9-PAHSA Prevents Mitochondrial Dysfunction and Increases the Viability of Steatotic Hepatocytes
Abstract
:1. Introduction
2. Results
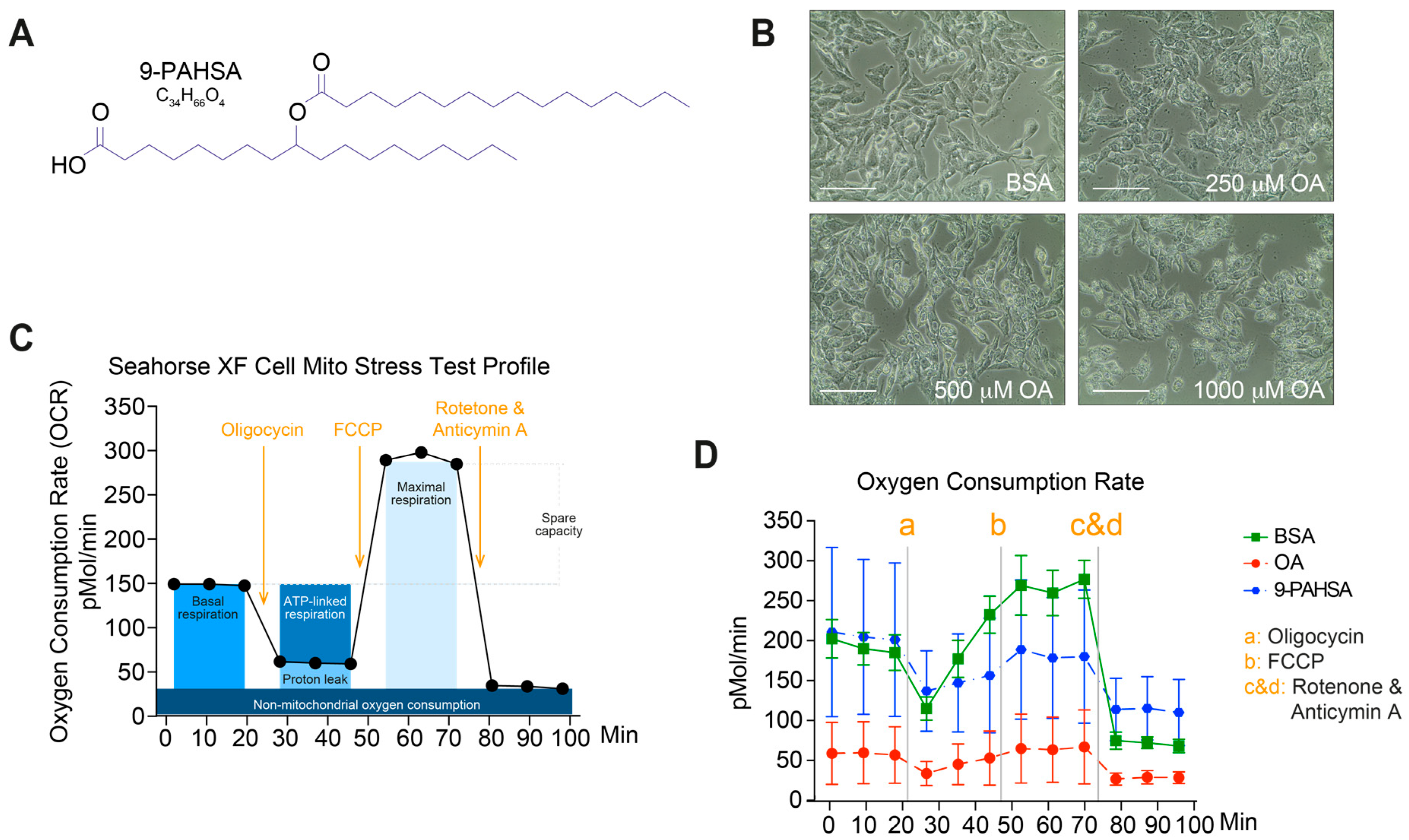
2.1. Pretreatment with 9-PAHSA Prevented Mitochondrial Dysfunction in Steatotic Cells
2.2. 9-PAHSA Treatment Improved the Viability of the Steatotic Primary Murine Hepatocytes (PMH)
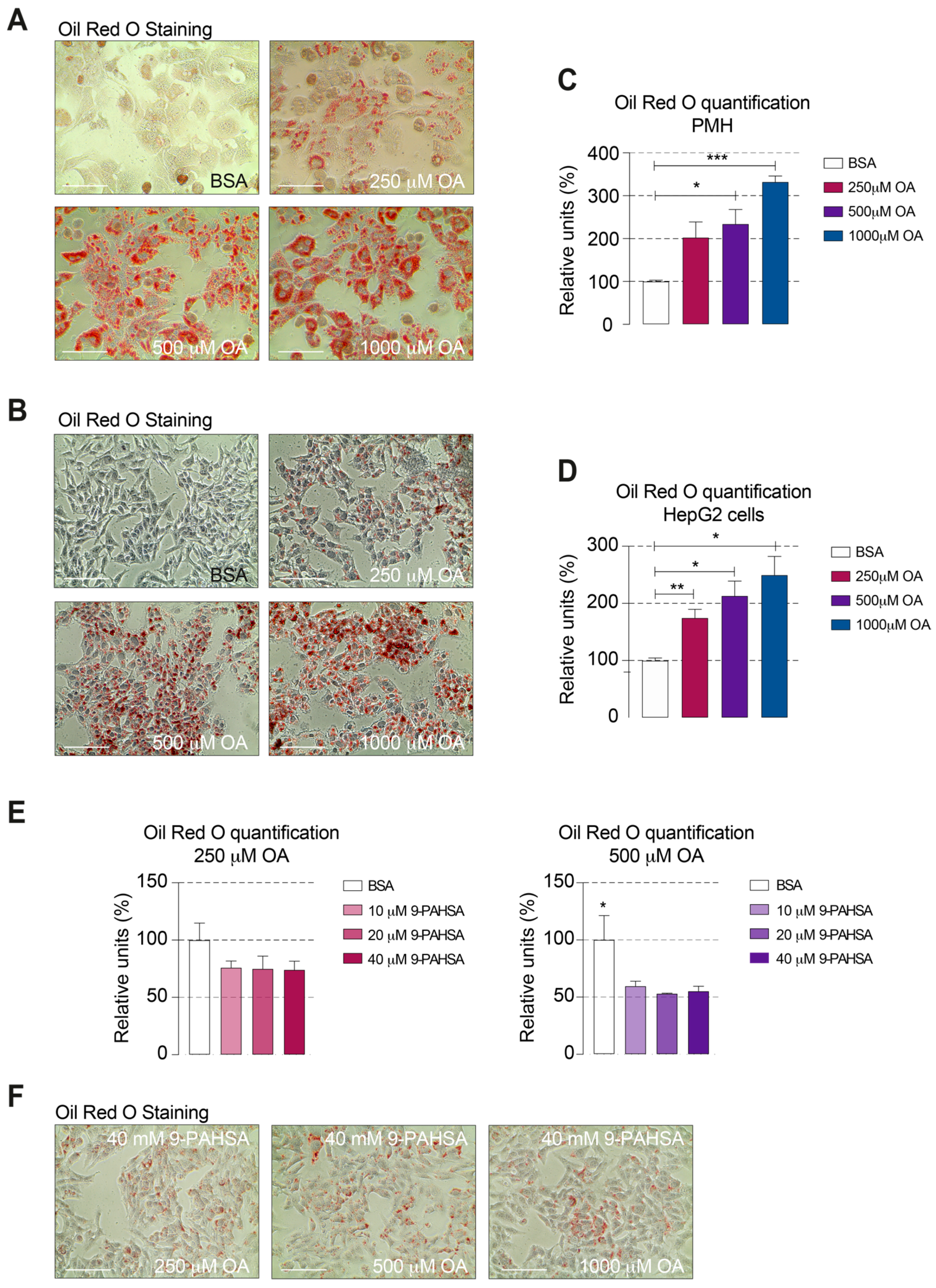
2.3. 9-PAHSA Treatment Reduced the Intracellular Lipid Accumulation in Steatotic Cells
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Primary Murine Hepatocyte Purification
4.3. Steatosis Induction
4.4. Red Oil O Staining and Quantification
4.5. 9-PAHSA Treatment
4.6. Cell Viability
4.7. Mitochondrial Respiration
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| 9-PAHSA | Palmitic-acid-9-hydroxy-stearic-acid |
| NAFL | Nonalcoholic fatty liver |
| FAHFA | Branched fatty acid esters of hydroxyl fatty acid |
| OA | Oleic acid |
| FCCP | Phenylhydrazone |
| OCR | Oxygen consumption rate |
| HFD | High fat diet |
| BSA | Bovine serum albumin |
References
- Bellentani, S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017, 37 (Suppl. 1), 81–84. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.-Y.; Watt, M.J.; Rensen, S.S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef] [Green Version]
- Roden, M. Mechanisms of Disease: Hepatic steatosis in type 2 diabetes—pathogenesis and clinical relevance. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 335–348. [Google Scholar] [CrossRef]
- Liu, W.; Baker, R.D.; Bhatia, T.; Zhu, L.; Baker, S.S. Pathogenesis of nonalcoholic steatohepatitis. Cell. Mol. Life Sci. 2016, 73, 1969–1987. [Google Scholar] [CrossRef]
- Argo, C.K.; Caldwell, S.H. Epidemiology and Natural History of Non-Alcoholic Steatohepatitis. Clin. Liver Dis. 2009, 13, 511–531. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-M.; Pu, C.-W.; Hou, Y.-H.; Chen, Z.; Alanazy, M.; Hebbard, L. Non alcoholic steatohepatitis a precursor for hepatocellular carcinoma development. World J. Gastroenterol. 2014, 20, 16464–16473. [Google Scholar] [CrossRef]
- Roeb, E.; Steffen, H.M.; Bantel, H.; Baumann, U.; Canbay, A.; Demir, M.; Drebber, U.; Geier, A.; Hampe, J.; Hellerbrand, C.; et al. S2k Guideline Non-Alcoholic Fatty Liver Disease. Z. Gastroenterol. 2015, 53, 668–723. [Google Scholar]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a Class of Endogenous Mammalian Lipids with Anti-Diabetic and Anti-inflammatory Effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef] [Green Version]
- Mouries, J.; Brescia, P.; Silvestri, A.; Spadoni, I.; Sorribas, M.; Wiest, R.; Mileti, E.; Galbiati, M.; Invernizzi, P.; Adorini, L.; et al. Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J. Hepatol. 2019, 71, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Moraes-Vieira, P.M.; Castoldi, A.; Aryal, P.; Yee, E.U.; Vickers, C.; Parnas, O.; Donaldson, C.J.; Saghatelian, A.; Kahn, B.B. Branched Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) Protect against Colitis by Regulating Gut Innate and Adaptive Immune Responses. J. Biol. Chem. 2016, 291, 22207–22217. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Santoro, A.; Peroni, O.D.; Nelson, A.T.; Saghatelian, A.; Siegel, D.; Kahn, B.B. PAHSAs enhance hepatic and systemic insulin sensitivity through direct and indirect mechanisms. J. Clin. Investig. 2019, 129, 4138–4150. [Google Scholar] [CrossRef] [PubMed]
- Perez-Carreras, M.; Del Hoyo, P.; Martin, M.A.; Rubio, J.C.; Martin, A.; Castellano, G.; Colina, F.; Arenas, J.; Solis-Herruzo, J.A. Defective Hepatic Mitochondrial Respiratory Chain in Patients with Nonalcoholic Steatohepatitis. Hepatology 2003, 38, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Syed, I.; Lee, J.; Moraes-Vieira, P.M.; Donaldson, C.J.; Sontheimer, A.; Aryal, P.; Wellenstein, K.; Kolar, M.J.; Nelson, A.T.; Siegel, D.; et al. Palmitic Acid Hydroxystearic Acids Activate GPR40, Which Is Involved in Their Beneficial Effects on Glucose Homeostasis. Cell Metab. 2018, 27, 419–427.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, I.; Rubin de Celis, M.F.; Mohan, J.F.; Moraes-Vieira, P.M.; Vijayakumar, A.; Nelson, A.T.; Siegel, D.; Saghatelian, A.; Mathis, D.; Kahn, B.B. Pahsas Attenuate Immune Responses and Promote Beta Cell Survival in Autoimmune Diabetic Mice. J. Clin. Investig. 2019, 129, 3717–3731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayakumar, A.; Aryal, P.; Wen, J.; Syed, I.; Vazirani, R.P.; Moraes-Vieira, P.M.; Camporez, J.P.; Gallop, M.R.; Perry, R.J.; Peroni, O.D.; et al. Absence of Carbohydrate Response Element Binding Protein in Adipocytes Causes Systemic Insulin Resistance and Impairs Glucose Transport. Cell Rep. 2017, 21, 1021–1035. [Google Scholar] [CrossRef] [Green Version]
- Pflimlin, E.; Bielohuby, M.; Korn, M.; Breitschopf, K.; Löhn, M.; Wohlfart, P.; Konkar, A.; Podeschwa, M.; Bärenz, F.; Pfenninger, A.; et al. Acute and Repeated Treatment with 5-PAHSA or 9-PAHSA Isomers Does Not Improve Glucose Control in Mice. Cell Metab. 2018, 28, 217–227.e13. [Google Scholar] [CrossRef] [Green Version]
- Syed, I.; Lee, J.; Peroni, O.D.; Yore, M.M.; Moraes-Vieira, P.M.; Santoro, A.; Wellenstein, K.; Smith, U.; McGraw, T.E.; Saghatelian, A.; et al. Methodological Issues in Studying PAHSA Biology: Masking PAHSA Effects. Cell Metab. 2018, 28, 543–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Chang, B.; Guo, Y.; Wu, X.; Liu, L. The role of oxidative stress-mediated apoptosis in the pathogenesis of uric acid nephropathy. Ren. Fail. 2019, 41, 616–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati-Čizmek, A.-M.; Biluš, M.; Brkić, A.L.; Barić, I.C.; Bakula, M.; Hozić, A.; Cindrić, M. Analysis of Fatty Acid Esters of Hydroxyl Fatty Acid in Selected Plant Food. Plant Foods Hum. Nutr. 2019, 74, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Moravcová, A.; Červinková, Z.; Kučera, O.; Mezera, V.; Rychtrmoc, D.; Lotková, H. The Effect of Oleic and Palmitic Acid on Induction of Steatosis and Cytotoxicity on Rat Hepatocytes in Primary Culture. Physiol. Res. 2015, 64 (Suppl. 5), S627–S636. [Google Scholar] [CrossRef]
- Wooltorton, E. Too much of a good thing? Toxic effects of vitamin and mineral supplements. CMAJ 2003, 169, 47–48. [Google Scholar] [PubMed]
- Liu, J.; Huang, X.; Werner, M.; Broering, R.; Yang, D.; Lu, M. Advanced Method for Isolation of Mouse Hepatocytes, Liver Sinusoidal Endothelial Cells, and Kupffer Cells. Methods Mol. Biol. 2016, 1540, 249–258. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schultz Moreira, A.R.; Rüschenbaum, S.; Schefczyk, S.; Hendgen-Cotta, U.; Rassaf, T.; Broering, R.; Hardtke-Wolenski, M.; Buitrago-Molina, L.E. 9-PAHSA Prevents Mitochondrial Dysfunction and Increases the Viability of Steatotic Hepatocytes. Int. J. Mol. Sci. 2020, 21, 8279. https://doi.org/10.3390/ijms21218279
Schultz Moreira AR, Rüschenbaum S, Schefczyk S, Hendgen-Cotta U, Rassaf T, Broering R, Hardtke-Wolenski M, Buitrago-Molina LE. 9-PAHSA Prevents Mitochondrial Dysfunction and Increases the Viability of Steatotic Hepatocytes. International Journal of Molecular Sciences. 2020; 21(21):8279. https://doi.org/10.3390/ijms21218279
Chicago/Turabian StyleSchultz Moreira, Adriana R., Sabrina Rüschenbaum, Stefan Schefczyk, Ulrike Hendgen-Cotta, Tienush Rassaf, Ruth Broering, Matthias Hardtke-Wolenski, and Laura Elisa Buitrago-Molina. 2020. "9-PAHSA Prevents Mitochondrial Dysfunction and Increases the Viability of Steatotic Hepatocytes" International Journal of Molecular Sciences 21, no. 21: 8279. https://doi.org/10.3390/ijms21218279
APA StyleSchultz Moreira, A. R., Rüschenbaum, S., Schefczyk, S., Hendgen-Cotta, U., Rassaf, T., Broering, R., Hardtke-Wolenski, M., & Buitrago-Molina, L. E. (2020). 9-PAHSA Prevents Mitochondrial Dysfunction and Increases the Viability of Steatotic Hepatocytes. International Journal of Molecular Sciences, 21(21), 8279. https://doi.org/10.3390/ijms21218279







