Implications of Splicing Alterations in the Onset and Phenotypic Variability of a Family with Subclinical Manifestation of Peutz–Jeghers Syndrome: Bioinformatic and Molecular Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Genetic Analysis
2.3. STK11 mRNA Qualitative and Quantitative Analysis
2.4. In Silico Sequence Analysis
2.5. Statistical Analysis
3. Results
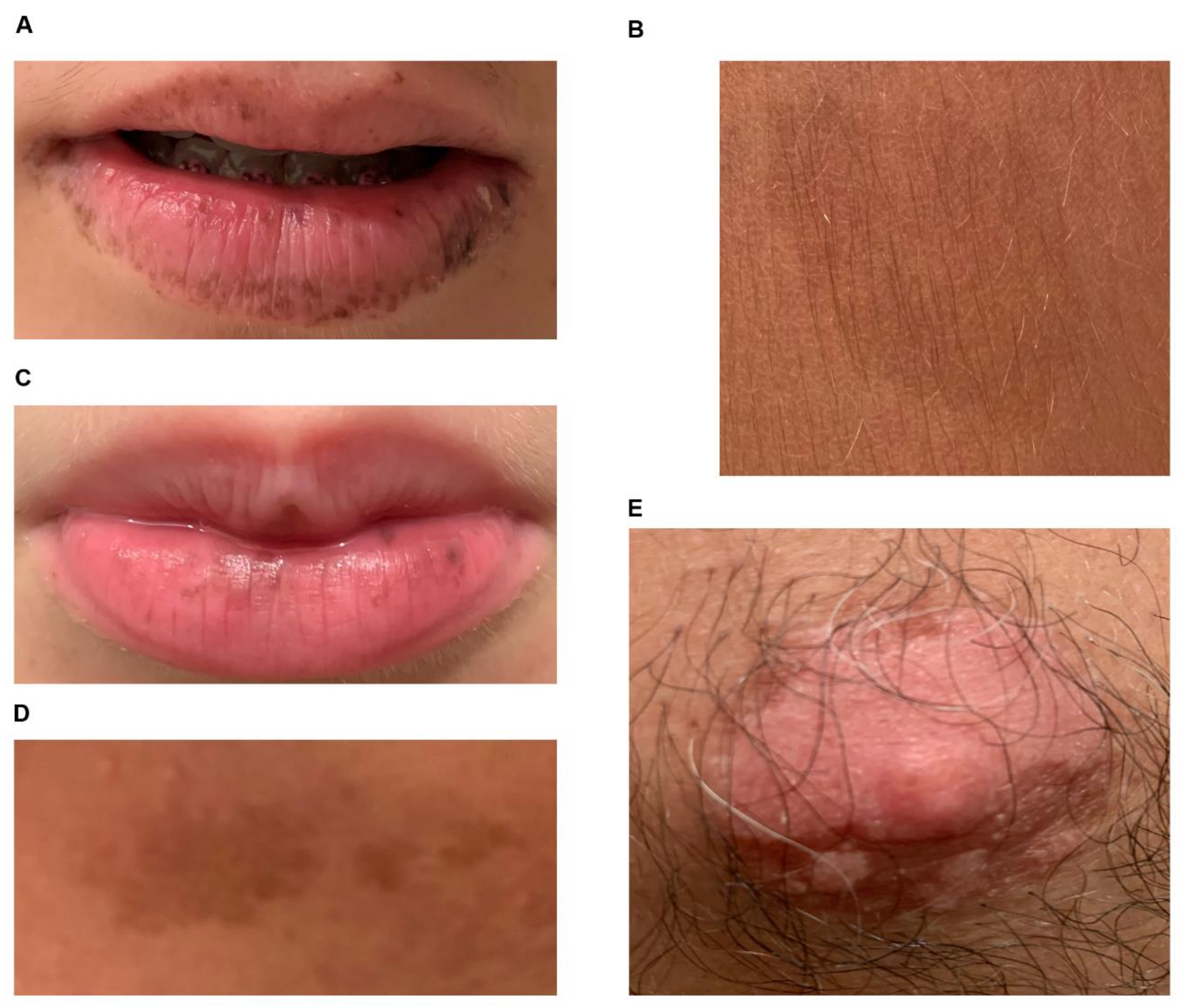
3.1. DNA Analysis
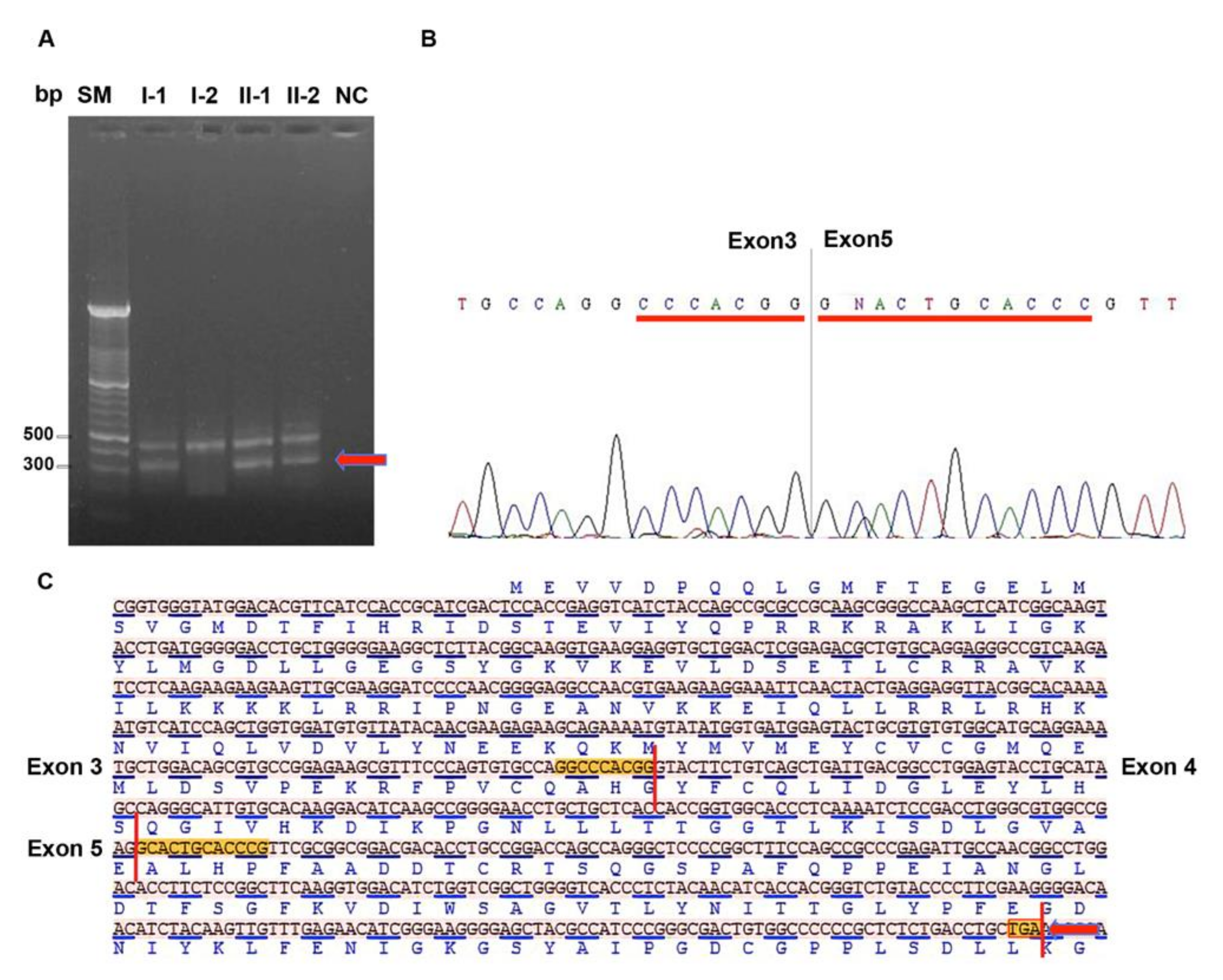
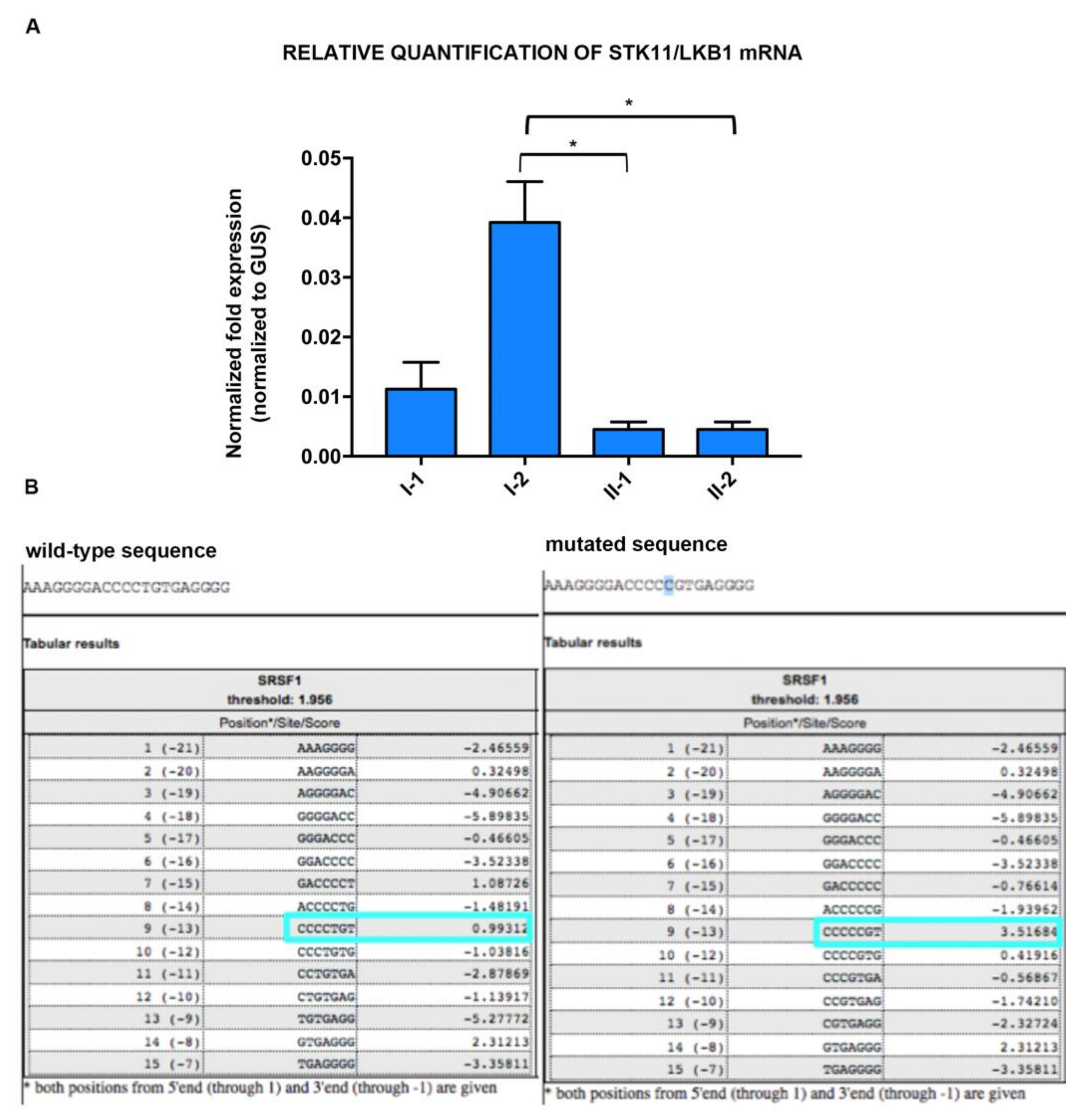
3.2. STK11 Isoforms Characterization and Expression Analysis
3.3. In Silico Analysis of STK11 Splicing Sequence
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Availability of Data and Materials
Ethics Approval and Consent to Participate
Patient Consent for Publication
References
- Tchekmedyian, A.; Amos, C.I.; Bale, S.J.; Zhu, D.; Arold, S.; Berrueta, J.; Nabon, N.; McGarrity, T. Findings From the Peutz–Jeghers Syndrome Registry of Uruguay. PLoS ONE 2013. [Google Scholar] [CrossRef]
- Meserve, E.E.K.; Nucci, M.R. Peutz–Jeghers Syndrome: Pathobiology, Pathologic Manifestations, and Suggestions for Recommending Genetic Testing in Pathology Reports. Surg. Pathol. Clin. 2016, 9, 243–268. [Google Scholar] [CrossRef] [PubMed]
- Sado, T.; Nakayama, Y.; Kato, S.; Homma, H.; Kusakari, M.; Hidaka, N.; Gomi, S.; Takamizawa, S.; Kosho, T.; Saito, S.; et al. Extremely Young Case of Small Bowel Intussusception Due to Peutz–Jeghers Syndrome with Nonsense Mutation of STK11. Clin. J. Gastroenterol. 2019, 12, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Jin, X.W.; Li, B.R.; Zhu, M.; Li, J.; Mao, G.P.; Zhang, Y.F.; Ning, S.B. Cancer Risk in Patients with Peutz–Jeghers Syndrome: A Retrospective Cohort Study of 336 Cases. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Tavusbay, C.; Acar, T.; Kar, H.; Kemal, A.; Kamer, E. The Patients with Peutz–Jeghers Syndrome Have a High Risk of Developing Cancer. Turk. J. Surg. 2018, 3, 162–164. [Google Scholar] [CrossRef]
- Nevozinskaya, Z.; Korsunskaya, I.; Sakaniya, L.; Perlamutrov, Y.; Sobolev, V. Peutz–Jeghers Syndrome in Dermatology. Acta Dermatovenerol. Alp. Pannonica Adriat. 2019, 28, 135–137. [Google Scholar] [CrossRef]
- Jenne, D.E.; Reimann, H.; Nezu, J.; Friedel, W.; Loffi, S.; Jeschke, R.; Mtiller, O.; Back, W.; Zimmer, M. Peutz–Jeghers Syndrome Is Caused by Mutations in a Novel Serine Threonine Kinase. Nat. Genet. 1998, 18, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Mehenni, H.; Gehrig, C.; Nezu, J.; Oku, A.; Shimane, M.; Rossier, C.; Guex, N.; Blouin, J.L.; Scott, H.S.; Antonarakis, S.E. Loss of LKB1 Kinase Activity in Peutz–Jeghers Syndrome, and Evidence for Allelic and Locus Heterogeneity. Am. J. Hum. Genet. 1998, 63, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, J.M.; Göransson, O.; Toth, R.; Deak, M.; Morrice, N.A.; Boudeau, J.; Hawley, S.A.; Udd, L.; Mäkelä, T.P.; Hardie, D.G.; et al. LKB1 Is a Master Kinase That Activates 13 Kinases of the AMPK Subfamily, Including MARK/PAR-1. EMBO J. 2004, 23, 833–843. [Google Scholar] [CrossRef]
- Orellana, P.; López-Köstner, F.; Heine, C.; Suazo, C.; Pinto, E.; Church, J.; Carvallo, P.; Alvarez, K. Large Deletions and Splicing-Site Mutations in the STK11 Gene in Peutz–Jeghers Chilean Families. Clin. Genet. 2013, 83, 365–369. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Wang, F.; Liu, C.; Lu, H.; Wan, H.; Wei, J.; Liu, J. One Novel Deletion and One Splicing Mutation of the LKB1 Gene in Two Chinese Patients with Peutz–Jeghers Syndrome. DNA Cell Biol. 2012, 31, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Xie, N.N.; Li, Q.Y.; Hu, Y.Q.; Ren, J.L.; Guleng, B. Exome Sequencing Revealed Novel Germline Mutations in Chinese Peutz–Jeghers Syndrome Patients. Dig. Dis. Sci. 2014, 59, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Zhao, Z.Y.; Li, B.R.; Wang, H.; Yu, E.D.; Ning, S.B. STK11 Gene Analysis Reveals a Significant Number of Splice Mutations in Chinese PJS Patients. Cancer Genet. 2019, 230, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.L.; Zhao, Z.Y.; Li, B.R.; Li, J.; Jin, X.W.; Yu, E.D.; Xu, X.D.; Ning, S.B. Early Screening the Small Bowel Is Key to Protect Peutz–Jeghers Syndrome Patients from Surgery: A Novel Mutation c.243delG in STK11 Gene. BMC Gastroenterol. 2019, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Galatola, M.; Quaglietta, L.; Miele, E.; De Palma, G.; Rossi, G.B.; Staiano, A.; Izzo, P. Alu-mediated Genomic Deletion of the Serine/Threonine Protein Kinase 11 (STK11) Gene in Peutz–Jeghers Syndrome. Gastroenterology 2010, 138, 2558–2560. [Google Scholar] [CrossRef]
- Amos, C.I.; Keitheri-Cheteri, M.B.; Sabripour, M.; Wei, C.; McGarrity, T.J.; Seldin, M.F.; Nations, L.; Lynch, P.M.; Fidder, H.H.; Friedman, E.; et al. Genotype-phenotype Correlations in Peutz–Jeghers Syndrome. J. Med. Genet. 2004, 41, 327–333. [Google Scholar] [CrossRef]
- Chiang, J.M.; Chen, T.C. Clinical Manifestations and STK11 Germline Mutations in Taiwanese Patients with Peutz–Jeghers Syndrome. Asian J. Surg. 2018, 41, 480–485. [Google Scholar] [CrossRef]
- Daniell, J.; Plazzer, J.P.; Perera, A.; Macrae, F. An Exploration of Genotype-Phenotype Link between Peutz–Jeghers Syndrome and STK11: A Review. Fam. Cancer 2018, 17, 421–427. [Google Scholar] [CrossRef]
- Zhang, Y.; Ke, Y.; Zheng, X.; Liu, Q.; Duan, X. Correlation between Genotype and Phenotype in Three Families with Peutz–Jeghers Syndrome. Exp. Ther. Med. 2017, 13, 507–514. [Google Scholar] [CrossRef]
- Kopacova, M.; Tacheci, I.; Rejchrt, S.; Bures, J. Peutz–Jeghers Syndrome: Diagnostic and Therapeutic Approach. World J. Gastroenterol. 2009, 15, 5397–5408. [Google Scholar] [CrossRef]
- Shen, N.; Li, D.; Zhu, Y.; Xie, H.; Lu, Y. Early Genetic Testing of STK11 Is Important for Management and Genetic Counseling for Peutz–Jeghers Syndrome. Dig. Liver Dis. 2019, 51, 1353–1355. [Google Scholar] [CrossRef]
- Miller, S.A.; Dykes, D.D.; Polesky, H.F. A Simple Salting Out Procedure for Extracting DNA from Human Nucleated Cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Turano, M.; Costabile, V.; Cerasuolo, A.; Duraturo, F.; Liccardo, R.; Delrio, P.; Pace, U.; Rega, D.; Dodaro, C.A.; Milone, M.; et al. Characterisation of Mesenchymal Colon Tumour-Derived Cells in Tumourspheres as a Model for Colorectal Cancer Progression. Int. J. Oncol. 2018, 53, 2379–2396. [Google Scholar] [CrossRef]
- Duraturo, F.; Liccardo, R.; Cavallo, A.; De Rosa, M.; Rossi, G.B.; Izzo, P. Multivariate Analysis as a Method for Evaluating the Pathogenicity of Novel Genetic MLH1 Variants in Patients with Colorectal Cancer and Microsatellite Instability. Int. J. Mol. Med. 2015, 36, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Galatola, M.; Paparo, L.; Duraturo, F.; Turano, M.; Rossi, G.B.; Izzo, P.; De Rosa, M. Beta Catenin and Cytokine Pathway Dysregulation in Patients with Manifestations of the “PTEN Hamartoma Tumor Syndrome”. BMC Med. Genet. 2012, 13, 28. [Google Scholar] [CrossRef]
- Smith, P.J.; Zhang, C.; Wang, J.; Zhang, M.Q.; Krainer, A.R. An Increased Specificity Score Matrix for the Prediction of SF2/ASF-specific Exonic Splicing Enhancers. Hum. Mol. Genet. 2006, 15, 2490–2508. [Google Scholar] [CrossRef]
- Cartegni, L.; Wang, J.; Zhu, Z.; Zhang, M.Q.; Krainer, A.R. ESEfinder: A Web Resource to Identify Exonic Splicing Enhancers. Nucleic Acids Res. 2003, 31, 3568–3571. [Google Scholar] [CrossRef]
- Desmet, F.O.; Hamroun, D.; Lalande, M.; Collod-Beroud, G.; Claustres, M.; Beroud, C. Human Splicing Finder: An Online Bioinformatics Tool to Predict Splicing Signals. Nucleic Acids Res. 2009, 37, e67. [Google Scholar] [CrossRef]
- De Rosa, M.; Galatola, M.; Borriello, S.; Duraturo, F.; Masone, S.; Izzo, P. Implication of Adenomatous Polyposis Coli and MUTYH Mutations in Familial Colorectal Polyposis. Dis. Colon Rectum 2009, 52, 268–274. [Google Scholar] [CrossRef]
- Achatz, M.I.; Porter, C.C.; Brugières, L.; Druker, H.; Frebourg, T.; Foulkes, W.D.; Kratz, C.P.; Kuiper, R.P.; Hansford, J.R.; Hernandez, H.S.; et al. Cancer Screening Recommendations and Clinical Management of Inherited Gastrointestinal Cancer Syndromes in Childhood. Clin. Cancer Res. 2017, 23, e107–e114. [Google Scholar] [CrossRef]
- Spoto, C.P.E.; Gullo, I.; Carneiro, F.; Montgomery, E.A.; Brosens, L.A.A. Hereditary Gastrointestinal Carcinomas and Their Precursors: An Algorithm for Genetic Testing. Semin. Diagn. Pathol. 2018, 35, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, R.G.; Cao, S.; Gao, Q.; Wendl, M.C.; Vo, N.S.; Reynolds, S.M.; Zhao, Y.; Gonzalez, H.G.; Chai, S.; Wang, F.; et al. Systematic Analysis of Splice-Site-Creating Mutations in Cancer. Cell Rep. 2018, 23, 270–281. [Google Scholar] [CrossRef]
- Abramowicz, A.; Gos, M. Splicing Mutations in Human Genetic Disorders: Examples, Detection, and Confirmation. J. Appl. Genet. 2018, 59, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Mechanistic Insights into Precursor Messenger RNA Splicing by the Spliceosome. Nat. Rev. Mol. Cell Biol. 2017, 18, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Rio, D.C. Mechanisms and Regulation of Alternative Pre-mRNA Splicing. Annu. Rev. Biochem. 2015, 84, 291–323. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Huang, B.O.; Xu, Y.M.; Li, J.; Huang, L.F.; Lin, J.; Zhang, J.; Min, Q.H.; Yang, W.M.; et al. Mechanism of Alternative Splicing and Its Regulation. Biomed. Rep. 2015, 3, 152–158. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Zhang, J. Distribution of Exonic Splicing Enhancer Elements in Human Genes. Genomics 2005, 3, 329–336. [Google Scholar] [CrossRef]
- Papp, J.; Kovacs, M.E.; Solyom, S.; Kasler, M.; Børresen-Dale, A.L.; Olah, E. High Prevalence of Germline STK11 Mutations in Hungarian Peutz–Jeghers Syndrome Patients. BMC Med. Genet. 2010, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Hosogi, H.; Nagayama, S.; Kawamura, J.; Koshiba, Y.; Nomura, A.; Itami, A.; Okabe, H.; Satoh, S.; Watanabe, G.; Sakai, Y. Molecular Insights Into Peutz–Jeghers Syndrome: Two Probands with a Germline Mutation of LKB1. J. Gastroenterol. 2008, 43, 492–497. [Google Scholar] [CrossRef]
- Abed, A.A.; Günther, K.; Kraus, C.; Ballhausen, W.G. Mutation Screening at the RNA Level of the STK11/LKB1 Gene in Peutz–Jeghers Syndrome Reveals Complex Splicing Abnormalities and a Novel mRNA Isoform (STK11 c.597(insertion mark)598insIVS4). Hum. Mutat. 2001, 18, 397–410. [Google Scholar] [CrossRef]
- Resta, N.; Stella, A.; Susca, F.C.; Di Giacomo, M.; Forleo, G.; Miccolis, I.; Rossini, F.P.; Genuardi, M.; Piepoli, A.; Grammatico, P.; et al. Two Novel Mutations and a New STK11/LKB1 Gene Isoform in Peutz–Jeghers Patients. Hum. Mutat. 2002, 20, 78–79. [Google Scholar] [CrossRef] [PubMed]

| Primers | Sequences (5′–3′) |
|---|---|
| 1FP | 5′-AACACAAGGAAGGACCGCTAC-3′ |
| 1RP | 5′-GACAGAACCATCAGCACCGTGAC-3′ |
| 2FP | 5′-CCTCCAGAGCCCCTTTTCT-3′ |
| 2RP | 5′-AAGGAGACGGGAAGAGGAC-3′ |
| 3aFP | 5′-CCTCCAGAGCCCCTTTTCT-3′ |
| 3aRP | 5′-ATCAGGACACAAGCAGTGTGGC-3′ |
| 3bFP | 5′-CCCCCTGAGCTGTGTGTC-3′ |
| 3bRP | 5′-AGTGTGGCCTCACGGAAA-3′ |
| 4FP | 5′-GTGTGCCTGGACTTCTGTGA-3′ |
| 5RP | 5′-GAGTGTGCGTGTGGTGAGTG-3′ |
| 6FP | 5′-AACCACCTTGACTGACCACGC-3′ |
| 6RP | 5′-GACACACCCCAACCCTACATTTCTG-3′ |
| 7FP | 5′-CGCCCCAGGGGGAATCCTC-3′ |
| 7RP | 5′-CTAGCGCCCGCTCAACCAG-3′ |
| 8FP | 5′-GGAGCTGGGTCGGAAAACTGGA-3′ |
| 8RP | 5′-TGCTCCCGTGGGACATCCTG-3′ |
| 9aFP | 5′-GTAAGTGCGTCCCCGTGGTG-3′ |
| 9aRP | 5′-CGGTCACCATGACTGACTAGC-3′ |
| 9bFP | 5′-CCTGTGGCTCTGGGGTTGC-3′ |
| 9bRP | 5′-CACGGCTGGCTGTGGCATC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerasuolo, A.; Cammarota, F.; Duraturo, F.; Staiano, A.; Martinelli, M.; Miele, E.; Izzo, P.; De Rosa, M. Implications of Splicing Alterations in the Onset and Phenotypic Variability of a Family with Subclinical Manifestation of Peutz–Jeghers Syndrome: Bioinformatic and Molecular Evidence. Int. J. Mol. Sci. 2020, 21, 8201. https://doi.org/10.3390/ijms21218201
Cerasuolo A, Cammarota F, Duraturo F, Staiano A, Martinelli M, Miele E, Izzo P, De Rosa M. Implications of Splicing Alterations in the Onset and Phenotypic Variability of a Family with Subclinical Manifestation of Peutz–Jeghers Syndrome: Bioinformatic and Molecular Evidence. International Journal of Molecular Sciences. 2020; 21(21):8201. https://doi.org/10.3390/ijms21218201
Chicago/Turabian StyleCerasuolo, Andrea, Francesca Cammarota, Francesca Duraturo, Annamaria Staiano, Massimo Martinelli, Erasmo Miele, Paola Izzo, and Marina De Rosa. 2020. "Implications of Splicing Alterations in the Onset and Phenotypic Variability of a Family with Subclinical Manifestation of Peutz–Jeghers Syndrome: Bioinformatic and Molecular Evidence" International Journal of Molecular Sciences 21, no. 21: 8201. https://doi.org/10.3390/ijms21218201
APA StyleCerasuolo, A., Cammarota, F., Duraturo, F., Staiano, A., Martinelli, M., Miele, E., Izzo, P., & De Rosa, M. (2020). Implications of Splicing Alterations in the Onset and Phenotypic Variability of a Family with Subclinical Manifestation of Peutz–Jeghers Syndrome: Bioinformatic and Molecular Evidence. International Journal of Molecular Sciences, 21(21), 8201. https://doi.org/10.3390/ijms21218201






