A Novel Multiplex RT-PCR Assay for Simultaneous Detection of Dengue and Chikungunya Viruses
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Reference Virus Strains, Enzymes, and Primers
2.3. Design and Selection of Primers for the mRT-PCR
2.4. Selection of Reverse Transcriptase and DNA Polymerase for mRT-PCR
2.5. Patient Sera
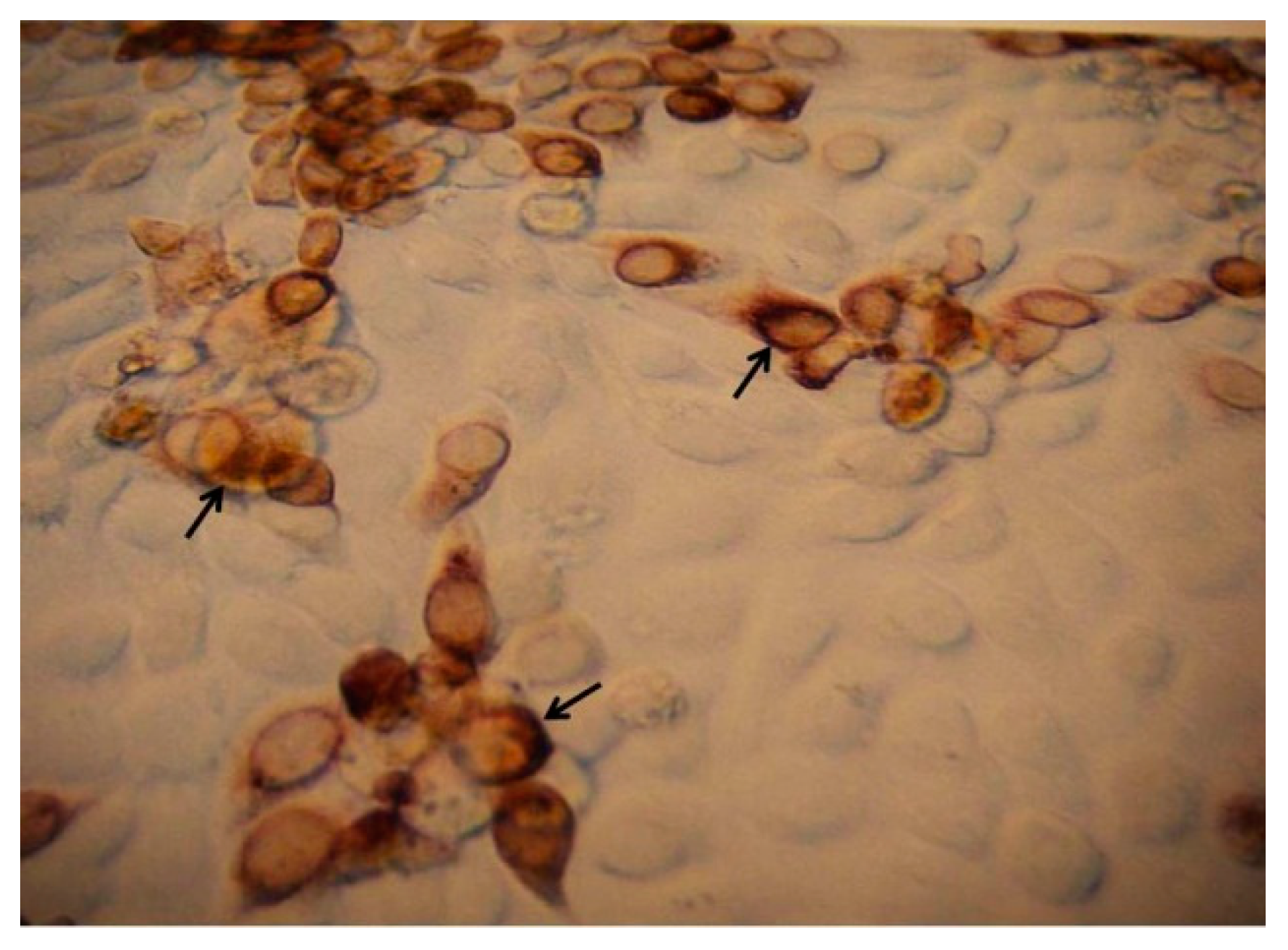
2.6. Virus Isolation, Stock Preparations, and Focus Assay
2.7. Extraction and Preparation of Spiked Viral RNA with Mosquito’s Tissue Homogenate
2.8. RT-PCR Reaction
2.9. mRT-PCR Reaction
2.10. Optimization of Thermal Profile to Increase the Specificity and Sensitivity of the mRT-PCR
2.11. Statistical Analysis
3. Results
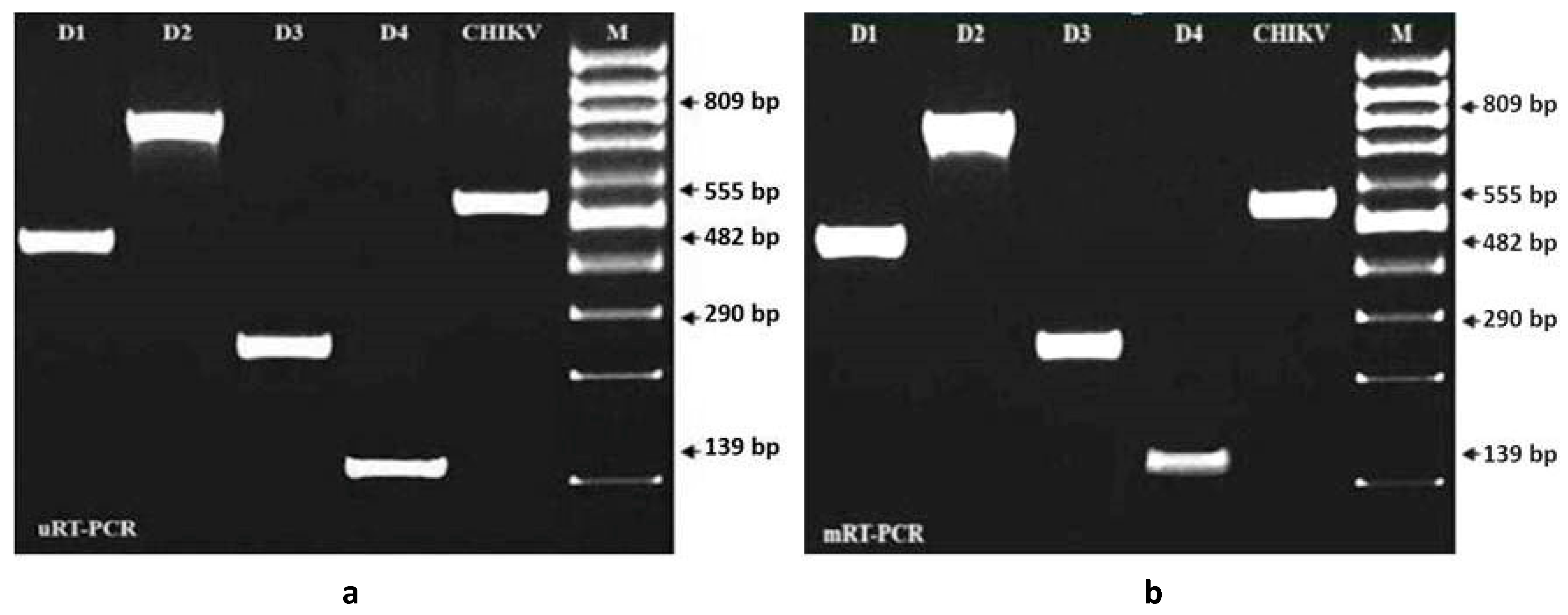
3.1. Comparing Specificity and Sensitivity of the Primers and Enzymes Designed for mRT-PCR
3.2. Evaluation of Specificity and Sensitivity of mRT-PCR for the Detection of Dengue and Chikungunya Viruses
3.3. Evaluation of Specificity and Sensitivity of mRT-PCR for the Detection of Dengue and Chikungunya Viruses Spiked with A. aegypti Tissue Homogenate
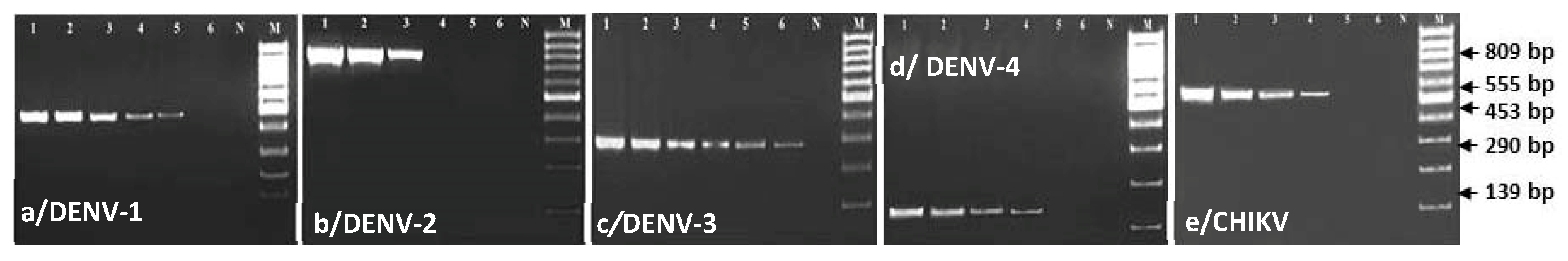
3.4. Comparing Specificity and Sensitivity of mRT-PCR and Virus Isolation and Validation of the Test for Serotyping of DENV and CHIKV Obtained from Clinical Samples
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Vu, D.M.; Jungkind, D.; Angelle, D.L. Chikungunya Virus. Clin. Lab. Med. 2019, 37, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Marques-Toledo, C.A.; Bendati, M.M.; Codeço, C.T.; Teixeira, M.M. Probability of dengue transmission and propagation in a non-endemic temperate area: Conceptual model and decision risk levels for early alert, prevention and control. Parasit. Vectors 2019, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Camilla, R.; Elaine, C.J. Emerging infectious diseases and international traveler. In The Travel and Tropical Medicine Manual, 5th ed.; Elsevier: Philadelphia, PA, USA, 2017; Volume 73, pp. 27–35. [Google Scholar]
- Burke, D.S.; Monath, T.P. Flavivirus. In Field Virology, 4th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 1043–1125. [Google Scholar]
- Solomon, T.; Mallewa, M. DEN and other emerging Flaviviruses. J. Infect. 2001, 42, 104–115. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. DEN and DEN Hemorrhagic Fever; Fact Sheet No. 117; WHO: Geneva, Switzerland, 2002; Available online: https://www.who.int/en/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 20 April 2020).
- Narain, J.P.; Dhariwal, A.C.; MacIntyre, C.R. Acute encephalitis in India: An unfolding tragedy. Indian J. Med. Res. 2017, 145, 584–587. [Google Scholar] [PubMed]
- Paulo, C.O.; Zé-Zé, L.; Jordão, S.; Pena, E.R.; Neves, I.; Alves, M.J. Dengue virus serotype 3 and Chikungunya virus co-infection in a traveller returning from India to Portugal, November 2016. IDCases 2017, 9, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.W. The Newala epidemic III. The virus: Isolation, pathogenic properties and relationship to the epidemic. J. Hyg. 1956, 54, 177–191. [Google Scholar] [CrossRef]
- Sarma, N. Emerging and re-emerging infectious diseases in South East Asia. Indian J. Dermatol. 2017, 62, 451–455. [Google Scholar] [PubMed]
- Halstead, S.B.; Nimmannitya, S.; Margiotta, M.R. Dengue and Chikungunya virus infection in man in Thailand. II. Observations on disease in outpatients. Am. J. Trop. Med. Hyg. 1969, 18, 972–983. [Google Scholar] [CrossRef]
- Jadhav, M.N.M.C.R.H.; Carey, D.E.; Myers, R.M. Chikungunya disease in infants and children in Vellore. A report on clinical and hematological features of virologically proved cases. Ind. J. Med. Res. 1965, 53, 764–776. [Google Scholar]
- De Ranitz, C.M.; Myers, R.M.; Varkey, M.J.; Isaac, Z.H.; Carey, D.E. Clinical impression of chikungunya in Vellore gained from study of adult patients. Ind. J. Med. Res. 1965, 53, 756–763. [Google Scholar]
- Myers, R.M.; Carey, D.E. Concurrent isolation from patient of two arboviruses, chikungunya and dengue type-2. Science 1967, 157, 1307–1308. [Google Scholar] [CrossRef] [PubMed]
- Morens, D.M. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin. Infect. Dis. 1994, 19, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue and dengue hemorrhagic fever: Its history and resurgence as a global public health problem. In Dengue and Dengue Hemorrhagic Fever; Gubler, D.J., Kuno, G., Eds.; CAB International: New York, NY, USA, 1997; pp. 1–22. [Google Scholar]
- Guzman, M.G.; Kouri, G.; Bravo, J.; Soler, M.; Vazquez, S.; Morier, L. Dengue hemorrhagic fever in Cuba, 1981: A retrospective seroepidemiologic study. Am. J. Trop. Med. Hyg. 1990, 42, 179–184. [Google Scholar] [CrossRef]
- Guzman, M.G.; Kouri, G. Advances in dengue diagnosis. Clin. Diag. lab. Immunol. 1996, 3, 621–627. [Google Scholar] [CrossRef]
- Vaughn, D.W.; Green, S.; Kalayannarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue viremia titer, antibody response pattern and virus serotype correlate with disease severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef]
- Wong, S.J.; Boyle, R.H.; Demarest, V.L.; Wood-mansee, A.N.; Kramer, L.D.; Drebot, H.L.i.-M.; Koski, K.A.; Fikrig, E.; Martin, D.A.; Shi, P.Y. Immunoassy targetting non-structural protein to differentiate West Nile virus infection from dengue and St. Louis encephalitis virus infections and from flavivirus vaccination. J. Clin. Microbiol. 2003, 41, 4217–4223. [Google Scholar] [CrossRef]
- Bundo, K.; Igarashi, A. Antibody-capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients. J. Virol. Methods 1985, 11, 15–22. [Google Scholar] [CrossRef]
- Valdes, K.; Alvarez, M.; Pupo, M.; Vazquez, S.; Rodriguez, R.; Guzman, M.G. Human dengue antibodies against structural and non-structural proteins. Clin. Diag. Lab. Immunol. 2000, 7, 856–857. [Google Scholar] [CrossRef]
- Innis, B.L.; Nisalak, A.; Nimmannitya, S.; Kusalerdchariya, S.; Chongswasadi, V.; Suntayakorn, S.; Puttisri, P.; Hoke, C.H. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am. J. Trop. Med. Hyg. 1989, 40, 418–427. [Google Scholar] [CrossRef]
- Kuno, G.; Gomez IGubler, D.J. An ELISA procedure for the diagnosis of dengue infections. J. Virol. Methods 1991, 33, 101–113. [Google Scholar] [CrossRef]
- Lam, S.K.; Devine, P.L. Evaluation of capture ELISA and rapid immunochromatographic test for the determination of IgM and IgG antibodies produced during dengue infection. Clin. Diag. Virol. 1998, 10, 75–81. [Google Scholar] [CrossRef]
- Shu, P.Y.; Chen, L.K.; Chang, S.F.; Sue, C.L.; Chien, L.J.; Chin, C.; Lin, T.H.; Huang, J.H. Dengue virus serotyping based on envelope/membrane (E/M) and non-structural protein NS1 serotype specific capture immunoglobulin M (IgM) enzyme-linked immunosorbent assays (ELISA). J. Clin. Microbiol. 2004, 42, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J.; Clark, G.G. Dengue/dengue hemorrhagic fever: The emergence of a global health problem. Emerg. Infect. Dis. 1995, 1, 55–57. [Google Scholar] [CrossRef]
- Batovska, J.; Mee, P.T.; Lynch, S.E.; Sawbridge, T.I.; Rodoni, B.C. Sensitivity and specificity of metatranscriptomics as an arbovirus surveillance tool. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Igarashi AMohamed, H.; Yusof, A.; Sinniah, M.; Tanaka, H. Production of type-2 dengue (D2) monoclonal antibody and cell culture derived d2 antigen for use in dengue IgM capture ELISA. Trop. Med. 1995, 37, 165–173. [Google Scholar]
- Henchal, E.A.; McSown, J.M.; Seguin, M.C.; Gentry, M.K.; Brandt, W.E. Rapid identification of dengue viruse isolates by using monoclonal antibodies in an indirect immunofluorescence assay. Am. J. Trop. Med. Hyg. 1983, 32, 164–169. [Google Scholar] [CrossRef]
- Igarashi, A. Isolation of a Singh’s Aedes albopictus cell clone sensitive to dengue and chikungynya viruses. J. Gen. Virol. 1978, 40, 531–544. [Google Scholar] [CrossRef]
- Igarashi, A. Mosquito cell cultures and the study of arthropod-borne toga viruses. Adv. Virus Res. 1987, 30, 21–39. [Google Scholar]
- Lanciotti, R.S.C.; Calisher, D.; Gubler, G.; Vorndam, V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 545–551. [Google Scholar] [CrossRef]
- He, T.; Kaplan, S.; Kamboj, M.; Tang, Y.W. Laboratory diagnosis of central nervous system infection. Curr. Infect. Dis. Rep. 2016, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Mawuntu, A.H.; Bernadus, J.B.; Dhenni, R.; Wiyatno, A.; Anggreani, R.; Yudhaputri, F.A.; Jaya, U.A.; Ma’roef, C.N.; Dewantari, A.K.; Fadhilah, A.; et al. Detection of central nervous system viral infections in adults in Manado, North Sulawesi, Indonesia. PLoS ONE 2018, 13, e0207440. [Google Scholar] [CrossRef]
- Harris, E.; Robert, T.G.; Smith, L.; Selle, J.; Kramer, L.D.; Valle, S.; Sandoval, E.; Balmaseda, A. Typing of dengue viruses in clinical species and mosquitoes by single tube multiplex reverse transcriptase PCR. J. Clin. Microbiol. 1998, 36, 2634–2639. [Google Scholar] [CrossRef] [PubMed]
- Chungue, E.; Roche, C.; Lefevre, M.F.; Barbazan, P.; Chanteau, S. Ultra-rapid, simple, sensitive, and economical silica method for extraction of dengue viral RNA from clinical specimens and mosquitoes by reverse transcriptase- polymerase chain reaction. J. Med. Virol. 1993, 40, 142–145. [Google Scholar] [CrossRef]
- Morita, K.; Tanaka, M.; Igarashi, A. Rapid identification of dengue virus serotypes by using polymerase chain reaction. J. Clin. Microbiol. 1991, 29, 2107–2110. [Google Scholar] [CrossRef]
- Kuno, G. Universal diagnostic RT-PCR protocol for arboviruses. J. Virol. Methods 1998, 72, 27–41. [Google Scholar] [CrossRef]
- Tanaka, M. Rapid identification of flavivirus using the polymerase chain reaction. J. Virol. Methods 1993, 41, 311–322. [Google Scholar] [CrossRef]
- Houng, H.H.; Chen, R.C.; Vaughn, M.D.W.; Kanesa-thasan, K. Development of a fluorogenic RT-PCR system for quantitative detection of dengue virus serotypes 1-4 using conserved and serotype specific 3′ noncoding sequence. J. Virol. Methods 2001, 95, 19–32. [Google Scholar] [CrossRef]
- Chow, V.T.K.; Chan, Y.C.; Yong, R.; Lee, K.M.; Lim, L.K.; Chung, Y.K.; Lam-phua, S.G.; Tan, B.T. Monitoring of dengue virus infield caught Aedes aegypti and Aedes albopictus mosquitoes by a type-specific polymerase chain reaction and cycle sequencing. Am. J. Trop. Med. Hyg. 1998, 58, 578–586. [Google Scholar] [CrossRef]
- Kumaria, R.; Chakravarti, A. Molecular detection and serotypic characterization of dengue viruses by single-tube multiplex reverse transcriptase-polymerase chain reaction. Diag. Microbiol. Infect. Dis. 2005, 52, 311–316. [Google Scholar] [CrossRef]
- Wilder-Smith, A.; Tissera, H.; AbuBakar, S.; Kittayapong, P.; Logan, J.; Neumayr, A.; Rocklöv, J.; Byass, P.; Louis, V.R.; Tozan, Y.; et al. Novel tools for the surveillance and control of dengue: Findings by the Dengue Tools research consortium. Glob. Health Action 2018, 11, 1549930. [Google Scholar] [CrossRef]
- Timothy, R.W.; Harshad, R.T. A sensitive method for quantification of vesicular stomatitis virus defective interfering particles: Focus forming assay. J. Gen. Virol. 1980, 48, 237–240. [Google Scholar]
- Islam, M.A.; Haque, M.E.; Sharif, M.M.; Islam, S.; Amin, M.R. Vector borne viral diseases: An emerging threat and their control strategies in Bangladesh perspectives. Int. Res. J. Med. Med. Sci. 2020, 8, 8–17. [Google Scholar] [CrossRef][Green Version]
- Mutsuddy, P.; Jhora, S.T.; Shamsuzzaman, A.K.M.; Kaisar, S.M.G.; Khan, M.N.A. Dengue situation in Bangladesh: An epidemiological shift in terms of morbidity and mortality. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 3516284. [Google Scholar] [CrossRef]
- Saxena, P.; Dash, P.K.; Santhosh, S.R.; Shrivastava, A.; Parida, M.; Rao, P.L. Development and evaluation of one step single tube multiplex RT-PCR for rapid detection and typing of dengue viruses. Virol. J. 2008, 5, 20. [Google Scholar] [CrossRef]

| Primers | Sequences (5′-3′) | Nucleotide Position | Orientation | Accession No. | Strain Name |
|---|---|---|---|---|---|
| * DC-S | TCAATATGCTGAAACGCGCGAGAAACCG | 134–161 | Sense | M84727 | DENV-2 16681 |
| * D1-C | CGTCTCAGTGATCCGGGGG | 586–568 | Complementary | M23027 | DENV-1 |
| D2-C | ACGCATTGTCATTGAGGGAG | 942–923 | Complementary | AF022434 | DENV-2 ThNH-7/93 |
| * D3-C | TAACATCATCATGAGACAGAGC | 421–400 | Complementary | L11423 | DENV-3 H-87 |
| D4-C | GGAAAGGACTCGCAAAAAC | 272–254 | Complementary | M14931 | DENV-4 |
| CHIKV-S | TACAGCACACAGCACCAT | 10,745–10,762 | Sense | AF369024 | CHIKV S27-African prototype |
| CHIKV-C | ACGCATAGCACCACGATTA | 11,294–11,276 | Complementary |
| DNA Polymerase | Reverse Transcriptase | Serotype Detection Limit of the mRT-PCR | ||||
|---|---|---|---|---|---|---|
| DENV-1 | DENV-2 | DENV-3 | DENV-4 | CHIKV | ||
| FFU Mean ± SE | FFU Mean ± SE | FFU Mean ± SE | FFU Mean ± SE | FFU Mean ± SE | ||
| LA-Taq+ | AMV-RT | 1.00 ± 0.00c | 20.00 ± 2.89b | 0.10 ± 0.00c | 10.00 ± 0.00b | 10.00 ± 0.00b |
| r-Taq+ | AMV-RT | 10.00 ± 2.89b | 20.00 ± 2.89b | 1.00 ± 0.00b | 20.00 ± 0.00a | 20.00 ± 2.89a |
| Tth+ | AMV-RT | 10.00 ± 2.89b | 50.00 ± 5.77a | 1.00 ± 0.00b | 20.00 ± 2.89a | 20.00 ± 2.89a |
| LA-Taq+ | RT-ACE | 20.00 ± 2.89a | 50.00 ± 2.89a | 1.00 ± 0.00b | 20.00 ± 0.00a | 20.00 ± 0.00a |
| r-Taq+ | RT-ACE | 20.00 ± 2.89a | 50.00 ± 1.15a | 10.00 ± 2.89a | 20.00 ± 2.89a | 20.00 ± 2.89a |
| Tth+ | RT-ACE | 20.00 ± 2.89a | 50.00 ± 2.89a | 10.00 ± 1.15a | 20.00 ± 2.89a | 20.00 ± 0.00a |
| p value | 0.001 | 0.001 | 0.001 | 0.023 | 0.023 | |
| Level of sig. | ** | ** | ** | * | * | |
| Country | Sampling Years | No. of Samples | No. of Positive Samples Using mRT-PCR | Total No. and % of Positive Samples | No. of Positive Samples Using Virus Isolation | Total No. and % of Positive Samples Using Virus Isolation | Total No. and % of Positive Samples Using Dengue NS1 Ag Kit | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | CHIKV | DENV | CHIKV | D1 | D4 | CHIKV | DENV | CHIKV | DENV | CHIKV | |||
| Bangladesh | 2016 | 200 | 0 | 10 | 90 | 0 | 5 | 100 (50%) | 5 (2.5%) | 0 | 0 | 0 | 25 (12.5%) | 0 | 85 (42%) | 0 |
| 2017 | 300 | 0 | 1 | 35 | 0 | 140 | 36 (12%) | 140 (46.7%) | 0 | 0 | 0 | 8 (2.7%) | 0 | 30 (10%) | 0 | |
| 2018 | 150 | 0 | 0 | 70 | 0 | 4 | 70 (46.7%) | 4 (2.7%) | 0 | 0 | 0 | 12 (8%) | 0 | 60 (40%) | 0 | |
| Total | 3 years | 650 | 0 | 11 | 195 | 0 | 149 | 206 (31.7%) | 149 (22.9%) | 0 | 0 | 0 | 45 (23.2%) | 0 | 175 (92%) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.A.; El Zowalaty, M.E.; Islam, S.; Sharif, M.; Rahman, M.R.; Amin, M.R.; Ali, M.M.; Rahman, M.T.; Morita, K.; Ashour, H.M. A Novel Multiplex RT-PCR Assay for Simultaneous Detection of Dengue and Chikungunya Viruses. Int. J. Mol. Sci. 2020, 21, 8281. https://doi.org/10.3390/ijms21218281
Islam MA, El Zowalaty ME, Islam S, Sharif M, Rahman MR, Amin MR, Ali MM, Rahman MT, Morita K, Ashour HM. A Novel Multiplex RT-PCR Assay for Simultaneous Detection of Dengue and Chikungunya Viruses. International Journal of Molecular Sciences. 2020; 21(21):8281. https://doi.org/10.3390/ijms21218281
Chicago/Turabian StyleIslam, Mohammed Alimul, Mohamed E. El Zowalaty, Sumaiya Islam, Mohiuddin Sharif, Md. Rajibur Rahman, Mohammad Robed Amin, Md. Mortuza Ali, Md. Tanvir Rahman, Kouichi Morita, and Hossam M. Ashour. 2020. "A Novel Multiplex RT-PCR Assay for Simultaneous Detection of Dengue and Chikungunya Viruses" International Journal of Molecular Sciences 21, no. 21: 8281. https://doi.org/10.3390/ijms21218281
APA StyleIslam, M. A., El Zowalaty, M. E., Islam, S., Sharif, M., Rahman, M. R., Amin, M. R., Ali, M. M., Rahman, M. T., Morita, K., & Ashour, H. M. (2020). A Novel Multiplex RT-PCR Assay for Simultaneous Detection of Dengue and Chikungunya Viruses. International Journal of Molecular Sciences, 21(21), 8281. https://doi.org/10.3390/ijms21218281









