Monoclonal Antibodies to Treat Multiple Myeloma: A Dream Come True
Abstract
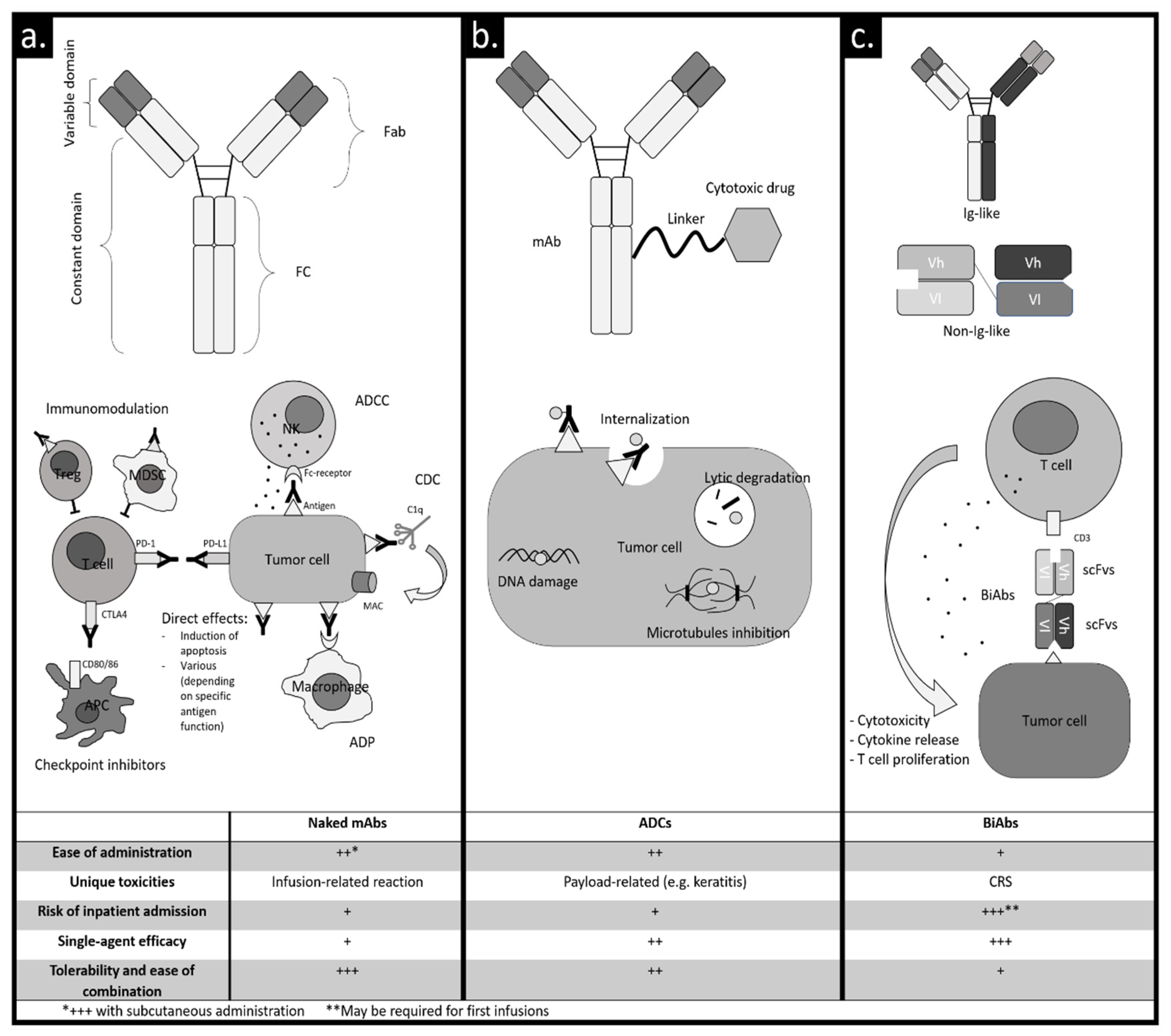
:1. Introduction
2. Naked Monoclonal Antibodies
3. ADCs
4. BiAbs
5. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADC | antibody–drug conjugate |
| ADCC | antibody-dependent cellular cytotoxicity |
| ADP | antibody-dependent phagocytosis |
| APC | antigen-presenting cell |
| BCMA | B-cell maturation antigen |
| belamaf | belantamab mafodotin |
| BiAb | bispecific antibody |
| BiTE | bispecific T-cell engager |
| CDC | complement-dependent cytotoxicity |
| CRS | cytokine release syndrome |
| CTLA-4 | cytotoxic T-lymphocyte antigen 4 |
| d, dex | dexamethasone |
| Dara | daratumumab |
| di-scFv | bivalent single-chain variable fragment |
| Elo | elotuzumab |
| EMA | European Medicines Agency |
| Fab | fragment antigen binding |
| Fc | fragment crystallizable region |
| FDA | US Food and Drug Administration |
| G | grade |
| Ig | immunoglobulin |
| IMiDs | immunomodulatory drugs |
| IRR | infusion-related reaction |
| Isa | isatuximab |
| iv | intravenous |
| K | carfilzomib |
| mAb | monoclonal antibody |
| MAC | membrane attack complex |
| MDSC | myeloid-derived suppressor cell |
| MM | multiple myeloma |
| MMAF | monomethyl auristatin F |
| MRD | minimal residual disease |
| NA | not available |
| NDMM | in newly diagnosed (ND) MM |
| NK | natural killer cell |
| NR | not reached |
| NTE | non-transplant-eligible |
| ORR | overall response rate |
| OS | overall survival |
| P, Poma | pomalidomide |
| PD-1 | programmed cell death protein 1 |
| PD-L1 | programmed death ligand 1 |
| PFS | progression-free survival |
| PI | proteasome inhibitor |
| R | lenalidomide |
| RRMM | relapsed/refractory multiple myeloma |
| sc | subcutaneous |
| scFv | single-chain variable fragment |
| sCR | stringent complete response |
| SLAMF7 | signaling lymphocytic activation molecule family 7 |
| T | thalidomide |
| TE | transplant-eligible |
| Treg | regulatory T cell |
| V | bortezomib |
| VMP | bortezomib-melphalan-prednisone |
References
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- D’agostino, M.; Bertamini, L.; Oliva, S.; Boccadoro, M.; Gay, F. Pursuing a curative approach in multiple myeloma: A review of new therapeutic strategies. Cancers 2019, 11, 2015. [Google Scholar] [CrossRef] [Green Version]
- Gay, F.; D’Agostino, M.; Giaccone, L.; Genuardi, M.; Festuccia, M.; Boccadoro, M.; Bruno, B. Immuno-oncologic Approaches: CAR-T Cells and Checkpoint Inhibitors. Clin. Lymphoma Myeloma Leuk. 2017, 17, 471–478. [Google Scholar] [CrossRef]
- D’Agostino, M.; Boccadoro, M.; Smith, E.L. Novel Immunotherapies for Multiple Myeloma. Curr. Hematol. Malig. Rep. 2017, 12, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buqué, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, M.; Raje, N. Anti-BCMA CAR T-cell therapy in multiple myeloma: Can we do better? Leukemia 2020, 34, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Morgan, B.P. Regulation of the complement membrane attack pathway. Crit. Rev. Immunol. 1999, 19, 173–198. [Google Scholar] [CrossRef]
- Wang, W.; Erbe, A.K.; Hank, J.A.; Morris, Z.S.; Sondel, P.M. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 2015, 6, 368. [Google Scholar] [CrossRef] [Green Version]
- Meyer, S.; Leusen, J.H.W.; Boross, P. Regulation of complement and modulation of its activity in monoclonal antibody therapy of cancer. MAbs 2014, 6, 1133–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gül, N.; Babes, L.; Siegmund, K.; Korthouwer, R.; Bögels, M.; Braster, R.; Vidarsson, G.; Ten Hagen, T.L.M.; Kubes, P.; Van Egmond, M. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Investig. 2014, 124, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Overdijk, M.B.; Jansen, J.H.M.; Nederend, M.; Lammerts van Bueren, J.J.; Groen, R.W.J.; Parren, P.W.H.I.; Leusen, J.H.W.; Boross, P. The Therapeutic CD38 Monoclonal Antibody Daratumumab Induces Programmed Cell Death via Fcγ Receptor–Mediated Cross-Linking. J. Immunol. 2016, 197, 807–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammerts van Bueren, J.; Jakobs, D.; Kaldenhoven, N.; Roza, M.; Hiddingh, S.; Meesters, J.; Voorhorst, M.; Gresnigt, E.; Wiegman, L.; Ortiz Buijsse, A.; et al. Direct In Vitro Comparison of Daratumumab with Surrogate Analogs of CD38 Antibodies MOR03087, SAR650984 and Ab79. Blood 2014, 124. Abstract #3474 [ASH 2014 56th Meeting]. [Google Scholar] [CrossRef]
- Oliva, S.; Troia, R.; D’Agostino, M.; Boccadoro, M.; Gay, F. Promises and pitfalls in the use of PD-1/PD-L1 inhibitors in multiple myeloma. Front. Immunol. 2018, 9, 2749. [Google Scholar] [CrossRef]
- D’Agostino, M.; Gazzera, G.; Cetani, G.; Bringhen, S.; Boccadoro, M.; Gay, F. Clinical and pharmacologic features of monoclonal antibodies and checkpoint blockade therapy in multiple myeloma. Curr. Med. Chem. 2019, 26, 5968–5981. [Google Scholar] [CrossRef]
- Bonello, F.; D’Agostino, M.; Moscvin, M.; Cerrato, C.; Boccadoro, M.; Gay, F. CD38 as an immunotherapeutic target in multiple myeloma. Expert Opin. Biol. 2018, 18, 1209–1221. [Google Scholar] [CrossRef]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.C.J.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, H.; Viskov, C.; Garcia-Echeverria, C. Antibody-drug conjugates—A new wave of cancer drugs. Bioorg. Med. Chem. Lett. 2014, 24, 5357–5363. [Google Scholar] [CrossRef] [Green Version]
- Skaletskaya, A.; Setiady, Y.Y.; Park, P.U.; Lutz, R.J. Lorvotuzumab mertansine (IMGN901) immune effector activity and its effect on human NK cells. Cancer Res. 2011, 71. Abstract #770 [AACR 2011 102nd Annual Meeting]. [Google Scholar]
- Fan, G.; Wang, Z.; Hao, M.; Li, J. Bispecific antibodies and their applications. J. Hematol. Oncol. 2015, 8, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.M.; Hamid, M. ScFv antibody: Principles and clinical application. Clin. Dev. Immunol. 2012, 2012, 980250. [Google Scholar] [CrossRef] [PubMed]
- Lokhorst, H.M.; Plesner, T.; Laubach, J.P.; Nahi, H.; Gimsing, P.; Hansson, M.; Minnema, M.C.; Lassen, U.; Krejcik, J.; Palumbo, A.; et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N. Engl. J. Med. 2015, 373, 1207–1219. [Google Scholar] [CrossRef]
- Lonial, S.; Weiss, B.M.; Usmani, S.Z.; Singhal, S.; Chari, A.; Bahlis, N.J.; Belch, A.; Krishnan, A.; Vescio, R.A.; Mateos, M.V.; et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 2016, 387, 1551–1560. [Google Scholar] [CrossRef]
- Chari, A.; Suvannasankha, A.; Fay, J.W.; Arnulf, B.; Kaufman, J.L.; Ifthikharuddin, J.J.; Weiss, B.M.; Krishnan, A.; Lentzsch, S.; Comenzo, R.; et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017, 130, 974–981. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Nahi, H.; Mateos, M.V.; van de Donk, N.W.C.J.; Chari, A.; Kaufman, J.L.; Moreau, P.; Oriol, A.; Plesner, T.; Benboubker, L.; et al. Subcutaneous delivery of daratumumab in relapsed or refractory multiple myeloma. Blood 2019, 134, 668–677. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.; Baz, R.; Benson, D.M.; Lendvai, N.; Wolf, J.; Munster, P.; Lesokhin, A.M.; Wack, C.; Charpentier, E.; Campana, F.; et al. A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood 2017, 129, 3294–3303. [Google Scholar] [CrossRef] [Green Version]
- Mikhael, J.; Richardson, P.; Usmani, S.Z.; Raje, N.; Bensinger, W.; Karanes, C.; Campana, F.; Kanagavel, D.; Dubin, F.; Liu, Q.; et al. A phase 1b study of isatuximab plus pomalidomide/dexamethasone in relapsed/refractory multiple myeloma. Blood 2019, 134, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Weisel, K.; Asemissen, A.M.; Besemer, B.; Haenel, M.; Blau, I.W.; Goerner, M.; Ko, Y.-D.; Dürig, J.; Staib, P.; Mann, C.; et al. Depth of response to isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in front-line treatment of high-risk multiple myeloma: Interim analysis of the GMMG-CONCEPT trial. J. Clin. Oncol. 2020, 38. Abstract #8508 [ASCO 2020 Annual Meeting]. [Google Scholar] [CrossRef]
- Raab, M.S.; Engelhardt, M.; Blank, A.; Goldschmidt, H.; Agis, H.; Blau, I.W.; Einsele, H.; Ferstl, B.; Schub, N.; Röllig, C.; et al. MOR202, a novel anti-CD38 monoclonal antibody, in patients with relapsed or refractory multiple myeloma: A first-in-human, multicentre, phase 1–2a trial. Lancet Haematol. 2020, 7, e381–e394. [Google Scholar] [CrossRef]
- Krishnan, A.Y.; Patel, K.K.; Hari, P.; Jagannath, S.; Niesvizky, R.; Silbermann, R.W.; Berg, D.T.; Li, Q.; Allikmets, K.; Stockerl-Goldstein, K. A phase Ib study of TAK-079, an investigational anti-CD38 monoclonal antibody (mAb) in patients with relapsed/ refractory multiple myeloma (RRMM): Preliminary results. J. Clin. Oncol. 2020, 38. Abstract #8539 [ASCO 2020 Annual Meeting]. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef]
- Mateos, M.V.; Orlowski, R.Z.; Ocio, E.M.; Rodríguez-Otero, P.; Reece, D.; Moreau, P.; Munshi, N.; Avigan, D.E.; Siegel, D.S.; Ghori, R.; et al. Pembrolizumab combined with lenalidomide and low-dose dexamethasone for relapsed or refractory multiple myeloma: Phase I KEYNOTE-023 study. Br. J. Haematol. 2019, 186, e117–e121. [Google Scholar] [CrossRef] [Green Version]
- Badros, A.; Hyjek, E.; Ma, N.; Lesokhin, A.; Dogan, A.; Rapoport, A.P.; Kocoglu, M.; Lederer, E.; Philip, S.; Milliron, T.; et al. Pembrolizumab, pomalidomide, and low-dose dexamethasone for relapsed/refractory multiple myeloma. Blood 2017, 130, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Anderson, L.D.; Sutherland, H.J.; Yong, K.; et al. Targeting B-cell maturation antigen with GSK2857916 antibody–drug conjugate in relapsed or refractory multiple myeloma (BMA117159): A dose escalation and expansion phase 1 trial. Lancet Oncol. 2018, 19, 1641–1653. [Google Scholar] [CrossRef]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody–drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.-O.A.; Callander, N.S.; Sborov, D.W.; Suvannasankha, A.; et al. Pivotal DREAMM-2 study: Single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma (RRMM) refractory to proteasome inhibitors (PIs), immunomodulatory agents, and refractory and/or intolerant to anti-CD38 monoclonal antibodies (mAbs). J. Clin. Oncol. 2020, 38. Abstract #8536 [ASCO 2020 Annual Meeting]. [Google Scholar]
- Cohen, A.D.; Trudel, S.; Lonial, S.; Libby, E.N.; Lee, H.C.; Besemer, B.; Facon, T.; Nooka, A.K.; Callander, N.S.; Chari, A.; et al. DREAMM-2: Single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma (RRMM) and high-risk (HR) cytogenetics. J. Clin. Oncol. 2020, 38. Abstract #8541 [ASCO 2020 Annual Meeting]. [Google Scholar] [CrossRef]
- Lee, H.C.; Cohen, A.D.; Chari, A.; Hultcrantz, M.; Nooka, A.K.; Callander, N.S.; Suvannasankha, A.; Badros, A.; Libby, E.N.; Trudel, S.; et al. DREAMM-2: Single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma (RRMM) and renal impairment. J. Clin. Oncol. 2020, 38. Abstract #8519 [ASCO 2020 Annual Meeting]. [Google Scholar] [CrossRef]
- Nooka, A.K.; Stockerl-Goldstein, K.; Quach, H.; Forbes, A.; Mateos, M.-V.; Khot, A.; Tan, A.; Abonour, R.; Chopra, B.; Rogers, R.; et al. DREAMM-6: Safety and tolerability of belantamab mafodotin in combination with bortezomib/dexamethasone in relapsed/refractory multiple myeloma (RRMM). J. Clin. Oncol. 2020, 38. Abstract #8502 [ASCO 2020 Annual Meeting]. [Google Scholar] [CrossRef]
- Topp, M.S.; Duell, J.; Zugmaier, G.; Attal, M.; Moreau, P.; Langer, C.; Krönke, J.; Facon, T.; Salnikov, A.V.; Lesley, R.; et al. Anti-B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J. Clin. Oncol. 2020, 38, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.S.; Jakubowiak, A.; Gasparetto, C.; Cornell, R.F.; Krupka, H.I.; Navarro, D.; Forgie, A.J.; Udata, C.; Basu, C.; Chou, J.; et al. Safety, Clinical Activity, Pharmacokinetics, and Pharmacodynamics from a Phase I Study of PF-06863135, a B-Cell Maturation Antigen (BCMA)-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2019, 134. Abstract #1869 [ASH 2019 61st Meeting]. [Google Scholar]
- Costa, L.J.; Wong, S.W.; Bermúdez, A.; de la Rubia, J.; Mateos, M.V.; Ocio, E.M.; Rodríguez-Otero, P.; San-Miguel, J.; Li, S.; Sarmiento, R.; et al. Interim results from the first phase 1 clinical study of the b-cell maturation antigen (BCMA) 2+1 T cell engager (TCE) cc-93269 in patients (PTS) with relapsed/refractory multiple myeloma (RRMM). HemaSphere 2020, 4, 59, [Abstract #S205, EHA 2020 25th Congress]. [Google Scholar]
- Usmani, S.Z.; Mateos, M.-V.; Nahi, H.; Krishnan, A.Y.; van de Donk, N.W.C.J.; San-Miguel, J.; Oriol, A.; Rosiñol, L.; Chari, A.; Adams, H.; et al. Phase I study of teclistamab, a humanized B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in relapsed/refractory multiple myeloma (R/R MM). J. Clin. Oncol. 2020, 38. Abstract #100 [ASCO 2020 Annual Meeting]. [Google Scholar] [CrossRef]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, M.; Quach, H.; Mateos, M.V.; Landgren, O.; Leleu, X.; Siegel, D.; Weisel, K.; Yang, H.; Klippel, Z.; Zahlten-Kumeli, A.; et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet 2020, 396, 186–197. [Google Scholar] [CrossRef]
- Mateos, M.V.; Cavo, M.; Blade, J.; Dimopoulos, M.A.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): A randomised, open-label, phase 3 trial. Lancet 2020, 395, 132–141. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Mateos, M.V.; Nahi, H.; Legiec, W.; Grosicki, S.; Vorobyev, V.; Spicka, I.; Hungria, V.; Korenkova, S.; Bahlis, N.; Flogegard, M.; et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA): A multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020, 7, e370–e380. [Google Scholar] [CrossRef]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.-A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hájek, R.; Spicka, I.; Risse, M.-L.; Asset, G.; et al. Isatuximab plus carfilzomib and dexamethasone vs carfilzomib and dexamethasone in relapsed/refractory multiple myeloma (ikema): Interim analysis of a phase 3, randomized, open-label study. In Proceedings of the EHA25 Virtual Congress, 13 November 2020; 2020. Late-Breaking Abstract #LB2603. [Google Scholar]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.-V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol. Rev. 2008, 88, 841–886. [Google Scholar] [CrossRef] [Green Version]
- Usmani, S.Z.; Weiss, B.M.; Plesner, T.; Bahlis, N.J.; Belch, A.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.M.; Richardson, P.G.; Chari, A.; et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016, 128, 37–44. [Google Scholar] [CrossRef]
- Spencer, A.; Lentzsch, S.; Weisel, K.; Avet-Loiseau, H.; Mark, T.M.; Spicka, I.; Masszi, T.; Lauri, B.; Levin, M.-D.; Bosi, A.; et al. Daratumumab plus bortezomib and dexamethasone versus bortezomib and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of CASTOR. Haematologica 2018, 103, 2079–2087. [Google Scholar] [CrossRef] [Green Version]
- Bahlis, N.J.; Dimopoulos, M.A.; White, D.J.; Benboubker, L.; Cook, G.; Leiba, M.; Ho, P.J.; Kim, K.; Takezako, N.; Moreau, P.; et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 2020, 34, 1875–1884. [Google Scholar] [CrossRef] [Green Version]
- Endell, J.; Boxhammer, R.; Steidl, S. Synergistic in Vitro Activity of MOR202, a Human CD38 Antibody, in Combination with Pomalidomide. Blood 2014, 124. Abstract #5712 [ASH 2014 65th Annual Meeting]. [Google Scholar] [CrossRef]
- San-Miguel, J.; Weisel, K.; Moreau, P.; Lacy, M.; Song, K.; Delforge, M.; Karlin, L.; Goldschmidt, H.; Banos, A.; Oriol, A.; et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 1055–1066. [Google Scholar] [CrossRef] [Green Version]
- Genmab Genmab Announces European Myeloma Network and Janssen Achieve Positive Topline Results from Phase 3 APOLLO Study of Daratumumab in Combination with Pomalidomide and Dexamethasone in Relapsed or Refractory Multiple Myeloma—Genmab A/S. Available online: https://ir.genmab.com/news-releases/news-release-details/genmab-announces-european-myeloma-network-and-janssen-achieve (accessed on 12 October 2020).
- Sonneveld, P.; Broijl, A.; Gay, F.; Boccadoro, M.; Einsele, H.; Blade, J.; Dimopoulos, M.A.; Delforge, M.; Spencer, A.; Hajek, R.; et al. Bortezomib, lenalidomide, and dexamethasone (VRd) ± daratumumab (DARA) in patients (pts) with transplant-eligible (TE) newly diagnosed multiple myeloma (NDMM): A multicenter, randomized, phase III study (PERSEUS). J. Clin. Oncol. 2019, 37. Abstract #TPS8055 [ASCO 2019 Annual Meeting]. [Google Scholar] [CrossRef]
- Janssen Biotech Inc. DARZALEX® (Daratumumab) Injection. Full Prescribing Information. Available online: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/DARZALEX-pi.pdf (accessed on 12 October 2020).
- Bittner, B.; Richter, W.; Schmidt, J. Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs 2018, 32, 425–440. [Google Scholar] [CrossRef] [Green Version]
- Chari, A.; Rodriguez-Otero, P.; McCarthy, H.; Suzuki, K.; Hungria, V.; Sureda Balari, A.; Perrot, A.; Hulin, C.; Magen, H.; Iida, S.; et al. Subcutaneous daratumumab plus standard treatment regimens in patients with multiple myeloma across lines of therapy (PLEIADES): An open-label Phase II study. Br. J. Haematol. 2020, 134, bjh.16980. [Google Scholar] [CrossRef]
- Jiang, H.; Acharya, C.; An, G.; Zhong, M.; Feng, X.; Wang, L.; Dasilva, N.; Song, Z.; Yang, G.; Adrian, F.; et al. SAR650984 directly induces multiple myeloma cell death via lysosomal-associated and apoptotic pathways, which is further enhanced by pomalidomide. Leukemia 2016, 30, 399–408. [Google Scholar] [CrossRef]
- Martin, T.; Strickland, S.; Glenn, M.; Charpentier, E.; Guillemin, H.; Hsu, K.; Mikhael, J. Phase I trial of isatuximab monotherapy in the treatment of refractory multiple myeloma. Blood Cancer J. 2019, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Orlowski, R.Z.; Goldschmidt, H.; Cavo, M.; Martin, T.G.; Paux, G.; Oprea, C.; Facon, T. Phase III (IMROZ) study design: Isatuximab plus bortezomib (V), lenalidomide (R), and dexamethasone (d) vs. VRd in transplant-ineligible patients (pts) with newly diagnosed multiple myeloma (NDMM). J. Clin. Oncol. 2018, 36. Abstract #TPS8055 [ASCO 2018 Annual Meeting]. [Google Scholar] [CrossRef]
- Tai, Y.-T.; Dillon, M.; Song, W.; Leiba, M.; Li, X.-F.; Burger, P.; Lee, A.I.; Podar, K.; Hideshima, T.; Rice, A.G.; et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood 2008, 112, 1329–1337. [Google Scholar] [CrossRef] [Green Version]
- Pazina, T.; James, A.M.; MacFarlane, A.W.; Bezman, N.A.; Henning, K.A.; Bee, C.; Graziano, R.F.; Robbins, M.D.; Cohen, A.D.; Campbell, K.S. The anti-SLAMF7 antibody elotuzumab mediates NK cell activation through both CD16-dependent and –independent mechanisms. Oncoimmunology 2017, 6, e1339853. [Google Scholar] [CrossRef]
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.C.; Efebera, Y.A.; Kwon, H.; Starling, G.C.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.L.; et al. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zonder, J.A.; Mohrbacher, A.F.; Singhal, S.; van Rhee, F.; Bensinger, W.I.; Ding, H.; Fry, J.; Afar, D.E.H.; Singhal, A.K. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood 2012, 120, 552–559. [Google Scholar] [CrossRef]
- Lonial, S.; Vij, R.; Harousseau, J.-L.; Facon, T.; Moreau, P.; Mazumder, A.; Kaufman, J.L.; Leleu, X.; Tsao, L.C.; Westland, C.; et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J. Clin. Oncol. 2012, 30, 1953–1959. [Google Scholar] [CrossRef]
- Richardson, P.G.; Jagannath, S.; Moreau, P.; Jakubowiak, A.J.; Raab, M.S.; Facon, T.; Vij, R.; White, D.; Reece, D.E.; Benboubker, L.; et al. Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: Final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. Lancet. Haematol. 2015, 2, e516–e527. [Google Scholar] [CrossRef]
- Bristol-Myers Squibb Company Press Release. Bristol Myers Squibb Reports Primary Results of ELOQUENT-1 Study Evaluating Empliciti (elotuzumab) Plus Revlimid (lenalidomide) and Dexamethasone in Patients with Newly Diagnosed, Untreated Multiple Myeloma Untreated Multiple Myeloma. Available online: https://news.bms.com/press-release/corporatefinancial-news/bristol-myers-squibb-reports-primary-results-eloquent-1-study- (accessed on 29 July 2020).
- Ribrag, V.; Avigan, D.E.; Green, D.J.; Wise-Draper, T.; Posada, J.G.; Vij, R.; Zhu, Y.; Farooqui, M.Z.H.; Marinello, P.; Siegel, D.S. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br. J. Haematol. 2019, 186, e41–e44. [Google Scholar] [CrossRef] [Green Version]
- Mateos, M.-V.; Blacklock, H.; Schjesvold, F.; Oriol, A.; Simpson, D.; George, A.; Goldschmidt, H.; Larocca, A.; Chanan-Khan, A.; Sherbenou, D.; et al. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e459–e469. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Schjesvold, F.; Oriol, A.; Karlin, L.; Cavo, M.; Rifkin, R.M.; Yimer, H.A.; LeBlanc, R.; Takezako, N.; McCroskey, R.D.; et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e448–e458. [Google Scholar] [CrossRef]
- Bezman, N.A.; Jhatakia, A.; Kearney, A.Y.; Brender, T.; Maurer, M.; Henning, K.; Jenkins, M.R.; Rogers, A.J.; Neeson, P.J.; Korman, A.J.; et al. PD-1 blockade enhances elotuzumab efficacy in mouse tumor models. Blood Adv. 2017, 1, 753–765. [Google Scholar] [CrossRef] [Green Version]
- Bezman, N.A.; Kinder, M.; Jhatakia, A.D.; Mattson, B.K.; Pizutti, D.; Thompson, E.W.; Capaldi, D.A.; Mendonca, M.W.; Anandam, A.; Dhar, G.; et al. Antitumor activity associated with dual targeting of CD38 and programmed death-1 (PD-1) pathways in preclinical models. In Proceedings of the Cancer Research; American Association for Cancer Research (AACR), Chicago, IL, USA, 14–18 April 2018; Volume 78. Abstract #1727. [Google Scholar]
- Carpenter, R.O.; Evbuomwan, M.O.; Pittaluga, S.; Rose, J.J.; Raffeld, M.; Yang, S.; Gress, R.E.; Hakim, F.T.; Kochenderfer, J.N. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin. Cancer Res. 2013, 19, 2048–2060. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.C.; Hering, M.; Peckham, D.; McDonagh, C.F.; Brown, L.; Kim, K.M.; Meyer, D.L.; Zabinski, R.F.; Grewal, I.S.; Carter, P.J. Antibody targeting of B-cell maturation antigen on malignant plasma cells. Mol. Cancer 2007, 6, 3009–3018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, A.-O.A.; Hoffman, J.E.; Schroeder, M.A.; Jacquemont, C.; Li, H.; Wang, Y.; Van Epps, H.; Campbell, M.S. SGNBCMA-001: A phase 1 study of SEA-BCMA, a non-fucosylated monoclonal antibody, in subjects with relapsed or refractory multiple myeloma. J. Clin. Oncol. 2019, 37. Abstract #TPS8054 [ASCO 2019 Annual Meeting]. [Google Scholar] [CrossRef]
- Bruins, W.S.C.; Zweegman, S.; Mutis, T.; van de Donk, N.W.C.J. Targeted Therapy With Immunoconjugates for Multiple Myeloma. Front. Immunol. 2020, 11, 1155. [Google Scholar] [CrossRef]
- Tai, Y.T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- De Oca, M.R.; Bhattacharya, S.; Vitali, N.; Patel, K.; Kaczynski, H.; Shi, H.Z.; Blackwell, C.; Seestaller-Wehr, L.; Cooper, D.; Jackson, H.; et al. The anti-bcma antibody-drug conjugate gsk2857916 drives immunogenic cell death and immune-mediated anti-tumor responses, and in combination with an ox40 agonist potentiates in vivo activity. HemaSphere 2019, 3, 231, [Abstract #PF558, EHA 2019 24th Congress]. [Google Scholar] [CrossRef]
- Zhao, H.; Atkinson, J.; Gulesserian, S.; Zeng, Z.; Nater, J.; Ou, J.; Yang, P.; Morrison, K.; Coleman, J.; Malik, F.; et al. Modulation of macropinocytosis-mediated internalization decreases ocular toxicity of antibody–drug conjugates. Cancer Res. 2018, 78, 2115–2126. [Google Scholar] [CrossRef] [Green Version]
- Caraccio, C.; Krishna, S.; Phillips, D.J.; Schürch, C.M. Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions. Front. Immunol. 2020, 11, 501. [Google Scholar] [CrossRef]
- Cho, S.-F.; Lin, L.; Xing, L.; Wen, K.; Yu, T.; Hsieh, P.A.; Li, Y.; Munshi, N.C.; Wahl, J.; Matthes, K.; et al. AMG 701 Potently Induces Anti-Multiple Myeloma (MM) Functions of T Cells and IMiDs Further Enhance Its Efficacy to Prevent MM Relapse In Vivo. Blood 2019, 134. Abstract #135 [ASH 2019 61st Annual Meeting]. [Google Scholar] [CrossRef]
- Kim, E.B.; Harrington, C.; Yee, A.; O’Donnell, E.; Branagan, A.; Burke, J.; Raje, N. Practical considerations and role of Daratumumab retreatment for relapsed refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, e263. [Google Scholar] [CrossRef]
- D’Agostino, M.; Mina, R.; Gay, F. Anti-CD38 monoclonal antibodies in multiple myeloma: Another cook in the kitchen? Lancet Haematol. 2020, 7, e355–e357. [Google Scholar] [CrossRef]
- Fedyk, E.R.; Zhao, L.; Koch, A.; Smithson, G.; Estevam, J.; Chen, G.; Lahu, G.; Roepcke, S.; Lin, J.; Mclean, L. Safety, tolerability, pharmacokinetics and pharmacodynamics of the anti-CD38 cytolytic antibody TAK-079 in healthy subjects. Br. J. Clin. Pharm. 2020, 86, 1314–1325. [Google Scholar] [CrossRef] [Green Version]
- Saltarella, I.; Desantis, V.; Melaccio, A.; Solimando, A.G.; Lamanuzzi, A.; Ria, R.; Storlazzi, C.T.; Mariggiò, M.A.; Vacca, A.; Frassanito, M.A. Mechanisms of Resistance to Anti-CD38 Daratumumab in Multiple Myeloma. Cells 2020, 9, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghose, J.; Viola, D.; Terrazas, C.; Caserta, E.; Troadec, E.; Khalife, J.; Gunes, E.G.; Sanchez, J.; McDonald, T.; Marcucci, G.; et al. Daratumumab induces CD38 internalization and impairs myeloma cell adhesion. Oncoimmunology 2018, 7, e1486948. [Google Scholar] [CrossRef] [Green Version]
- Nijhof, I.S.; Groen, R.W.J.; Lokhorst, H.M.; Van Kessel, B.; Bloem, A.C.; Van Velzen, J.; De Jong-Korlaar, R.; Yuan, H.; Noort, W.A.; Klein, S.K.; et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia 2015, 29, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- García-Guerrero, E.; Gogishvili, T.; Danhof, S.; Schreder, M.; Pallaud, C.; Pérez-Simón, J.A.; Einsele, H.; Hudecek, M. Panobinostat induces CD38 upregulation and augments the antimyeloma efficacy of daratumumab. Blood 2017, 129, 3386–3388. [Google Scholar] [CrossRef]
- Madduri, D.; Usmani, S.Z.; Jagannath, S.; Singh, I.; Zudaire, E.; Yeh, T.-M.; Allred, A.J.; Banerjee, A.; Goldberg, J.D.; Schecter, J.M.; et al. Results from CARTITUDE-1: A Phase 1b/2 Study of JNJ-4528, a CAR-T Cell Therapy Directed Against B-Cell Maturation Antigen (BCMA), in Patients with Relapsed and/or Refractory Multiple Myeloma (R/R MM). Blood 2019, 134. Abstract #577 [ASH 2019 61st Annual Meeting]. [Google Scholar] [CrossRef]
- D’Agostino, M.; Zaccaria, G.M.; Ziccheddu, B.; Rustad, E.H.; Genuardi, E.; Capra, A.; Oliva, S.; Auclair, D.; Yesil, J.; Colucci, P.; et al. Early Relapse Risk in Patients with Newly Diagnosed Multiple Myeloma Characterized by Next-generation Sequencing. Clin. Cancer Res. 2020, 26, 4832–4841. [Google Scholar] [CrossRef]
| MAb Class | Molecule (Targets) | Study | Treatment | Setting | Toxicities (≥G3) | ORR (MRD Negativity Rate; NGS, Sensitivity 10−5) | PFS | OS |
|---|---|---|---|---|---|---|---|---|
| Naked | Daratumumab (anti-CD38) | GEN501 + SIRIUS [24,25] NCT00574288 NCT01985126 | Daratumumab single agent | RRMM | Anemia (17.6%); back pain g3 (2.7%); fatigue g3 (2%) | 31.1% | 4 | 20.1 |
| Naked | Daratumumab (anti-CD38) | EQUULEUS [26] NCT01998971 | Dara-Poma-dex | RRMM | Neutropenia (77%); fatigue (12%); dyspnea (8%) | 60% (6%) | 8.8 | 17.5 |
| Naked | Daratumumab (anti-CD38) | GRIFFIN [27] NCT02874742 | Dara-VRd vs. VRd | NDMM | Neutropenia (41.4% vs. 21.6%); peripheral neuropathy (7.1% vs. 7.8%); diarrhea (7.1% vs. 3.9%) | 99% vs. 91.8% (51% vs. 20.4%) | NR vs. NR | NR vs. NR |
| Naked | Daratumumab (anti-CD38) | PAVO [28] NCT02519452 | Subcutaneous administration of daratumumab single agent* | RRMM | Anemia (15.6%); hypertension (8.9%); pneumonia (4.4%); hyponatremia (4.4%); respiratory syncytial virus infection (4.4%); device-related infection (4.4%) | 42.2% | NA | NA |
| Naked | Isatuximab (anti-CD38) | TCD11863 [29] NCT01749969 | Isa-Rd | RRMM | Neutropenia (60%); pneumonia (9%); fatigue (7%) | 56% | 8.5 | NR |
| Naked | Isatuximab (anti-CD38) | TCD14079 [30] NCT02283775 | Isa-Pd | RRMM | Neutropenia (84%); pneumonia (18%); fatigue (7%); urinary tract infection (7%); traumatic fracture (7%); syncope (7%); dyspnea (7%); hypertension (7%) | 62.2% (0%) * | 17.6 | NR |
| Naked | Isatuximab (anti-CD38) | GMMC-CONCEPT [31] NCT03104842 | Isa-KRD | High risk NDMM | Neutropenia (34%); hypertension (12%); cardiac failure (4%) | 100% (40%) ** | NA | NA |
| Naked | MOR202 (anti-CD38) | MOR202C101 [32] NCT01421186 | MOR202+dexamethasone | RRMM | Anemia (39%); hypertension (11%); bronchitis (6%); pneumonia (6%); hyperglycemia (6%) | 28% | 8.4 | NA |
| MOR202-Rd | Lymphopenia (59%); hypophosphatemia (12%); hypertension (12%) | 65% | NR | NA | ||||
| MOR202-Pd | Neutropenia (71%); pneumonia (24%); hypertension (19%) | 48% | 17.5 | NA | ||||
| Naked | TAK-079 (anti-CD38) | TAK-079–1501 [33] NCT03439280 | TAK-079 single agent | RRMM | Neutropenia (5%); parainfluenza virus infection (5%); diverticulitis (5%) | 33% | NR | NA |
| Naked | Elotuzumab (anti-SLAMF7) | ELOQUENT-3 [34] NCT02654132 | Elo-Pd vs. Pd | RRMM | Neutropenia (13% vs. 27%); infections (13% vs. 22%); hyperglycemia (8% vs. 7%) | 53% vs. 26% | 10.3 vs. 4.7 | NA |
| Naked | Pembrolizumab (anti-PD-1) | KEYNOTE-023 [35] NCT02036502 | Pembrolizumab-Rd | RRMM | Neutropenia (27.4%); hyperglycemia (6.5%); pneumonia (6.5%); atrial fibrillation (3.2%); insomnia (3.2%) | 44% | 7.2 | NR |
| Naked | Pembrolizumab (anti-PD-1) | HP-00061522 [36] NCT02289222 | Pembrolizumab-Pd | RRMM | Neutropenia (42%); hyperglycemia (21%); fatigue (15%); pneumonia 15%) | 60% | 17.4 | NR |
| ADC | Belantamab mafodotin (anti-BCMA, monomethyl auristatin F payload) | DREAMM-1 [37,38] NCT02064387 | Belamaf single agent | RRMM | Thrombocytopenia (35%); keratopathy (14%); diarrhea (12%) | 60% *** | 12 | NR |
| ADC | Belantamab mafodotin (anti-BCMA, monomethyl auristatin F payload) | DREAMM-2 [39,40,41,42] NCT03525678 | Belamaf single agent (data on the 2.5 mg/kg cohort are shown) | RRMM | Thrombocytopenia (20%); keratopathy (27%); hypercalcemia (7%) | 31% | 2.9 | 14.9 |
| ADC | Belantamab mafodotin (anti-BCMA, monomethyl auristatin F payload) | DREAMM-6 [43] NCT03544281 | Belamaf-Vd | RRMM | Thrombocytopenia (61%); keratopathy (56%); hypercalcemia (7%) | 78% | NA | NA |
| BiAb | AMG 420 (anti-BCMA/anti-CD3) | 1351.1 [44] NCT02514239 | AMG 420 single agent | RRMM | Infections (24%) neuropathy (5%) CRS (2%) | 70% *** | NA | NA |
| BiAb | PF-3135 (anti-BCMA/anti-CD3) | C1071001 [45] NCT03269136 | PF-3135 single agent | RRMM | Increased liver enzymes (6%) neutropenia (6%), lymphopenia (6%) | 0% *** | NA | NA |
| BiAb | CC-93269 (anti-BCMA/anti-CD3) | CC-93269-MM-001 [46] NCT03486067 | CC-93269 single agent | RRMM | Neutropenia (43%), infections (30%), general physical deterioration (10%) | 89% *** (78%) ** | NA | NA |
| BiAb | Teclistamab (anti-BCMA/anti-CD3) | CR108206 [47] NCT03145181 | Teclistamab single agent | RRMM | Neutropenia (48%), infections (21%), neurotoxicity (3%) | 67% *** | NA | NA |
| Molecule (Target) | Study | Treatment Schema | Setting | Toxicities (≥G3) | ORR (MRD Negativity Rate, NGS, Sensitivity 10−5) | PFS | OS |
|---|---|---|---|---|---|---|---|
| Daratumumab (anti-CD38) | CASTOR [48] NCT02136134 | Dara-Vd vs. Vd | RRMM | Thrombocytopenia (45.7% vs. 32.9%); pneumonia (9.9% vs. 10.1%); hypertension (6.6% vs. 0.8%) | 83.8% vs. 63.2% (11.6% vs. 2.4%) | 16.7 vs. 7.1 | NA |
| Daratumumab (anti-CD38) | POLLUX [49] NCT02076009 | Dara-Rd vs. Rd | RRMM | Neutropenia (55.5% vs. 41.6%); pneumonia (15.2% vs. 10%); diarrhea (9.9% vs. 3.9%); | 92.9% vs. 76.4% (30.4% vs. 5.3%) | 44.5 vs. 17.5 | 1-year OS 92.1% vs. 86.8% |
| Daratumumab (anti-CD38) | CANDOR [50] NCT03158688 | Dara-Kd vs. Kd | RRMM | Thrombocytopenia (24% vs. 16%); respiratory tract infection (29% vs. 16%); hypertension (18% vs. 13%) | 84% vs. 75% (14% vs. 3%) | NR vs. 15.8 | NR vs. NR |
| Daratumumab (anti-CD38) | ALCYONE [51] NCT02195479 | Dara-VMP vs. VMP | NDMM | Neutropenia (39.9% vs. 38.7%); infections (23.1% vs. 14.7%); any infusion-related reaction (4.9% vs. na) | 90.9% vs. 73.9% (22.3% vs. 6.2%) | NR vs. 18.1 | 36-month rate: 78% vs. 67.9% |
| Daratumumab (anti-CD38) | MAIA [52] NCT02252172 | Dara-Rd vs. Rd | NDMM | Neutropenia (50% vs. 35.3%); infections (32.1% vs. 23.3%); fatigue (8% vs. 3.8%) | 92.9% vs. 81.3% (24.2% vs. 7.3%) | NR vs. 31.9 | NA |
| Daratumumab (anti-CD38) | CASSIOPEIA [53] NCT02541383 | Dara-VTd vs. VTd | NDMM | Neutropenia (28% vs. 15%); stomatitis (13% vs. 16%); peripheral sensory neuropathy (9% vs. 9%) | 92.6% vs. 89.9% (64% vs. 44%) * | NA | NA |
| Daratumumab (anti-CD38) | COLUMBA [54] NCT03277105 | Subcutaneous vs. intravenous administration of daratumumab | RRMM | Thrombocytopenia (14% vs. 13%); hypertension (3% vs. 6%); febrile neutropenia (2% vs. 3%); back pain (2% vs. 3%) | 41% vs. 37% | 5.6 vs. 6.1 | NA |
| Isatuximab (anti-CD38) | ICARIA-MM [55] NCT02990338 | Isa-Pd vs. Pd | RRMM | Neutropenia (85% vs. 70%); pneumonia (16% vs. 14%); dyspnea (4% vs. 1%) | 60% vs. 35% (5% vs. 0%) | 11.5 vs. 6.5 | NA |
| Isatuximab (anti-CD38) | IKEMA [56] NCT03275285 | Isa-Kd vs. Kd | RRMM | Respiratory infections (32.2% vs. 23.8%); cardiac failure (4% vs. 4.1%); thrombocytopenia (29.9% vs. 23.8%); neutropenia (19.2% vs. 7.4%) kd, respectively. | 86.6% vs. 82.9% (29.6% vs. 13%) | NR vs. 19.2 | NA |
| Elotuzumab (anti-SLAMF7) | ELOQUENT-2 [57] NCT01239797 | Elo-Rd vs. Rd | RRMM | Lymphocytopenia (79% vs. 49%); infections (33% vs. 26%); pneumonia (14% vs. 10%) | 79% vs. 66% | 19.4 vs. 14.9 | 48 vs. 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Agostino, M.; Innorcia, S.; Boccadoro, M.; Bringhen, S. Monoclonal Antibodies to Treat Multiple Myeloma: A Dream Come True. Int. J. Mol. Sci. 2020, 21, 8192. https://doi.org/10.3390/ijms21218192
D’Agostino M, Innorcia S, Boccadoro M, Bringhen S. Monoclonal Antibodies to Treat Multiple Myeloma: A Dream Come True. International Journal of Molecular Sciences. 2020; 21(21):8192. https://doi.org/10.3390/ijms21218192
Chicago/Turabian StyleD’Agostino, Mattia, Salvatore Innorcia, Mario Boccadoro, and Sara Bringhen. 2020. "Monoclonal Antibodies to Treat Multiple Myeloma: A Dream Come True" International Journal of Molecular Sciences 21, no. 21: 8192. https://doi.org/10.3390/ijms21218192
APA StyleD’Agostino, M., Innorcia, S., Boccadoro, M., & Bringhen, S. (2020). Monoclonal Antibodies to Treat Multiple Myeloma: A Dream Come True. International Journal of Molecular Sciences, 21(21), 8192. https://doi.org/10.3390/ijms21218192





