Downregulation of CTRP-3 by Weight Loss In Vivo and by Bile Acids and Incretins in Adipocytes In Vitro
Abstract
1. Introduction
- the detailed CTRP-3 mRNA expression profile in subcutaneous and visceral adipose tissue samples obtained from severely obese patients undergoing bariatric surgery,
- basal CTRP-3 serum concentrations in obese patients and their changes after bariatric surgery or during LCD,
- correlations of adipose tissue CTRP-3 gene expression with its circulating protein concentrations and with a broad panel of anthropometric and biochemical parameters by univariate and multivariate analysis,
- effects of bile acids and incretins on CTRP-3 expression in adipocytes in vitro.
2. Results
2.1. Study Populations
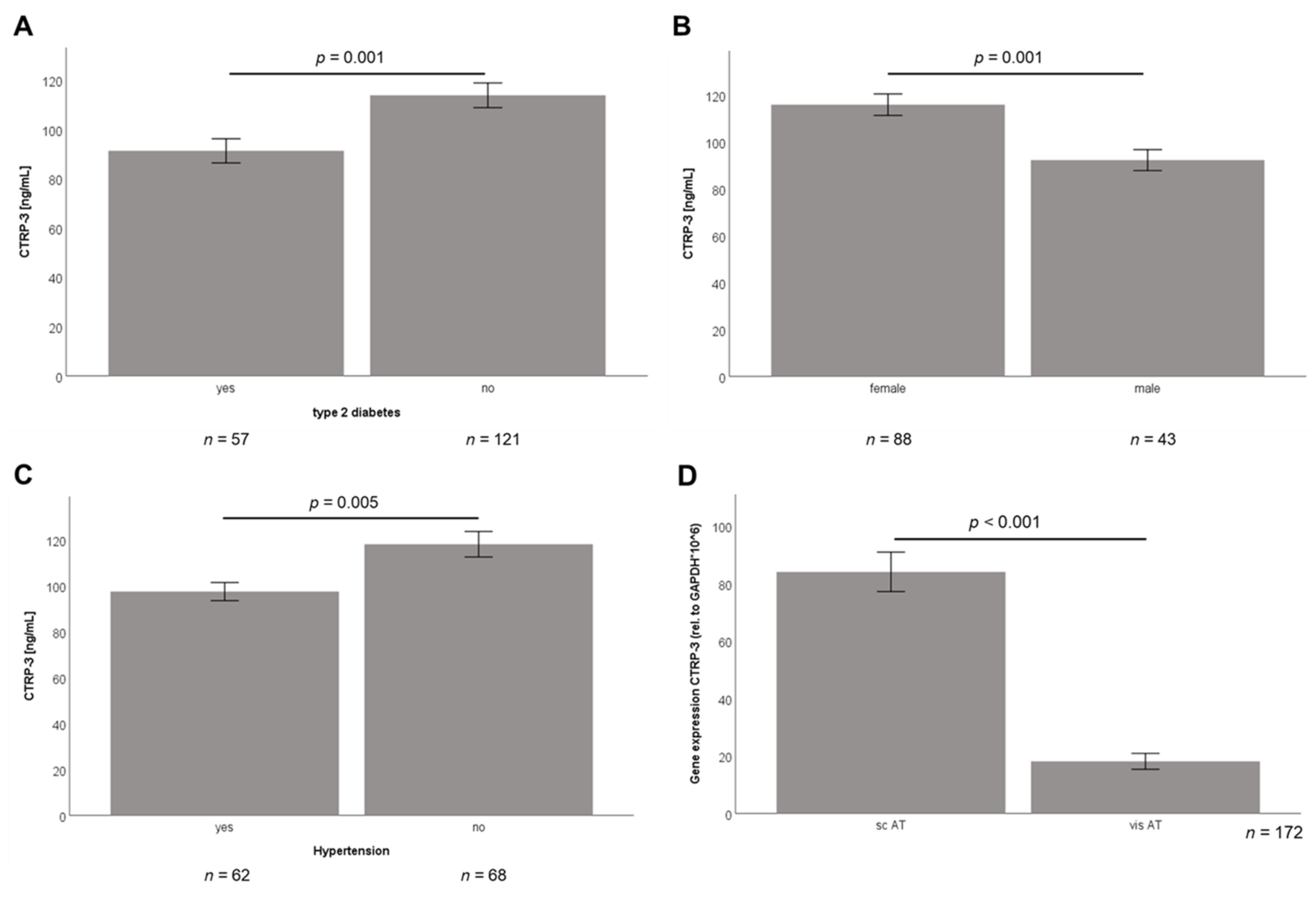
2.2. Baseline CTRP-3 Serum Concentrations and CTRP-3 Gene Expression in Adipose Tissue of Obese Subjects
2.3. Correlation Analysis of Baseline Data
2.4. Significant Decline of CTRP-3 Serum Concentrations After Intervention
2.5. Correlation Analysis of Adipose Tissue CTRP-3 and Bile Acid Receptor Gene Expression
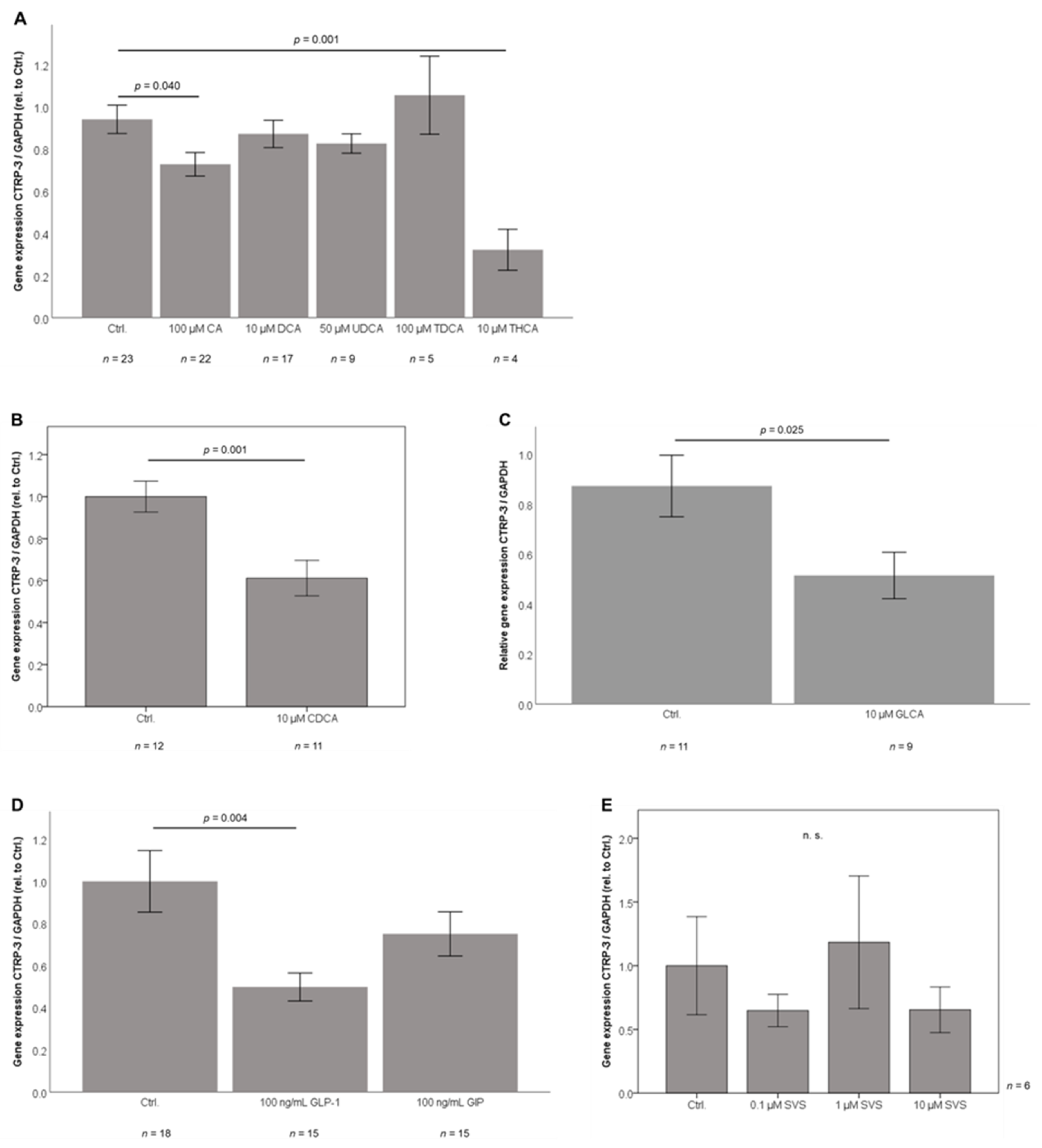
2.6. Bile Acid Sub-Species Exert Differential Effects on CTRP-3 mRNA Expression in Adipocytes In Vitro
2.7. GLP-1 Treatment Downregulates CTRP-3 Gene Expression in Adipocytes
2.8. Adipocyte CTRP-3 mRNA Expression is Unresponsive to Simvastatin
2.9. CTRP-3 and Bile Acid Receptor Gene Expression in the Murine System
3. Discussion
4. Materials and Methods
4.1. The ROBS Study Cohort (Research in Obesity and Bariatric Surgery)
4.2. Serum Measurement of CTRP-3 Concentrations
4.3. Cell Culture Experiments
4.4. Adipose Tissue and Adipocyte mRNA Extraction
4.5. Real-Time PCR Analysis of CTRP-3 and Bile Acid Receptor mRNA Expression
- Human CTRP-3: 5′-GCCCCAGTATCAGGTGTGTA-3′/5′-GCAAAGGTGGAGAAGCGTTG-3′;
- Murine CTRP-3: 5′-GGGGTTCTTTATGGAGCATT-3′/5′-AAATGCATCCTTTGAGGTGA-3′;
- Human TGR5: 5′-CACTGTTGTCCCTCCTCTCC-3′/5′-ACACTGCTTTGGCTGCTTG-3′;
- Murine TGR5: 5′-GAGCGTCGCCCACCACTAGG-3′/5′-CGCTGATCACCCAGCCCCATG-3′;
- Murine FXR I (for adipocyte mRNA): 5′-TGGGCTCCGAATCCTCTTAGA-3′/5′-TGGTCCTCAAATAAGATCCTTGG-3′;
- Murine FXR II (for adipose tissue mRNA): 5′-GTACAAGTGTAAGAACGGGG-3′/5′-CTTGGTTGTGGAGGTCACTT-3′.
4.6. Animals
4.6.1. Tissues from Wildtype and CTRP-3 Knockout Mice
4.6.2. Isolation and Cell Culture of Primary Murine Adipocytes
4.6.3. Intraperitoneal Application of Recombinant CTRP-3 in Mice
4.6.4. Data Base and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Cohort | Mean BMI (kg/m2) | Mean CTRP-3 Serum Concentrations (ng/ml) | ELISA Supplier | Study | Reference |
|---|---|---|---|---|---|
| Bariatric surgery (n = 179) | 53.3 ± 6.9 | 106.5 ± 51.0 | CTRP-3 DuoSet ELISA, R&D system, Wiesbaden, Germany | Schmid, A. et al. | present |
| Low calorie diet (n = 131) | 43.5 ± 6.7 | 108.1 ± 40.5 | CTRP-3 DuoSet ELISA, R&D system, Wiesbaden, Germany | Schmid, A. et al. | present |
| Obesity subgroup (n = 180) | 28.02 ± 1.65 | 94.53 ± 43.94 | CTRP-3 ELISA Aviscera Bioscience, Santa Clara, CA, USA | Deng, W. et al. | [14] |
| Obesity subgroup (n = 44) | 45.2 ± 1.1 | 405 ± 8.3 | CTRP-3 ELISA AdipoGen, Incheon, Korea | Wolf, R.M. et al. | [13] |
| Obesity subgroup (n = 30) | 33.7 ± 4.4 | 258.22 ± 147.62 | CTRP-3 ELISA Wuhan El Aab Science Co. | Jain, N.B. et al. | [24] |
References
- Schäffler, A.; Buechler, C. CTRP family: Linking immunity to metabolism. Trends Endocrinol. Metab. 2012, 23, 194–204. [Google Scholar] [CrossRef]
- Wong, G.W.; Krawczyk, S.A.; Kitidis-Mitrokostas, C.; Revett, T.; Gimeno, R.; Lodish, H.F. Molecular, biochemical and functional characterizations of C1q/TNF family members: Adipose-tissue-selective expression patterns, regulation by PPAR-γ agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem. J. 2008, 416, 161–177. [Google Scholar] [CrossRef]
- Akiyama, H.; Furukawa, S.; Wakisaka, S.; Maeda, T. CTRP3/cartducin promotes proliferation and migration of endothelial cells. Mol. Cell. Biochem. 2007, 304, 243–248. [Google Scholar] [CrossRef]
- Maeda, T.; Wakisaka, S. CTRP3/cartducin is induced by transforming growth factor-β1 and promotes vascular smooth muscle cell proliferation. Cell Biol. Int. 2010, 34, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Otani, M.; Furukawa, S.; Wakisaka, S.; Maeda, T. A novel adipokine C1q/TNF-related protein 3 is expressed in developing skeletal muscle and controls myoblast proliferation and differentiation. Mol. Cell. Biochem. 2015, 409, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.M.; Seldin, M.M.; Wei, Z.; Aja, S.; Wong, G.W. CTRP3 attenuates diet-induced hepatic steatosis by regulating triglyceride metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G214–G224. [Google Scholar] [CrossRef]
- Wolf, R.M.; Lei, X.; Yang, Z.C.; Nyandjo, M.; Tan, S.Y.; William Wong, G. CTRP3 deficiency reduces liver size and alters IL-6 and TGFβ levels in obese mice. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E332–E345. [Google Scholar] [CrossRef] [PubMed]
- Kopp, A.; Bala, M.; Buechler, C.; Falk, W.; Gross, P.; Neumeier, M.; Schölmerich, J.; Schäffler, A. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 2010, 151, 5267–5278. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Kopp, A.; Hanses, F.; Karrasch, T.; Schäffler, A. C1q/TNF-related protein-3 (CTRP-3) attenuates lipopolysaccharide (LPS)-induced systemic inflammation and adipose tissue Erk-1/-2 phosphorylation in mice in vivo. Biochem. Biophys. Res. Commun. 2014, 452, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Murayama, M.A.; Kakuta, S.; Maruhashi, T.; Shimizu, K.; Seno, A.; Kubo, S.; Sato, N.; Saijo, S.; Hattori, M.; Iwakura, Y. CTRP3 plays an important role in the development of collagen-induced arthritis in mice. Biochem. Biophys. Res. Commun. 2014, 443, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.M.; Wei, Z.; Wong, G.W. C1q/TNF-related protein-3 (CTRP3), a novel adipokine that regulates hepatic glucose output. J. Biol. Chem. 2010, 285, 39691–39701. [Google Scholar] [CrossRef] [PubMed]
- Compton, S.A.; Cheatham, B. CTRP-3: Blocking a toll booth to obesity-related inflammation. Endocrinology 2010, 151, 5095–5097. [Google Scholar] [CrossRef]
- Wolf, R.M.; Steele, K.E.; Peterson, L.A.; Magnuson, T.H.; Schweitzer, M.A.; Wong, G.W. Lower circulating C1q/TNF-Related Protein-3 (CTRP3) Levels are associated with obesity: A cross-sectional study. PLoS ONE 2015, 10, e0133955. [Google Scholar] [CrossRef]
- Deng, W.; Li, C.; Zhang, Y.; Zhao, J.; Yang, M.; Tian, M.; Li, L.; Zheng, Y.; Chen, B.; Yang, G. Serum C1q/TNF-related protein-3 (CTRP3) levels are decreased in obesity and hypertension and are negatively correlated with parameters of insulin resistance. Diabetol. Metab. Syndr. 2015, 7, 33. [Google Scholar] [CrossRef]
- Chen, T.; Wang, F.; Chu, Z.; Shi, X.; Sun, L.; Lv, H.; Zhou, W.; Shen, J.; Chen, L.; Hou, M. Serum CTRP3 Levels In Obese Children: A Potential Protective Adipokine Of Obesity, Insulin Sensitivity And Pancreatic β Cell Function. Diabetes. Metab. Syndr. Obes. Targets Ther. 2019, 12, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Berghoff, M.; Hochberg, A.; Schäffler, A.; Karrasch, T. CTRP-3 is permeable to the blood–brain barrier and is not regulated by glucose or lipids in vivo. Eur. J. Clin. Invest. 2017, 47, 203–212. [Google Scholar] [CrossRef]
- Wagner, R.M.; Sivagnanam, K.; Clark, W.A.; Peterson, J.M. Divergent relationship of circulating CTRP3 levels between obesity and gender: A cross-sectional study. PeerJ 2016, 4, e2573. [Google Scholar] [CrossRef] [PubMed]
- Abdeen, G.; le Roux, C.W. Mechanism Underlying the Weight Loss and Complications of Roux-en-Y Gastric Bypass. Rev. Obes. Surg. 2016, 26, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.H.; Jahansouz, C.; Abraham, A.A.; Leslie, D.B.; Ikramuddin, S. The future of the Roux-en-Y gastric bypass. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 777–784. [Google Scholar] [CrossRef]
- Ahmad, N.N.; Pfalzer, A.; Kaplan, L.M. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int. J. Obes. 2013, 37, 1553–1559. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Flynn, C.R.; Cai, S.; Xiao, Y.; Tamboli, R.A.; Abumrad, N.N. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J. Clin. Endocrinol. Metab. 2015, 100, E1225–E1233. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, S.; Campos, V.; Egli, L.; Toepel, U.; Carrel, G.; Cariou, B.; Rainteau, D.; Schneiter, P.; Tappy, L.; Giusti, V. Long-term effects of Roux-en-Y gastric bypass on postprandial plasma lipid and bile acids kinetics in female non diabetic subjects: A cross-sectional pilot study. Clin. Nutr. 2015, 34, 911–917. [Google Scholar] [CrossRef]
- Brock, J.; Schmid, A.; Karrasch, T.; Pfefferle, P.; Schlegel, J.; Busse, I.; Hauenschild, A.; Schmidt, B.; Koukou, M.; Arapogianni, E.; et al. Progranulin serum levels and gene expression in subcutaneous vs visceral adipose tissue of severely obese patients undergoing bariatric surgery. Clin. Endocrinol. 2019, 91, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.B.; Yadav, A.; Shankar, N.; Ahmed, R. Correlation of C1q/TNF-related protein-3 with cardiac autonomic tone and metabolic parameters in obesity. Int. J. Endocrinol. Metab. 2019, 17, e74250. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Schlegel, J.; Thomalla, M.; Karrasch, T.; Schäffler, A. Evidence of functional bile acid signaling pathways in adipocytes. Mol. Cell. Endocrinol. 2019, 483, 1–10. [Google Scholar] [CrossRef]
- Ban, B.; Bai, B.; Zhang, M.; Hu, J.; Ramanjaneya, M.; Tan, B.K.; Chen, J. Low serum cartonectin/CTRP3 concentrations in newly diagnosed type 2 diabetes mellitus: In vivo regulation of cartonectin by glucose. PLoS ONE 2014, 9, e112931. [Google Scholar] [CrossRef]
- Šimonyte, K.; Olsson, T.; Näslund, I.; Angelhed, J.E.; Lönn, L.; Mattsson, C.; Rask, E. Weight loss after gastric bypass surgery in women is followed by a metabolically favorable decrease in 11β-hydroxysteroid dehydrogenase 1 expression in subcutaneous adipose tissue. J. Clin. Endocrinol. Metab. 2010, 95, 3527–3531. [Google Scholar] [CrossRef]
- Shrestha, C.; He, H.; Liu, Y.; Zhu, S.; Xiong, J.; Mo, Z. Changes in adipokines following laparoscopic roux-en-Y gastric bypass surgery in chinese individuals with type 2 diabetes mellitus and BMI of 22–30 kg·m−2. Int. J. Endocrinol. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Hutch, C.R.; Sandoval, D. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology 2017, 158, 4139–4151. [Google Scholar] [CrossRef]
- Shankar, S.S.; Mixson, L.A.; Chakravarthy, M.; Chisholm, R.; Acton, A.J.; Jones, R.M.; Mattar, S.G.; Miller, D.L.; Petry, L.; Beals, C.R.; et al. Metabolic improvements following Rouxen-Y surgery assessed by solid meal test in subjects with short duration type 2 diabetes. BMC Obes. 2017, 4, 10. [Google Scholar] [CrossRef]
- Kindel, T.L.; Krause, C.; Helm, M.C.; McBride, C.L.; Oleynikov, D.; Thakare, R.; Alamoudi, J.; Kothari, V.; Alnouti, Y.; Kohli, R. Increased glycine-amidated hyocholic acid correlates to improved early weight loss after sleeve gastrectomy. Surg. Endosc. 2018, 32, 805–812. [Google Scholar] [CrossRef]
- Green, H.; Kehinde, O. An established preadipose cell line and its differentiation in culture II. Factors affecting the adipose conversion. Cell 1975, 5, 19–27. [Google Scholar] [CrossRef]
- Zaitsu, H.; Serrero, G. Pedersen fetuin contains three adipogenic factors with distinct biochemical characteristics. J. Cell. Physiol. 1990, 144, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, M.; Loffler, G. Adipogenic activities in commercial preparations of fetuin. Horm. Metab. Res. 1994, 26, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J. Cell. Physiol. 1979, 101, 169–171. [Google Scholar] [CrossRef]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef]
- MacDougald, O.A.; Lane, M.D. Transcriptional Regulation of Gene Expression During Adipocyte Differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef]
- Cornelius, P.; MacDougald, O.A.; Lane, M.D. Regulation of Adipocyte Development. Annu. Rev. Nutr. 1994, 14, 99–129. [Google Scholar] [CrossRef]
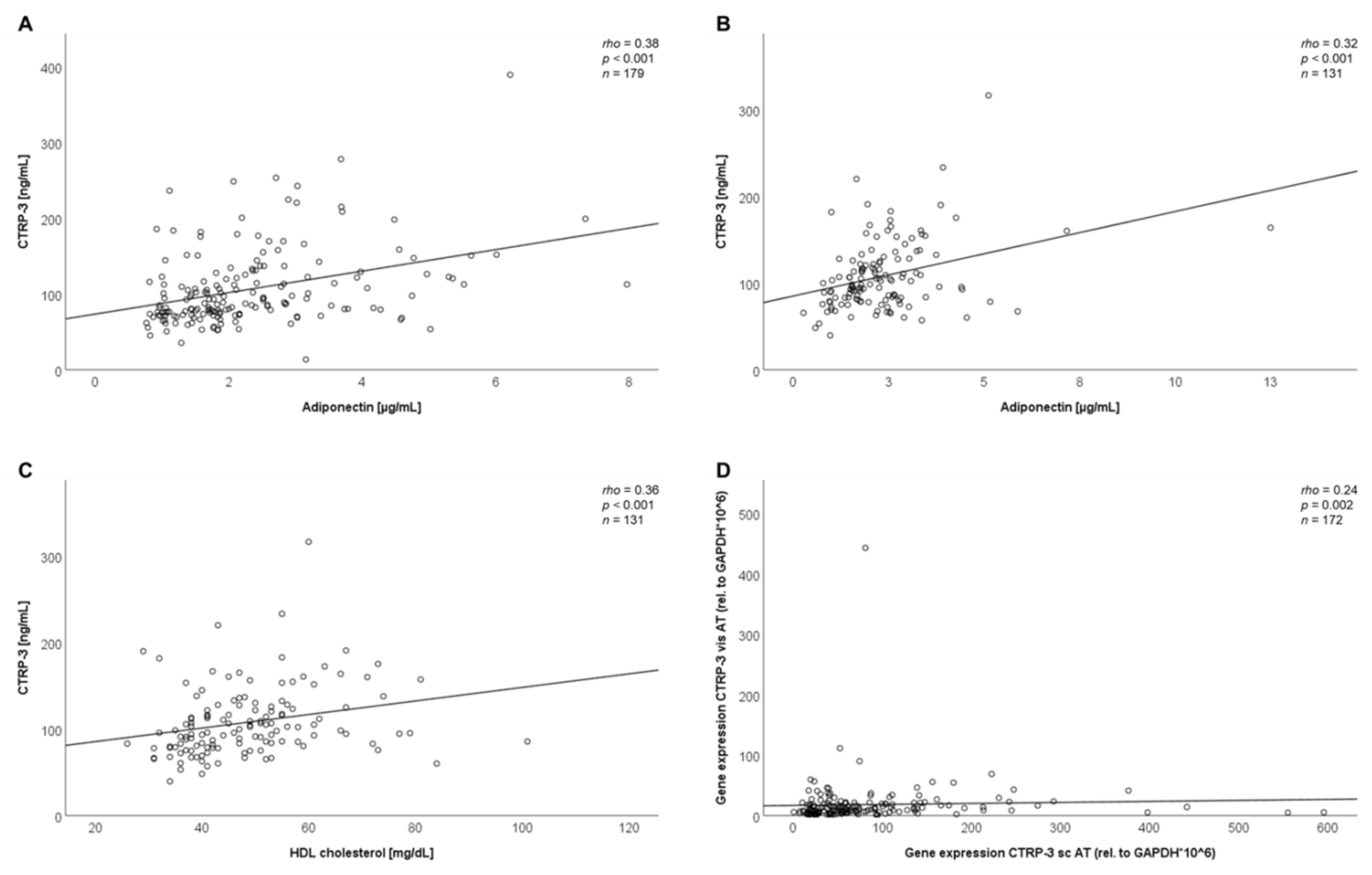


| A—Correlation Analysis of | rho | p |
| Serum CTRP-3 concentrations in BS patients with | ||
| CTRP-3 gene expression in sc AT | +0.23 | 0.003 |
| CTRP-3 gene expression in vis AT | +0.16 | 0.033 |
| Adiponectin (µg/ml) | +0.38 | <0.001 |
| Leptin (ng/ml) | +0.16 | 0.033 |
| B—Correlation Analysis of | rho | p |
| Serum CTRP-3 concentrations in LCD patients with | ||
| Adiponectin (µg/mL) | +0.32 | <0.001 |
| Leptin (ng/mL) | +0.23 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, A.; Gehl, J.; Thomalla, M.; Hochberg, A.; Kreiß, A.; Patz, M.; Karrasch, T.; Schäffler, A. Downregulation of CTRP-3 by Weight Loss In Vivo and by Bile Acids and Incretins in Adipocytes In Vitro. Int. J. Mol. Sci. 2020, 21, 8168. https://doi.org/10.3390/ijms21218168
Schmid A, Gehl J, Thomalla M, Hochberg A, Kreiß A, Patz M, Karrasch T, Schäffler A. Downregulation of CTRP-3 by Weight Loss In Vivo and by Bile Acids and Incretins in Adipocytes In Vitro. International Journal of Molecular Sciences. 2020; 21(21):8168. https://doi.org/10.3390/ijms21218168
Chicago/Turabian StyleSchmid, Andreas, Jonas Gehl, Miriam Thomalla, Alexandra Hochberg, Anja Kreiß, Marissa Patz, Thomas Karrasch, and Andreas Schäffler. 2020. "Downregulation of CTRP-3 by Weight Loss In Vivo and by Bile Acids and Incretins in Adipocytes In Vitro" International Journal of Molecular Sciences 21, no. 21: 8168. https://doi.org/10.3390/ijms21218168
APA StyleSchmid, A., Gehl, J., Thomalla, M., Hochberg, A., Kreiß, A., Patz, M., Karrasch, T., & Schäffler, A. (2020). Downregulation of CTRP-3 by Weight Loss In Vivo and by Bile Acids and Incretins in Adipocytes In Vitro. International Journal of Molecular Sciences, 21(21), 8168. https://doi.org/10.3390/ijms21218168




