Alternatives to Insulin for the Regulation of Blood Sugar Levels in Type 2 Diabetes
Abstract
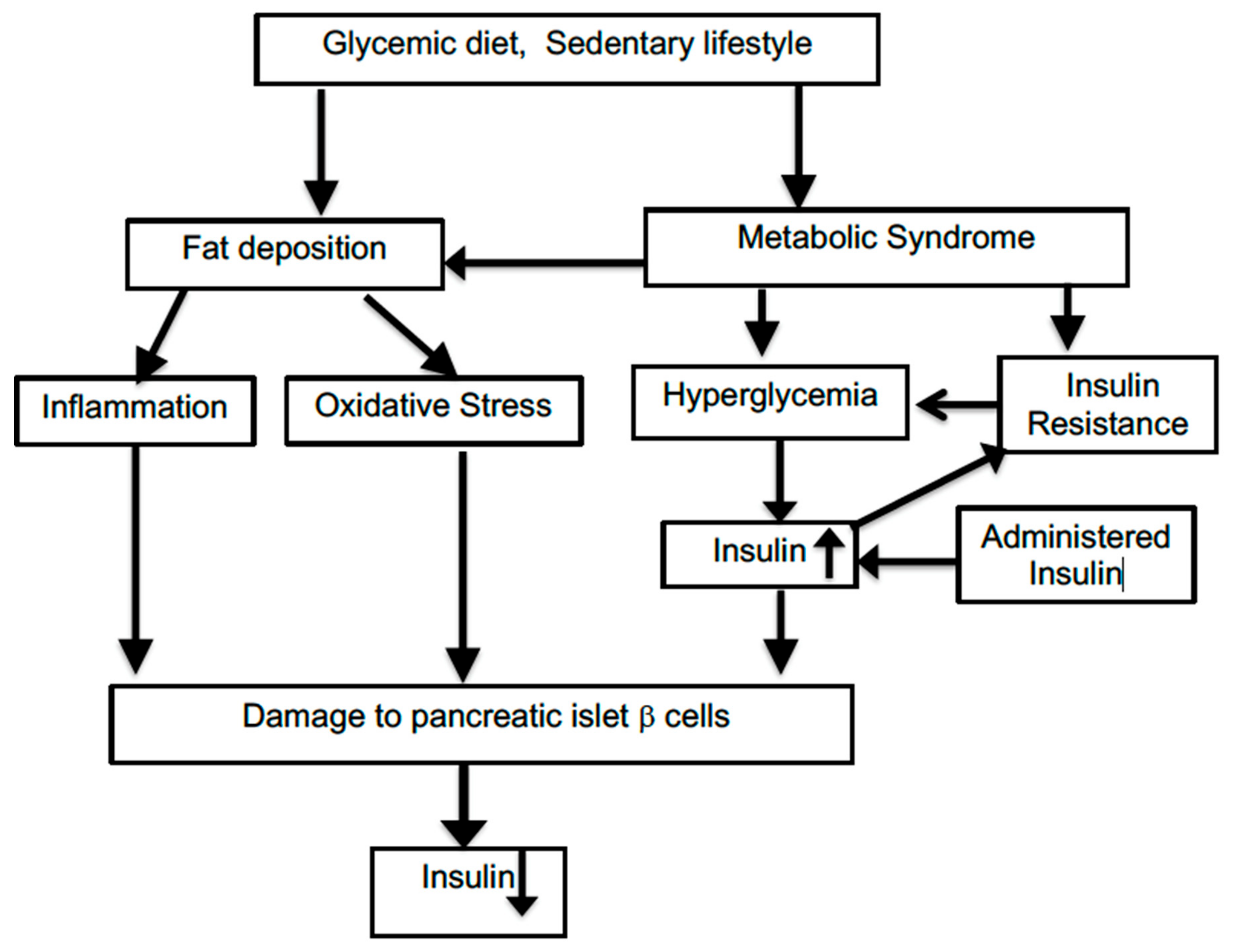
:1. Introduction
1.1. History of Diabetes and of Insulin as a Medication
1.2. Intent of This Review
2. Subtypes of Diabetes Mellitus
2.1. Type 1 Diabetes
2.2. Gestational Diabetes
2.3. Type 2 Diabetes (T2D)
2.4. Type 3 Diabetes
3. Growing Incidence of Type 2 Diabetes Mellitus
4. Association of T2D with Other Conditions
4.1. Diet and Weight
4.2. Indices of Wellbeing
4.3. Environmental Factors
4.4. Dementia
5. Role of Oxidative Stress and Inflammation in T2D
5.1. Oxidative Stress
5.2. Inflammation
6. Benefits of Insulin Therapy
7. Shortcomings of Use of Exogenous Insulin
7.1. Insulin Resistance
7.2. Mortality and the Extent of Insulin Use
8. T2D Treatment without Use of Exogenous Insulin
8.1. Behavioral and Dietary Changes
8.2. Pharmacological Treatment of Diabetes
8.2.1. MicroRNA (miRNA)
8.2.2. Sirtuin Activation
8.2.3. Glucagon-Like Peptide-1 Receptor Agonists
8.2.4. Inhibitors of the Sodium-Glucose Co-Tranpsorter-2 (SGLT2)
8.3. Broadly Acting Materials
8.4. Treatment of T2D with Antioxidant and Anti-Inflammatory Agents
9. Conclusions
Funding
Conflicts of Interest
References
- Karamanou, M.; Protogerou, A.; Tsoucalas, G.; Androutsos, G.; Poulakou-Rebelakou, E. Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stefan, M.; Zhang, W.; Concepcion, E.; Yi, Z.; Tomer, Y. DNA methylation profiles in type 1 diabetes twins point to strong epigenetic effects on etiology. J. Autoimmun. 2014, 50, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chafe, R.; Aslanov, R.; Sarkar, A.; Gregory, P.; Comeau, A.; Newhook, L.A. Association of type 1 diabetes and concentrations of drinking water components in Newfoundland and Labrador, Canada. BMJ Open Diabetes Res. Care 2018, 6, e000466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unsworth, R.; Wallace, S.; Oliver, N.S.; Yeung, S.; Kshirsagar, A.; Naidu, H.; Kwong, R.M.W.; Kumar, P.; Logan, K.M. New-Onset Type 1 Diabetes in Children During COVID-19: Multicenter Regional Findings in the U.K. Diabetes Care 2020, dc201551. [Google Scholar] [CrossRef]
- Yang, J.-K.; Lin, S.-S.; Ji, X.-J.; Guo, L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010, 47, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, M.; Kroger, C.J.; Tisch, R. Type 1 Diabetes: A Chronic Anti-Self-Inflammatory Response. Front. Immunol. 2017, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Sun, L.; Fu, P.; Xie, J.; Lu, J.; Zhou, J.; Yu, D.; Whelton, P.K.; He, J.; Gu, D. Prevalence and risk factors for type 2 diabetes mellitus in the Chinese adult population: The InterASIA Study. Diabetes Res. Clin. Pract. 2009, 84, 288–295. [Google Scholar] [CrossRef]
- Lauritzen, H.P. In vivo imaging of GLUT4 translocationThis paper is one of a selection of papers published in this Special Issue, entitled 14th International Biochemistry of Exercise Conference—Muscles as Molecular and Metabolic Machines, and has undergone the Journal’s usual peer review process. Appl. Physiol. Nutr. Metab. 2009, 34, 420–423. [Google Scholar] [CrossRef]
- De La Monte, S.M. The Full Spectrum of Alzheimer’s Disease Is Rooted in Metabolic Derangements That Drive Type 3 Diabetes. Polyglutamine Disord. 2019, 1128, 45–83. [Google Scholar] [CrossRef]
- Maruthur, N.M. The Growing Prevalence of Type 2 Diabetes: Increased Incidence or Improved Survival? Curr. Diabetes Rep. 2013, 13, 786–794. [Google Scholar] [CrossRef]
- Matsuda, A.; Kuzuya, T. Relationship between obesity and concordance rate for Type 2 (non-insulin-dependent) diabetes mellitus among twins. Diabetes Res. Clin. Pract. 1994, 26, 137–143. [Google Scholar] [CrossRef]
- Willemsen, G.; Ward, K.J.; Bell, C.G.; Christensen, K.; Bowden, J.; Dalgård, C.; Harris, J.R.; Kaprio, J.; Lyle, R.; Magnusson, P.K.; et al. The Concordance and Heritability of Type 2 Diabetes in 34,166 Twin Pairs From International Twin Registers: The Discordant Twin (DISCOTWIN) Consortium. Twin Res. Hum. Genet. 2015, 18, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Czech, M.P. Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 2017, 23, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.J.; Golden, S.H. Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2016, 1391, 20–34. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Li, T.; Yan, Y.; Xian, H.; Al-Aly, Z. The 2016 global and national burden of diabetes mellitus attributable to PM2·5 air pollution. Lancet Planet Health 2018, 2, e301–e312. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.C.; Thurston, G.D. Air Pollution, Oxidative Stress, and Diabetes: A Life Course Epidemiologic Perspective. Curr. Diabetes Rep. 2019, 19, 58. [Google Scholar] [CrossRef]
- Esposito, K.; Petrizzo, M.; Maiorino, M.I.; Bellastella, G.; Giugliano, D. Particulate matter pollutants and risk of type 2 diabetes: A time for concern? Endocrinology 2015, 51, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.-C.; Kile, M.L.; Seow, W.J.; Lin, X.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Mostofa, G.; Lü, Q.; Christiani, D.C. Genetic Susceptible Locus in NOTCH2 Interacts with Arsenic in Drinking Water on Risk of Type 2 Diabetes. PLoS ONE 2013, 8, e70792. [Google Scholar] [CrossRef] [Green Version]
- Fabricio, G.; Malta, A.; Chango, A.; Mathias, P.C.D.F. Environmental Contaminants and Pancreatic Beta-Cells. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 257–263. [Google Scholar] [CrossRef]
- Ninomiya, T. Diabetes Mellitus and Dementia. Curr. Diabetes Rep. 2014, 14, 487. [Google Scholar] [CrossRef]
- Bharadwaj, P.; Wijesekara, N.; Liyanapathirana, M.; Newsholme, P.; Ittner, L.; Fraser, P.; Verdile, G. The Link between Type 2 Diabetes and Neurodegeneration: Roles for Amyloid-β, Amylin, and Tau Proteins. J. Alzheimer’s Dis. 2017, 59, 421–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [Green Version]
- Maiese, K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2015, 2015, 875961. [Google Scholar] [CrossRef] [Green Version]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Kaneto, H.; Matsuoka, T.-A.; Katakami, N.; Kawamori, D.; Miyatsuka, T.; Yoshiuchi, K.; Yasuda, T.; Sakamoto, K.; Yamasaki, Y.; Matsuhisa, M. Oxidative stress and the JNK pathway are involved in the development of type 1 and type 2 diabetes. Curr. Mol. Med. 2007, 7, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P.; Harmon, J.S. Diabetes, glucose toxicity, and oxidative stress: A case of double jeopardy for the pancreatic islet beta cell. Free Radic. Biol. Med. 2005, 41, 177–184. [Google Scholar] [CrossRef]
- Uruno, A.; Furusawa, Y.; Yagishita, Y.; Fukutomi, T.; Muramatsu, H.; Negishi, T.; Sugawara, A.; Kensler, T.W.; Yamamoto, M. The Keap1-Nrf2 System Prevents Onset of Diabetes Mellitus. Mol. Cell. Biol. 2013, 33, 2996–3010. [Google Scholar] [CrossRef] [Green Version]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2020, 70, 809–824. [Google Scholar]
- Cecilia, O.-M.; Alberto, C.-G.J.; José, N.-P.; Germán, C.-M.E.; Karen, L.-C.A.; Miguel, R.-P.L.; Raúl, R.-R.R.; Daniel, R.-C.A. Oxidative Stress as the Main Target in Diabetic Retinopathy Pathophysiology. J. Diabetes Res. 2019, 2019, 8562408. [Google Scholar] [CrossRef] [Green Version]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eguchi, K.; Nagai, R. Islet inflammation in type 2 diabetes and physiology. J. Clin. Investig. 2017, 127, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Diabetes and ageing-induced vascular inflammation. J. Physiol. 2015, 594, 2125–2146. [Google Scholar] [CrossRef] [Green Version]
- Berghe, G.V.D. How does blood glucose control with insulin save lives in intensive care? J. Clin. Investig. 2004, 114, 1187–1195. [Google Scholar] [CrossRef] [Green Version]
- Jacobi, J.; Bircher, N.; Krinsley, J.; Agus, M.; Braithwaite, S.S.; Deutschman, C.; Freire, A.X.; Geehan, D.; Kohl, B.; Nasraway, S.A.; et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit. Care Med. 2012, 40, 3251–3276. [Google Scholar] [CrossRef] [PubMed]
- Cahn, A.; Miccoli, R.; Dardano, A.; Del Prato, S. New forms of insulin and insulin therapies for the treatment of type 2 diabetes. Lancet Diabetes Endocrinol. 2015, 3, 638–652. [Google Scholar] [CrossRef]
- Umpierrez, G.E.; Klonoff, D.C. Diabetes Technology Update: Use of Insulin Pumps and Continuous Glucose Monitoring in the Hospital. Diabetes Care 2018, 41, 1579–1589. [Google Scholar] [CrossRef] [Green Version]
- Delarue, J.; Magnan, C. Free fatty acids and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Bertolotto, M.B.; Vuilleumier, N.; Franciosi, U.; Puddu, A.; Minetti, S.; DelRio, A.; Quercioli, A.; Bergamini, E.; Ottonello, L.; et al. Acipimox reduces circulating levels of insulin and associated neutrophilic inflammation in metabolic syndrome. Am. J. Physiol. Metab. 2011, 300, E681–E690. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19–39. [Google Scholar]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I., Jr.; Newsholme, P. Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and β-Cell Dysfunction. Oxid. Med. Cell. Longev. 2015, 2015, 181643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, S.J.; Garvey, W. Insulin action and insulin resistance: Diseases involving defects in insulin receptors, signal transduction, and the glucose transport effector system. Am. J. Med. 1998, 105, 331–345. [Google Scholar] [CrossRef]
- Ertek, S.; Cetinkalp, S. Is there U-turn from insulin back to pills in diabetes? Curr. Vasc. Pharmacol. 2014, 12, 617–626. [Google Scholar] [CrossRef]
- Fluitt, M.B.; Rizvi, S.; Li, L.; Alunan, A.; Lee, H.; Tiwari, S.; Ecelbarger, C.M. Chronic Insulin Infusion Down-Regulates Circulating and Urinary Nitric Oxide (NO) Levels Despite Molecular Changes in the Kidney Predicting Greater Endothelial NO Synthase Activity in Mice. Int. J. Mol. Sci. 2018, 19, 2880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanik, M.H.; Xu, Y.; Škrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin Resistance and Hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31, S262–S268. [Google Scholar] [CrossRef] [Green Version]
- Gamble, J.-M.; Simpson, S.H.; Eurich, D.T.; Majumdar, S.R.; Johnson, J.A. Insulin use and increased risk of mortality in type 2 diabetes: A cohort study. Diabetes Obes. Metab. 2010, 12, 47–53. [Google Scholar] [CrossRef]
- Vijan, S.; Sussman, J.B.; Yudkin, J.S.; Hayward, R.A. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern. Med. 2014, 174, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Herman, M.E.; O’Keefe, J.H.; Mb, D.S.H.B.; Schwartz, S.S. Insulin Therapy Increases Cardiovascular Risk in Type 2 Diabetes. Prog. Cardiovasc. Dis. 2017, 60, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Mendez, C.E.; Walker, R.; Eiler, C.R.; Mishriky, B.M.; Egede, L. Insulin therapy in patients with type 2 diabetes and high insulin resistance is associated with increased risk of complications and mortality. Postgrad. Med. 2019, 131, 376–382. [Google Scholar] [CrossRef]
- Ansari, A.M.; Osmani, L.; Matsangos, A.E.; Li, Q.K. Current insight in the localized insulin-derived amyloidosis (LIDA): Clinico-pathological characteristics and differential diagnosis. Pathol. Res. Pract. 2017, 213, 1237–1241. [Google Scholar] [CrossRef]
- Furmli, S.; Elmasry, R.; Ramos, M.; Fung, J. Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin. BMJ Case Rep. 2018, 2018, bcr2017221854. [Google Scholar] [CrossRef] [Green Version]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2019, 69. [Google Scholar] [CrossRef]
- Chauveau, A.; Kaufmann, M. Experiénces pour la determination du coefficient de l’activité nutritive et respiratoire des muscles en repos et en travail. Compt. Rend. Acad. Sci. 1887, 104, 1126–1132. [Google Scholar]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [Green Version]
- Soto-Mota, A.; Norwitz, N.G.; Clarke, K. Why a d-β-hydroxybutyrate monoester? Biochem. Soc. Trans. 2020, 48, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Miao, D.; Liu, Z.; Liu, K.; Zhang, B.; Li, J.; Li, Y.; Qi, J. β-hydroxybutyrate antagonizes aortic endothelial injury by promoting generation of VEGF in diabetic rats. Tissue Cell 2020, 64, 101345. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Gupta, L.; Khandelwal, D.; Gupta, P.; Dutta, D.; Aggarwal, S. Ketogenic diet in endocrine disorders: Current perspectives. J. Postgrad. Med. 2017, 63, 242–251. [Google Scholar] [CrossRef]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010, 24, 4948–4959. [Google Scholar] [CrossRef] [Green Version]
- Vallianou, N.; Stratigou, T.; Tsagarakis, S. Microbiome and diabetes: Where are we now? Diabetes Res. Clin. Pract. 2018, 146, 111–118. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [Green Version]
- Chambers, E.S.; Byrne, C.S.; Frost, G. Carbohydrate and human health: Is it all about quality? Lancet 2019, 393, 384–386. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Dendi, V.S.R.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emadi, S.S.; Soufi, F.G.; Khamaneh, A.M.; Alipour, M.R. MicroRNA-146a expression and its intervention in NF-?B signaling pathway in diabetic rat aorta. Endocr. Regul. 2014, 48, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, Q.A.; Pinti, M.V.; Durr, A.J.; Waris, S.; Shepherd, D.L.; Hollander, J.M. Regulating microRNA expression: At the heart of diabetes mellitus and the mitochondrion. Am. J. Physiol. Circ. Physiol. 2018, 314, H293–H310. [Google Scholar] [CrossRef]
- Grueter, C.E.; Van Rooij, E.; Johnson, B.A.; DeLeon, S.M.; Sutherland, L.B.; Qi, X.; Gautron, L.; Elmquist, J.K.; Bassel-Duby, R.; Olson, E.N. A Cardiac MicroRNA Governs Systemic Energy Homeostasis by Regulation of MED13. Cell 2012, 149, 671–683. [Google Scholar] [CrossRef] [Green Version]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschöop, M.H. Sirtuin 1 and Sirtuin 3: Physiological Modulators of Metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [Green Version]
- Pacifici, F.; Di Cola, D.; Pastore, D.; Abete, P.; Guadagni, F.; Donadel, G.; Bellia, A.; Esposito, E.; Salimei, C.; Salimei, P.S.; et al. Proposed Tandem Effect of Physical Activity and Sirtuin 1 and 3 Activation in Regulating Glucose Homeostasis. Int. J. Mol. Sci. 2019, 20, 4748. [Google Scholar] [CrossRef] [Green Version]
- Bonkowski, M.S.; Sinclair, M.S.B.D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef]
- Song, J.; Yang, B.; Jia, X.; Li, M.; Tan, W.; Ma, S.; Shi, X.; Feng, L. Distinctive Roles of Sirtuins on Diabetes, Protective or Detrimental? Front. Endocrinol. 2018, 9, 724. [Google Scholar] [CrossRef]
- Trujillo, J.M.; Nuffer, W. GLP-1 Receptor Agonists for Type 2 Diabetes Mellitus: Recent Developments and Emerging Agents. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2014, 34, 1174–1186. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Taub, P.R.; Ciaraldi, T.P.; Nogueira, L.; Coe, T.; Perkins, G.; Hogan, M.C.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. (−)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. Int. J. Cardiol. 2013, 168, 3982–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Xu, X.; Du, M.; Zhao, T.; Wang, J. A preclinical overview of metformin for the treatment of type 2 diabetes. Biomed. Pharmacother. 2018, 106, 1227–1235. [Google Scholar] [CrossRef]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, T.; Singh, A.K.; Haratipour, P.; Sah, A.N.; Pandey, A.K.; Naseri, R.; Juyal, V.; Farzaei, M.H. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J. Cell. Physiol. 2019, 234, 17212–17231. [Google Scholar] [CrossRef]
- Ceriello, A.; Testa, R. Antioxidant Anti-Inflammatory Treatment in Type 2 Diabetes. Diabetes Care 2009, 32, S232–S236. [Google Scholar] [CrossRef] [Green Version]
- Abdali, D.; Samson, S.E.; Grover, A.K. How Effective Are Antioxidant Supplements in Obesity and Diabetes? Med. Princ. Pract. 2015, 24, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Ramos, R.; Laura, G.-L.A.; Elina, M.-C.B.; Donají, B.-A.A. Vitamins and Type 2 Diabetes Mellitus. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Punithavathi, V.R.; Anuthama, R.; Prince, P.S.M. Combined treatment with naringin and vitamin C ameliorates streptozotocin-induced diabetes in male Wistar rats. J. Appl. Toxicol. 2008, 28, 806–813. [Google Scholar] [CrossRef]
- Ghelani, H.; Razmovski-Naumovski, V.; Nammi, S. Chronic treatment of (R)-α-lipoic acid reduces blood glucose and lipid levels in high-fat diet and low-dose streptozotocin-induced metabolic syndrome and type 2 diabetes in Sprague-Dawley rats. Pharmacol. Res. Perspect. 2017, 5, e00306. [Google Scholar] [CrossRef]
- Karkabounas, S.; Papadopoulos, N.; Anastasiadou, C.; Gubili, C.; Peschos, D.; Daskalou, T.; Fikioris, N.; Simos, Y.V.; Kontargiris, E.; Gianakopoulos, X.; et al. Effects of α-Lipoic Acid, Carnosine, and Thiamine Supplementation in Obese Patients with Type 2 Diabetes Mellitus: A Randomized, Double-Blind Study. J. Med. Food 2018, 21, 1197–1203. [Google Scholar] [CrossRef]
- Blum, S.; Vardi, M.; Brown, J.B.; Russell, A.; Milman, U.; Shapira, C.; Levy, N.S.; Miller-Lotan, R.; Asleh, R.; Levy, A.P. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2-2 genotype. Pharmacogenomics 2010, 11, 675–684. [Google Scholar] [CrossRef] [Green Version]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE Pathway and Oxidative Stress as a Therapeutic Target in Type II Diabetes Mellitus. J. Diabetes Res. 2017, 4826724. [Google Scholar] [CrossRef]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar]
- Prasad, K.N. Micronutrients in Prevention and Improvement of the Standard Therapy in Diabetes; Informa UK Limited: London, UK, 2019; pp. 95–129. [Google Scholar]
| Goal of Treatment | Mechanism Involved | Treatment |
|---|---|---|
| Prevent hyperglycemia | Lower levels of circulating glucose | Insulin |
| Inhibition of the renal sodium-glucose co-transporter-2 (SGLT2) | ||
| Glucagon-like peptide-1 receptor agonists to stimulate insulin release | ||
| Reduced rate of carbohydrate absorption | Diminished consumption of refined or simple carbohydrates | |
| Reduced hepatic gluconeogenesis, increased glucose uptake by muscle | Metformin | |
| Reduction of levels of inflammation and oxidative stress | Increased antioxidant and anti-inflammatory milieu | Activation of Nrf/KEAP/ARE pathway |
| Antioxidant vitamins (e.g., lipoate, α-tocopherol, and ascorbate) | ||
| Use of phytochemicals with a broad range of properties (e.g., curcumin, resveratrol, and catechins) | ||
| Ketogenic diet | ||
| SIRT activation | Rapamycin | |
| Moderation of fat deposition | Lower accumulation of fat | Physical activity |
| Blocking of miR-208a with an antisense oligonucleotide | ||
| Modulation of gut biome | Ketogenic diet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondy, S.C.; Wu, M.; Prasad, K.N. Alternatives to Insulin for the Regulation of Blood Sugar Levels in Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 8302. https://doi.org/10.3390/ijms21218302
Bondy SC, Wu M, Prasad KN. Alternatives to Insulin for the Regulation of Blood Sugar Levels in Type 2 Diabetes. International Journal of Molecular Sciences. 2020; 21(21):8302. https://doi.org/10.3390/ijms21218302
Chicago/Turabian StyleBondy, Stephen C., Meixia Wu, and Kedar N. Prasad. 2020. "Alternatives to Insulin for the Regulation of Blood Sugar Levels in Type 2 Diabetes" International Journal of Molecular Sciences 21, no. 21: 8302. https://doi.org/10.3390/ijms21218302





