Neurotransmitter Modulation of Carotid Body Germinal Niche
Abstract
:1. Introduction
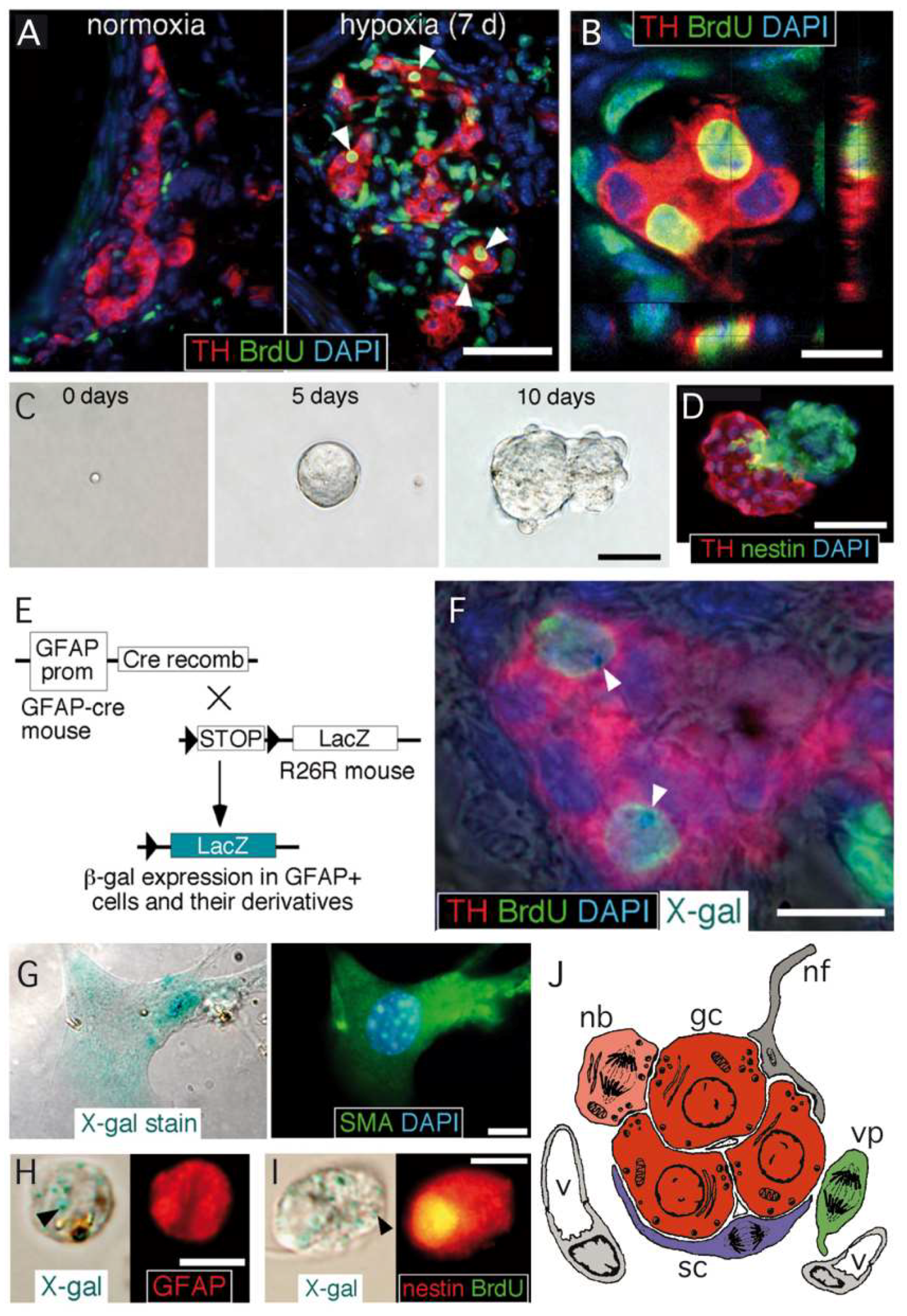
2. The Carotid Body Germinal Niche
3. Neurotransmitter Modulation of Carotid Body Progenitor Cells
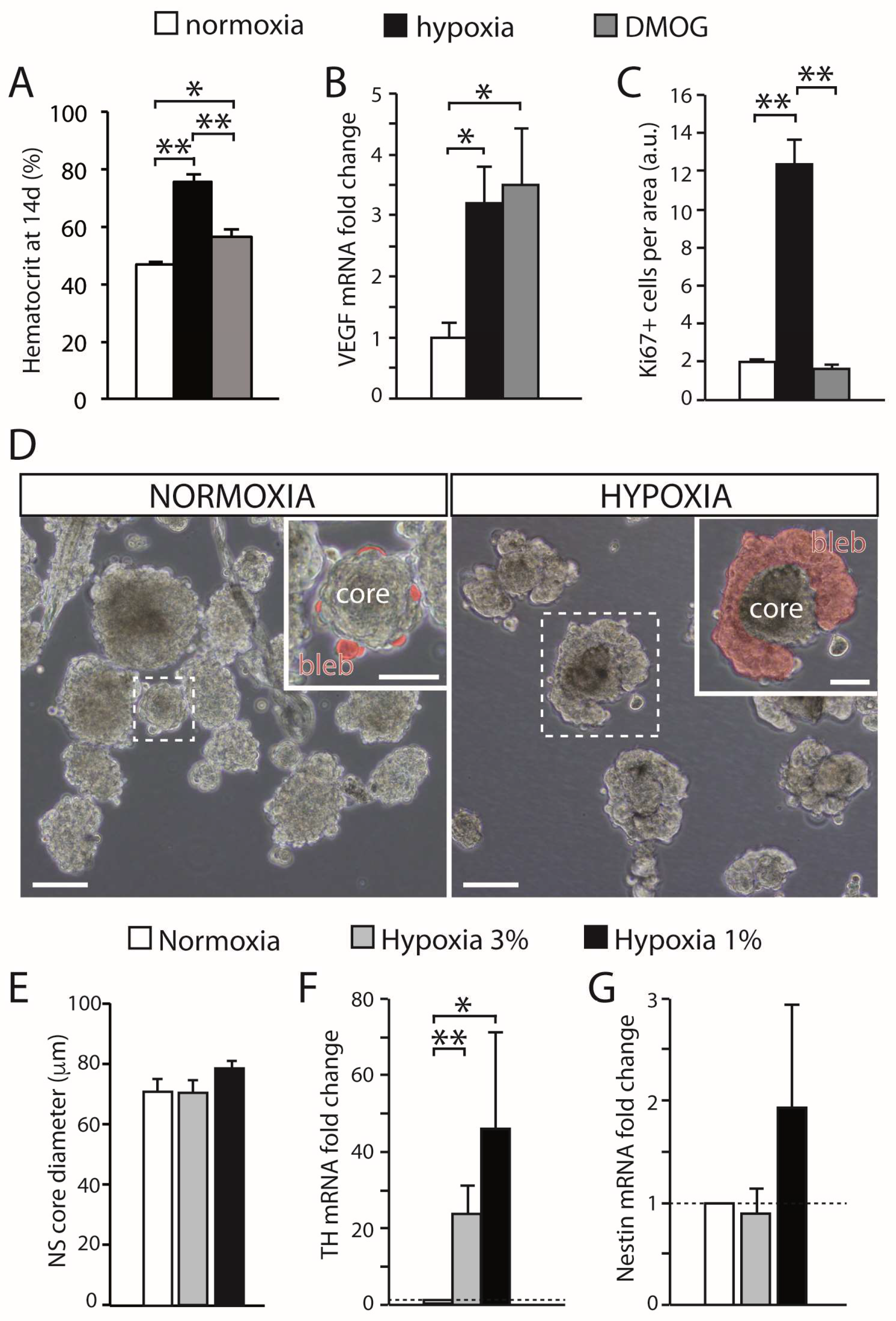
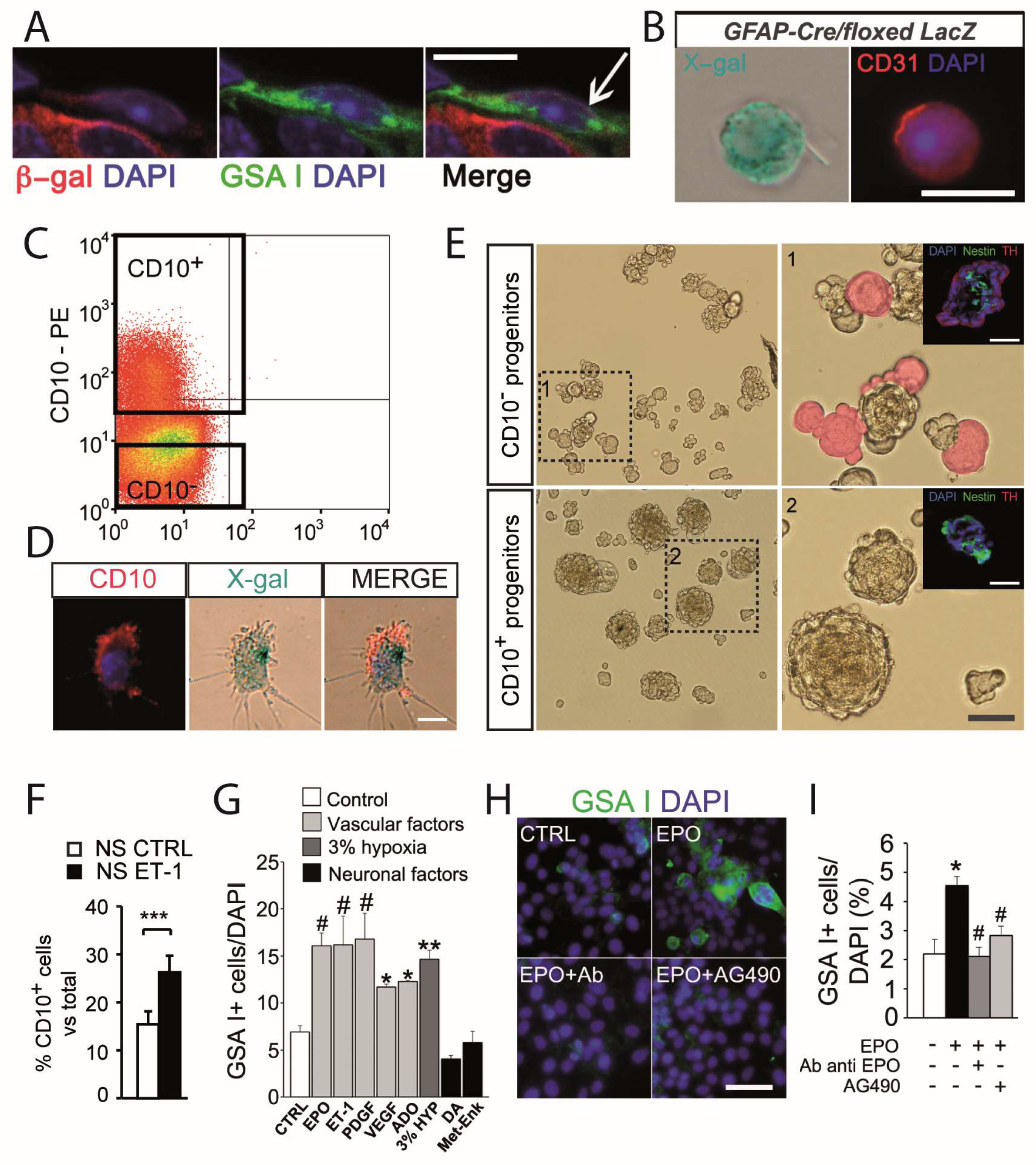
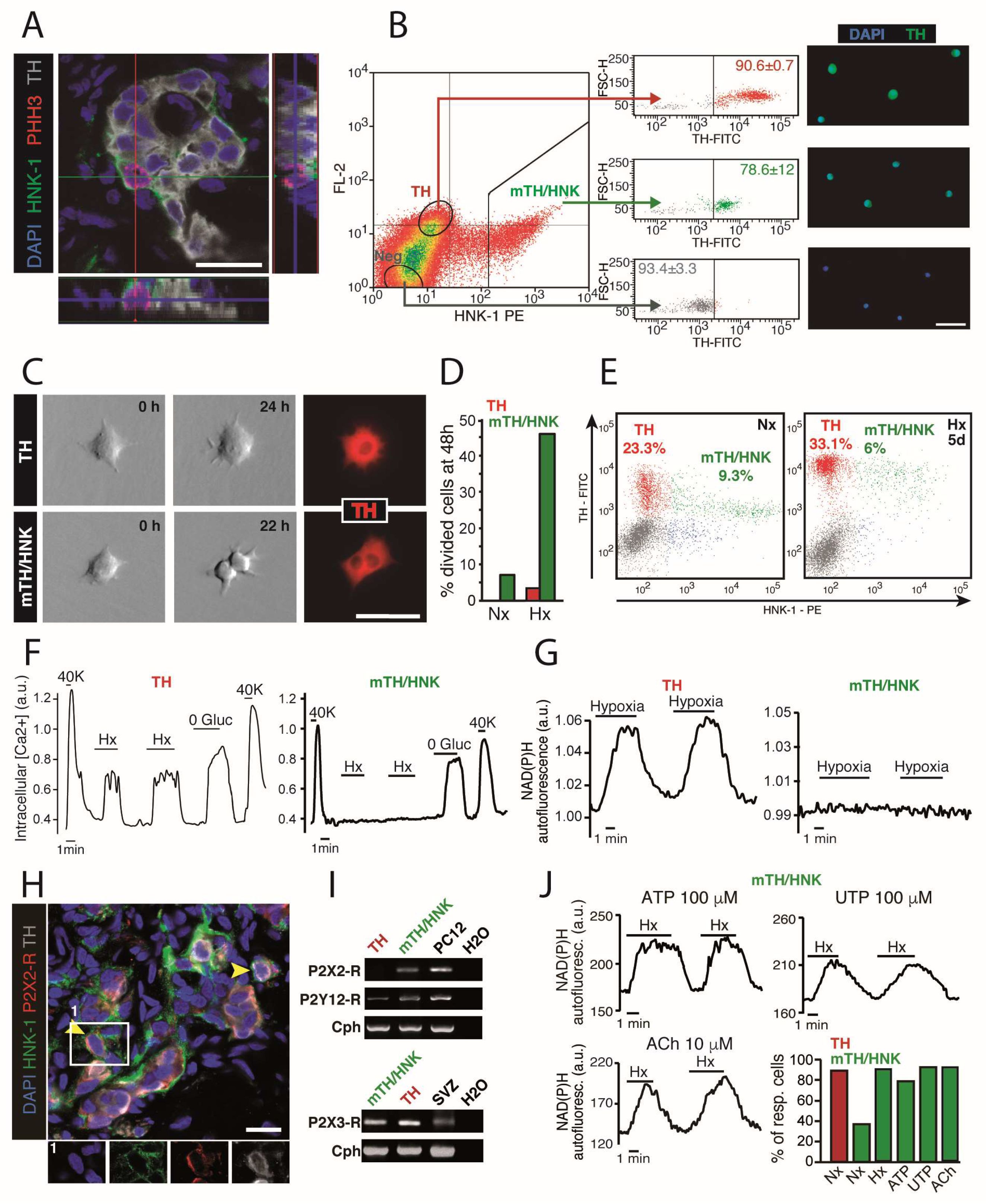
3.1. Carotid Body Multipotent Progenitors. Activation by Oxygen-Sensitive Glomus Cells
3.2. Angiogenesis and Glomus Cell-Released Vascular Cytokines
3.3. Maturation of Carotid Body Neuroblasts in Response to Glomus Cell Activity
4. Concluding Remarks
Funding
Conflicts of Interest
References
- Ortega-Sáenz, P.; López-Barneo, J. Physiology of the Carotid Body: From Molecules to Disease. Annu. Rev. Physiol. 2020, 82, 127–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, E.K.; López-Barneo, J.; Buckler, K.J.; Archer, S.L. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 2005, 353, 2042–2055. [Google Scholar] [CrossRef] [PubMed]
- Timmers, H.J.; Wieling, W.; Karemaker, J.M.; Lenders, J.W. Denervation of carotid baro- and chemoreceptors in humans. J. Physiol. 2003, 553, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Biscoe, T.J.; Stehbens, W.E. Ultrastructure of the carotid body. J. Cell Biol. 1966, 30, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Hellström, S. Morphometric studies of dense-cored vesicles in type I cells of rat carotid body. J. Neurocytol. 1975, 4, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Duchen, M.R.; Caddy, K.W.; Kirby, G.C.; Patterson, D.L.; Ponte, J.; Biscoe, T.J. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience 1988, 26, 291–311. [Google Scholar] [CrossRef]
- López-Barneo, J.; López-López, J.R.; Ureña, J.; González, C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science 1988, 241, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Buckler, K.J.; Vaughan-Jones, R.D. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J. Physiol. 1994, 476, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Peers, C. Hypoxic suppression of K+ currents in type I carotid body cells: Selective effect on the Ca2(+)-activated K+ current. Neurosci. Lett. 1990, 119, 253–256. [Google Scholar] [CrossRef]
- Stea, A.; Nurse, C.A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflug. Arch. 1991, 418, 93–101. [Google Scholar] [CrossRef]
- Ureña, J.; Fernández-Chacón, R.; Benot, A.R.; Alvarez de Toledo, G.A.; López-Barneo, J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc. Natl. Acad. Sci. USA 1994, 91, 10208–10211. [Google Scholar] [CrossRef] [Green Version]
- López-Barneo, J.; Macías, D.; Platero-Luengo, A.; Ortega-Sáenz, P.; Pardal, R. Carotid body oxygen sensing and adaptation to hypoxia. Pflug. Arch. 2016, 468, 59–70. [Google Scholar] [CrossRef]
- Gao, L.; Bonilla-Henao, V.; Garcia-Flores, P.; Arias-Mayenco, I.; Ortega-Saenz, P.; Lopez-Barneo, J. Gene expression analyses reveal metabolic specifications in acute O2-sensing chemoreceptor cells. J. Physiol. 2017, 595, 6091–6120. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Domínguez, A.; Ortega-Sáenz, P.; Gao, L.; Colinas, O.; García-Flores, P.; Bonilla-Henao, V.; Aragonés, J.; Hüttemann, M.; Grossman, L.I.; Weissmann, N.; et al. Acute O(2) sensing through HIF2α-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci. Signal. 2020, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Matsunami, H. Lessons from single-cell transcriptome analysis of oxygen-sensing cells. Cell Tissue Res. 2017, 372, 403–415. [Google Scholar] [CrossRef]
- Arias-Mayenco, I.; González-Rodríguez, P.; Torres-Torrelo, H.; Gao, L.; Fernández-Agüera, M.C.; Bonilla-Henao, V.; Ortega-Sáenz, P.; López-Barneo, J. Acute O(2) Sensing: Role of Coenzyme QH(2)/Q Ratio and Mitochondrial ROS Compartmentalization. Cell Metab. 2018, 28, 145–158.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Agüera, M.C.; Gao, L.; González-Rodríguez, P.; Pintado, C.O.; Arias-Mayenco, I.; García-Flores, P.; García-Pergañeda, A.; Pascual, A.; Ortega-Sáenz, P.; López-Barneo, J. Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab. 2015, 22, 825–837. [Google Scholar] [CrossRef] [Green Version]
- Nurse, C.A. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J. Physiol. 2014, 592, 3419–3426. [Google Scholar] [CrossRef]
- Pardal, R.; Ortega-Sáenz, P.; Durán, R.; López-Barneo, J. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell 2007, 131, 364–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Stella, J.; Valcarcel, J. Chief cell hyperplasia in the human carotid body at high altitudes; physiologic and pathologic significance. Hum. Pathol. 1976, 7, 361–373. [Google Scholar] [CrossRef]
- McGregor, K.H.; Gil, J.; Lahiri, S. A morphometric study of the carotid body in chronically hypoxic rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1430–1438. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Bisgard, G.E. Chronic hypoxia-induced morphological and neurochemical changes in the carotid body. Microsc. Res. Tech. 2002, 59, 168–177. [Google Scholar] [CrossRef]
- Edwards, C.; Heath, D.; Harris, P. The carotid body in emphysema and left ventricular hypertrophy. J. Pathol. 1971, 104, 1–13. [Google Scholar] [CrossRef]
- Heath, D.; Smith, P.; Jago, R. Hyperplasia of the carotid body. J. Pathol. 1982, 138, 115–127. [Google Scholar] [CrossRef]
- Pequignot, J.M.; Hellström, S.; Johansson, C. Intact and sympathectomized carotid bodies of long-term hypoxic rats: A morphometric ultrastructural study. J. Neurocytol. 1984, 13, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Joseph, V.; Pequignot, J.M. Breathing at high altitude. Cell. Mol. Life Sci. 2009, 66, 3565–3573. [Google Scholar] [CrossRef]
- Lopez-Barneo, J.; Pardal, R.; Ortega-Sáenz, P. Cellular mechanism of oxygen sensing. Annu. Rev. Physiol. 2001, 63, 259–287. [Google Scholar] [CrossRef]
- Sobrino, V.; Annese, V.; Navarro-Guerrero, E.; Platero-Luengo, A.; Pardal, R. The carotid body: A physiologically relevant germinal niche in the adult peripheral nervous system. Cell. Mol. Life Sci. 2019, 76, 1027–1039. [Google Scholar] [CrossRef]
- Annese, V.; Navarro-Guerrero, E.; Rodríguez-Prieto, I.; Pardal, R. Physiological Plasticity of Neural-Crest-Derived Stem Cells in the Adult Mammalian Carotid Body. Cell Rep. 2017, 19, 471–478. [Google Scholar] [CrossRef] [Green Version]
- Navarro-Guerrero, E.; Platero-Luengo, A.; Linares-Clemente, P.; Cases, I.; López-Barneo, J.; Pardal, R. Gene Expression Profiling Supports the Neural Crest Origin of Adult Rodent Carotid Body Stem Cells and Identifies CD10 as a Marker for Mesectoderm-Committed Progenitors. Stem Cells 2016, 34, 1637–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Saenz, P.; Pardal, R.; Levitsky, K.; Villadiego, J.; Munoz-Manchado, A.B.; Duran, R.; Bonilla-Henao, V.; Arias-Mayenco, I.; Sobrino, V.; Ordonez, A.; et al. Cellular properties and chemosensory responses of the human carotid body. J. Physiol. 2013, 591, 6157–6173. [Google Scholar] [CrossRef]
- Sobrino, V.; González-Rodríguez, P.; Annese, V.; López-Barneo, J.; Pardal, R. Fast neurogenesis from carotid body quiescent neuroblasts accelerates adaptation to hypoxia. EMBO Rep. 2018, 19. [Google Scholar] [CrossRef]
- Sobrino, V.; Annese, V.; Pardal, R. Progenitor Cell Heterogeneity in the Adult Carotid Body Germinal Niche. Adv. Exp. Med. Biol. 2019, 1123, 19–38. [Google Scholar] [CrossRef]
- Fielding, J.W.; Hodson, E.J.; Cheng, X.; Ferguson, D.J.P.; Eckardt, L.; Adam, J.; Lip, P.; Maton-Howarth, M.; Ratnayaka, I.; Pugh, C.W.; et al. PHD2 inactivation in Type I cells drives HIF-2α-dependent multilineage hyperplasia and the formation of paraganglioma-like carotid bodies. J. Physiol. 2018, 596, 4393–4412. [Google Scholar] [CrossRef]
- Platero-Luengo, A.; González-Granero, S.; Durán, R.; Díaz-Castro, B.; Piruat, J.I.; García-Verdugo, J.M.; Pardal, R.; López-Barneo, J. An O2-sensitive glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell 2014, 156, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Pardal, R.; Ludewig, U.; Garcia-Hirschfeld, J.; Lopez-Barneo, J. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc. Natl. Acad. Sci. USA 2000, 97, 2361–2366. [Google Scholar] [CrossRef] [Green Version]
- Alcayaga, J.; Cerpa, V.; Retamal, M.; Arroyo, J.; Iturriaga, R.; Zapata, P. Adenosine triphosphate-induced peripheral nerve discharges generated from the cat petrosal ganglion in vitro. Neurosci. Lett. 2000, 282, 185–188. [Google Scholar] [CrossRef]
- Zhang, M.; Zhong, H.; Vollmer, C.; Nurse, C.A. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J. Physiol. 2000, 525 Pt 1, 143–158. [Google Scholar] [CrossRef]
- Leonard, E.M.; Salman, S.; Nurse, C.A. Sensory Processing and Integration at the Carotid Body Tripartite Synapse: Neurotransmitter Functions and Effects of Chronic Hypoxia. Front. Physiol. 2018, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Shirahata, M.; Balbir, A.; Otsubo, T.; Fitzgerald, R.S. Role of acetylcholine in neurotransmission of the carotid body. Respir. Physiol. Neurobiol. 2007, 157, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Vollmer, C.; Nurse, C.A. Adenosine and dopamine oppositely modulate a hyperpolarization-activated current I(h) in chemosensory neurons of the rat carotid body in co-culture. J. Physiol. 2018, 596, 3101–3117. [Google Scholar] [CrossRef] [Green Version]
- Benot, A.R.; López-Barneo, J. Feedback Inhibition of Ca2+ Currents by Dopamine in Glomus Cells of the Carotid Body. Eur. J. Neurosci. 1990, 2, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, L.; Dinger, B.; Stensaas, L.; Fidone, S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L1314–L1323. [Google Scholar] [CrossRef]
- Koyama, Y.; Michinaga, S. Regulations of astrocytic functions by endothelins: Roles in the pathophysiological responses of damaged brains. J. Pharmacol. Sci. 2012, 118, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Bonano, M.; Tríbulo, C.; De Calisto, J.; Marchant, L.; Sánchez, S.S.; Mayor, R.; Aybar, M.J. A new role for the Endothelin-1/Endothelin-A receptor signaling during early neural crest specification. Dev. Biol. 2008, 323, 114–129. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.K.; Levorse, J.M.; Ingram, R.S.; Tilghman, S.M. The temporal requirement for endothelin receptor-B signalling during neural crest development. Nature 1999, 402, 496–501. [Google Scholar] [CrossRef]
- Elton, T.S.; Oparil, S.; Taylor, G.R.; Hicks, P.H.; Yang, R.H.; Jin, H.; Chen, Y.F. Normobaric hypoxia stimulates endothelin-1 gene expression in the rat. Am. J. Physiol. 1992, 263, R1260–R1264. [Google Scholar] [CrossRef]
- Camenisch, G.; Stroka, D.M.; Gassmann, M.; Wenger, R.H. Attenuation of HIF-1 DNA-binding activity limits hypoxia-inducible endothelin-1 expression. Pflug. Arch. 2001, 443, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Ureña, J.; López-López, J.; González, C.; López-Barneo, J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J. Gen. Physiol. 1989, 93, 979–999. [Google Scholar] [CrossRef]
- Xu, J.; Tse, F.W.; Tse, A. ATP triggers intracellular Ca2+ release in type II cells of the rat carotid body. J. Physiol. 2003, 549, 739–747. [Google Scholar] [CrossRef]
- Murali, S.; Zhang, M.; Nurse, C.A. Angiotensin II mobilizes intracellular calcium and activates pannexin-1 channels in rat carotid body type II cells via AT1 receptors. J. Physiol. 2014, 592, 4747–4762. [Google Scholar] [CrossRef]
- Tse, A.; Yan, L.; Lee, A.K.; Tse, F.W. Autocrine and paracrine actions of ATP in rat carotid body. Can. J. Physiol. Pharmacol. 2012, 90, 705–711. [Google Scholar] [CrossRef]
- Murali, S.; Nurse, C.A. Purinergic signalling mediates bidirectional crosstalk between chemoreceptor type I and glial-like type II cells of the rat carotid body. J. Physiol. 2016, 594, 391–406. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Piskuric, N.A.; Vollmer, C.; Nurse, C.A. P2Y2 receptor activation opens pannexin-1 channels in rat carotid body type II cells: Potential role in amplifying the neurotransmitter ATP. J. Physiol. 2012, 590, 4335–4350. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.Y.; Tipoe, G.L.; Fung, M.L. Upregulation of erythropoietin and its receptor expression in the rat carotid body during chronic and intermittent hypoxia. Adv. Exp. Med. Biol. 2009, 648, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; He, L.; Liu, X.; Dinger, B.; Stensaas, L.; Fidone, S. Effect of the endothelin receptor antagonist bosentan on chronic hypoxia-induced morphological and physiological changes in rat carotid body. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L1257–L1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paciga, M.; Vollmer, C.; Nurse, C. Role of ET-1 in hypoxia-induced mitosis of cultured rat carotid body chemoreceptors. Neuroreport 1999, 10, 3739–3744. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Olson, E.B., Jr.; Bjorling, D.E.; Mitchell, G.S.; Bisgard, G.E. Sustained hypoxia-induced proliferation of carotid body type I cells in rats. J. Appl. Physiol. 2008, 104, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.L.; Yates, R.D. Two types of glomus cell in the rat carotid body as revealed by alpha-bungarotoxin binding. J. Neurocytol. 1984, 13, 281–302. [Google Scholar] [CrossRef]
- McDonald, D.M.; Mitchell, R.A. The innervation of glomus cells, ganglion cells and blood vessels in the rat carotid body: A quantitative ultrastructural analysis. J. Neurocytol. 1975, 4, 177–230. [Google Scholar] [CrossRef]
- Conde, S.V.; Monteiro, E.C.; Rigual, R.; Obeso, A.; Gonzalez, C. Hypoxic intensity: A determinant for the contribution of ATP and adenosine to the genesis of carotid body chemosensory activity. J. Appl. Physiol. 2012, 112, 2002–2010. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Xu, J.; Tse, F.W.; Tse, A. Adenosine stimulates depolarization and rise in cytoplasmic [Ca2+] in type I cells of rat carotid bodies. Am. J. Physiol. Cell Physiol. 2006, 290, C1592–C1598. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Clarke, K.; Zhong, H.; Vollmer, C.; Nurse, C.A. Postsynaptic action of GABA in modulating sensory transmission in co-cultures of rat carotid body via GABA(A) receptors. J. Physiol. 2009, 587, 329–344. [Google Scholar] [CrossRef]
- Gao, L.; Ortega-Sáenz, P.; García-Fernández, M.; González-Rodríguez, P.; Caballero-Eraso, C.; López-Barneo, J. Glucose sensing by carotid body glomus cells: Potential implications in disease. Front. Physiol. 2014, 5, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, J.F.; Sobotka, P.A.; Fudim, M.; Engelman, Z.J.; Hart, E.C.; McBryde, F.D.; Abdala, A.P.; Marina, N.; Gourine, A.V.; Lobo, M.; et al. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 2013, 61, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Cramer, J.A.; Wiggins, R.H.; Fudim, M.; Engelman, Z.J.; Sobotka, P.A.; Shah, L.M. Carotid body size on CTA: Correlation with comorbidities. Clin. Radiol. 2014, 69, e33–e36. [Google Scholar] [CrossRef]
- McBryde, F.D.; Abdala, A.P.; Hendy, E.B.; Pijacka, W.; Marvar, P.; Moraes, D.J.; Sobotka, P.A.; Paton, J.F. The carotid body as a putative therapeutic target for the treatment of neurogenic hypertension. Nat. Commun. 2013, 4, 2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, R.; Marcus, N.J.; Schultz, H.D. Carotid chemoreceptor ablation improves survival in heart failure: Rescuing autonomic control of cardiorespiratory function. J. Am. Coll. Cardiol. 2013, 62, 2422–2430. [Google Scholar] [CrossRef] [Green Version]
- Narkiewicz, K.; Ratcliffe, L.E.; Hart, E.C.; Briant, L.J.; Chrostowska, M.; Wolf, J.; Szyndler, A.; Hering, D.; Abdala, A.P.; Manghat, N.; et al. Unilateral Carotid Body Resection in Resistant Hypertension: A Safety and Feasibility Trial. JACC Basic Transl. Sci. 2016, 1, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.J.; Sacramento, J.F.; Gonzalez, C.; Guarino, M.P.; Monteiro, E.C.; Conde, S.V. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 2013, 62, 2905–2916. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobrino, V.; Platero-Luengo, A.; Annese, V.; Navarro-Guerrero, E.; González-Rodríguez, P.; López-Barneo, J.; Pardal, R. Neurotransmitter Modulation of Carotid Body Germinal Niche. Int. J. Mol. Sci. 2020, 21, 8231. https://doi.org/10.3390/ijms21218231
Sobrino V, Platero-Luengo A, Annese V, Navarro-Guerrero E, González-Rodríguez P, López-Barneo J, Pardal R. Neurotransmitter Modulation of Carotid Body Germinal Niche. International Journal of Molecular Sciences. 2020; 21(21):8231. https://doi.org/10.3390/ijms21218231
Chicago/Turabian StyleSobrino, Verónica, Aida Platero-Luengo, Valentina Annese, Elena Navarro-Guerrero, Patricia González-Rodríguez, José López-Barneo, and Ricardo Pardal. 2020. "Neurotransmitter Modulation of Carotid Body Germinal Niche" International Journal of Molecular Sciences 21, no. 21: 8231. https://doi.org/10.3390/ijms21218231
APA StyleSobrino, V., Platero-Luengo, A., Annese, V., Navarro-Guerrero, E., González-Rodríguez, P., López-Barneo, J., & Pardal, R. (2020). Neurotransmitter Modulation of Carotid Body Germinal Niche. International Journal of Molecular Sciences, 21(21), 8231. https://doi.org/10.3390/ijms21218231




