Hoop, M.; Chen, X.-Z.; Ferrari, A.; Mushtaq, F.; Ghazaryan, G.; Tervoort, T.; Poulikakos, D.; Nelson, B.; Pané, S. Ultrasound-mediated piezoelectric differentiation of neuron-like PC12 cells on PVDF membranes. Sci. Rep. 2017, 7, 4028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, S.; Lee, J.S.; Ha, L.; Lim, J.Y. Inducing Neurite Outgrowth by Mechanical Cell Stretch. BioRes. Open Access 2013, 2, 212–216. [Google Scholar] [CrossRef]
- Kudo, T.; Kanetaka, H.; Mochizuki, K.; Tominami, K.; Nunome, S.; Abe, G.; Kosukegawa, H.; Abe, T.; Mori, H.; Mori, K.; et al. Induction of Neurite Outgrowth in PC12 Cells Treated with Temperature-Controlled Repeated Thermal Stimulation. PLoS ONE 2015, 10, e0124024. [Google Scholar] [CrossRef] [Green Version]
- Gill, J.S.; Schenone, A.E.; Podratz, J.L.; Windebank, A.J. Autocrine regulation of neurite outgrowth from PC12 cells by nerve growth factor. Mol. Brain Res. 1998, 57, 123–131. [Google Scholar] [CrossRef]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rømer, M.; Lademann, U.A. Regulation of programmed cell death by plasminogen activator inhibitor type 1 (PAI-1). Thromb. Haemost. 2008, 100, 1041–1046. [Google Scholar] [CrossRef] [Green Version]
- Radio, N.M.; Mundy, W.R. Developmental neurotoxicity testing in vitro: Models for assessing chemical effects on neurite outgrowth. Neurotoxicology 2008, 29, 361–376. [Google Scholar] [CrossRef] [Green Version]
- Vaudry, D.; Stork, P.J.S.; Lazarovici, P.; Eiden, L.E. Signaling Pathways for PC12 Cell Differentiation: Making the Right Connections. Science 2002, 296, 1648–1649. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, B.; Que, H.; Lin, Q.; Lv, S.; Liu, S. Neurons derived from PC12 cells have the potential to develop synapses with primary neurons from rat cortex. Acta Neurobiol. Exp. 2006, 66, 105–112. [Google Scholar]
- Leinhauser, I.; Richter, A.; Lee, M.; Höfig, I.; Anastasov, N.; Fend, F.; Ercolino, T.; Mannelli, M.; Gimenez-Roqueplo, A.-P.; Robledo, M.; et al. Oncogenic features of the bone morphogenic protein 7 (BMP7) in pheochromocytoma. Oncotarget 2015, 6, 39111–39126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, L.A.; Rukenstein, A. Regulation of acetylcholinesterase activity by nerve growth factor. Role of transcription and dissociation from effects on proliferation and neurite outgrowth. J. Biol. Chem. 1981, 256, 6363–6367. [Google Scholar] [PubMed]
- Hayashi, H.; Ishisaki, A.; Suzuki, M.; Imamura, T. BMP-2 augments FGF-induced differentiation of PC12 cells through upregulation of FGF receptor-1 expression. J. Cell Sci. 2001, 114, 1387–1395. [Google Scholar]
- Iwasaki, S.; Hattori, A.; Sato, M.; Tsujimoto, M.; Kohno, M. Characterization of the bone morphogenetic protein-2 as a neurotrophic factor. Induction of neuronal differentiation of PC12 cells in the absence of mitogen-activated protein kinase activation. J. Biol. Chem. 1996, 271, 17360–17365. [Google Scholar]
- Kudo, T.; Kanetaka, H.; Mizuno, K.; Ryu, Y.; Miyamoto, Y.; Nunome, S.; Zhang, Y.; Kano, M.; Shimizu, Y.; Hayashi, H. Dorsomorphin stimulates neurite outgrowth in PC12 cells via activation of a protein kinase A-dependent MEK-ERK1/2 signaling pathway. Genes Cells 2011, 16, 1121–1132. [Google Scholar] [CrossRef]
- Althini, S.; Usoskin, D.; Kylberg, A.; Dijke, P.T.; Ebendal, T. Bone morphogenetic protein signalling in NGF-stimulated PC12 cells. Biochem. Biophys. Res. Commun. 2003, 307, 632–639. [Google Scholar] [CrossRef]
- Iwasaki, S.; Iguchi, M.; Watanabe, K.; Hoshino, R.; Tsujimoto, M.; Kohno, M. Specific Activation of the p38 Mitogen-activated Protein Kinase Signaling Pathway and Induction of Neurite Outgrowth in PC12 Cells by Bone Morphogenetic Protein-2. J. Biol. Chem. 1999, 274, 26503–26510. [Google Scholar] [CrossRef] [Green Version]
- Kao, S.-C.; Jaiswal, R.K.; Kolch, W.; Landreth, G.E. Identification of the Mechanisms Regulating the Differential Activation of the MAPK Cascade by Epidermal Growth Factor and Nerve Growth Factor in PC12 Cells. J. Biol. Chem. 2001, 276, 18169–18177. [Google Scholar] [CrossRef] [Green Version]
- Rakhit, S.; Pyne, S.; Pyne, N.J. Nerve growth factor stimulation of p42/p44 mitogen-activated protein kinase in PC12 cells: Role of G(i/o), G protein-coupled receptor kinase 2, beta-arrestin I, and endocytic processing. Mol. Pharmacol. 2001, 60, 63–70. [Google Scholar] [CrossRef]
- Zhang, S.; Uludağ, H. Nanoparticulate Systems for Growth Factor Delivery. Pharm. Res. 2009, 26, 1561–1580. [Google Scholar] [CrossRef]
- Chen, P.J.-C.; Su, C.-M.; Chen, G.-S.; Lai, C.-C.; Chen, C.-Y.; Lin, K.; Lin, F.-H.; Dong, G.-C. Enhancement of Neurite Outgrowth by Warming Biomaterial Ultrasound Treatment. Int. J. Mol. Sci. 2020, 21, 2236. [Google Scholar] [CrossRef] [Green Version]
- Kudo, T.; Kanetaka, H.; Shimizu, Y.; Abe, T.; Mori, H.; Mori, K.; Suzuki, E.; Takagi, T.; Izumi, S. Induction of neuritogenesis in PC12 cells by a pulsed electromagnetic field via MEK-ERK1/2 signaling. Cell Struct. Funct. 2013, 38, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Einarson, M.B.; Chao, M.V. Regulation of Id1 and its association with basic helix-loop-helix proteins during nerve growth factor-induced differentiation of PC12 cells. Mol. Cell. Biol. 1995, 15, 4175–4183. [Google Scholar] [CrossRef] [Green Version]
- Lonn, P.; Zaia, K.; Israelsson, C.; Althini, S.; Usoskin, D.; Kylberg, A.; Ebendal, T. BMP Enhances Transcriptional Responses to NGF During PC12 Cell Differentiation. Neurochem. Res. 2005, 30, 753–765. [Google Scholar] [CrossRef]
- Cuny, G.D.; Yu, P.B.; Laha, J.K.; Xing, X.; Liu, J.-F.; Lai, C.S.; Deng, D.Y.; Sachidanandan, C.; Bloch, K.D.; Peterson, R.T. Structure–activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 4388–4392. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, A.; Okada, M.; Tamada, A.; Okuda, S.; Nozumi, M.; Ito, Y.; Kobayashi, D.; Yamasaki, T.; Yokoyama, R.; Shibata, T.; et al. Growth Cone Phosphoproteomics Reveals that GAP-43 Phosphorylated by JNK Is a Marker of Axon Growth and Regeneration. iScience 2018, 4, 190–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morooka, T.; Nishida, E. Requirement of p38 mitogen-activated protein kinase for neuronal differentiation in PC12 cells. J. Biol. Chem. 1998, 273, 24285–24288. [Google Scholar] [CrossRef] [Green Version]
- Fantetti, K.N.; Fekete, D.M. Members of the BMP, Shh, and FGF morphogen families promote chicken statoacoustic ganglion neurite outgrowth and neuron survival in vitro. Dev. Neurobiol. 2012, 72, 1213–1228. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudo, T.-a.; Tominami, K.; Izumi, S.; Hayashi, Y.; Noguchi, T.; Matsuzawa, A.; Hong, G.; Nakai, J. Characterization of PC12 Cell Subclones with Different Sensitivities to Programmed Thermal Stimulation. Int. J. Mol. Sci. 2020, 21, 8356. https://doi.org/10.3390/ijms21218356
Kudo T-a, Tominami K, Izumi S, Hayashi Y, Noguchi T, Matsuzawa A, Hong G, Nakai J. Characterization of PC12 Cell Subclones with Different Sensitivities to Programmed Thermal Stimulation. International Journal of Molecular Sciences. 2020; 21(21):8356. https://doi.org/10.3390/ijms21218356
Chicago/Turabian StyleKudo, Tada-aki, Kanako Tominami, Satoshi Izumi, Yohei Hayashi, Takuya Noguchi, Atsushi Matsuzawa, Guang Hong, and Junichi Nakai. 2020. "Characterization of PC12 Cell Subclones with Different Sensitivities to Programmed Thermal Stimulation" International Journal of Molecular Sciences 21, no. 21: 8356. https://doi.org/10.3390/ijms21218356








