How the Pathological Microenvironment Affects the Behavior of Mesenchymal Stem Cells in the Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
2. Results
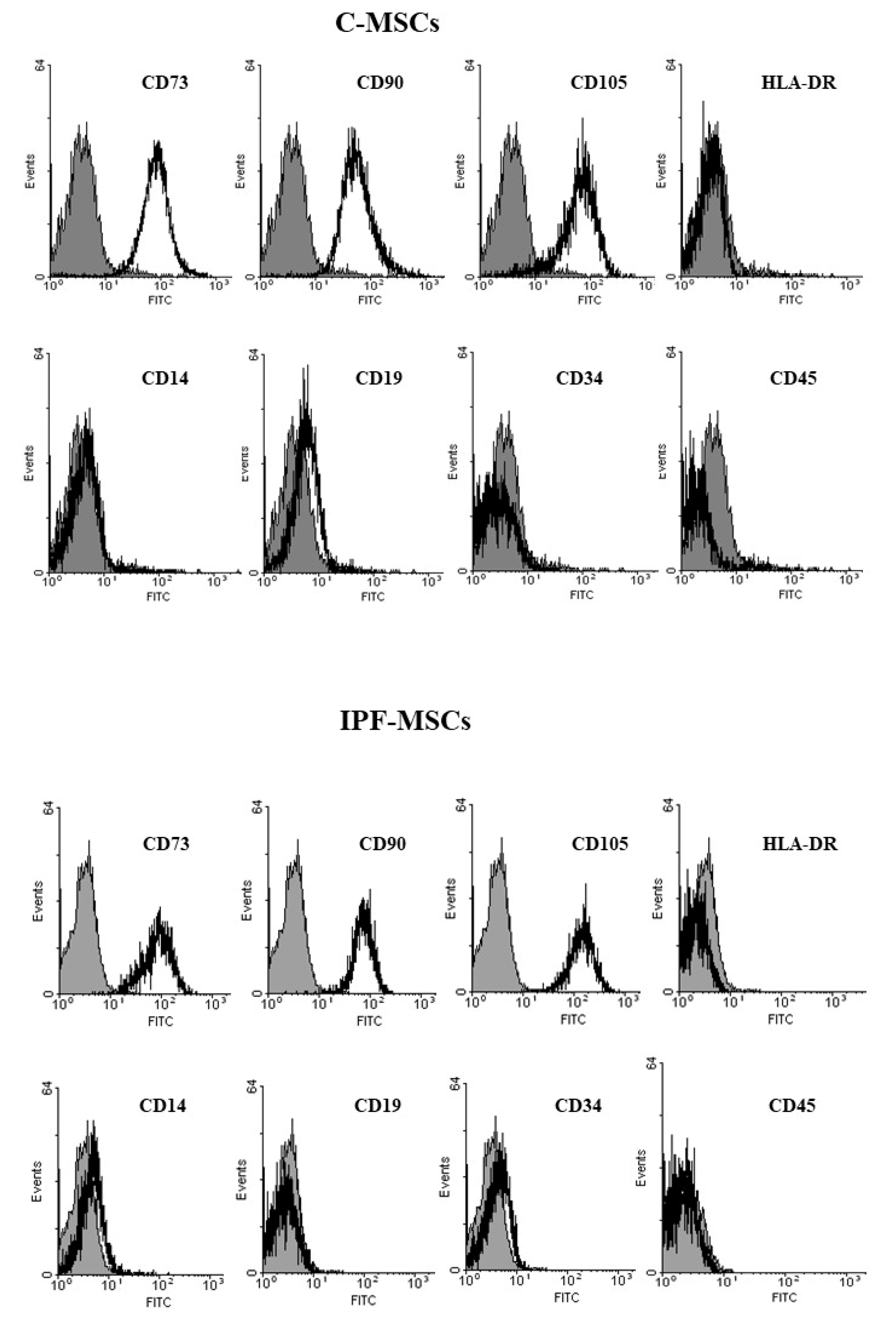
2.1. MSCs Isolation and Characterization from Healthy and Fibrotic Lung
2.2. α-SMA, Collagen Type 1, Fibronectin, and TGF-β1 Expression
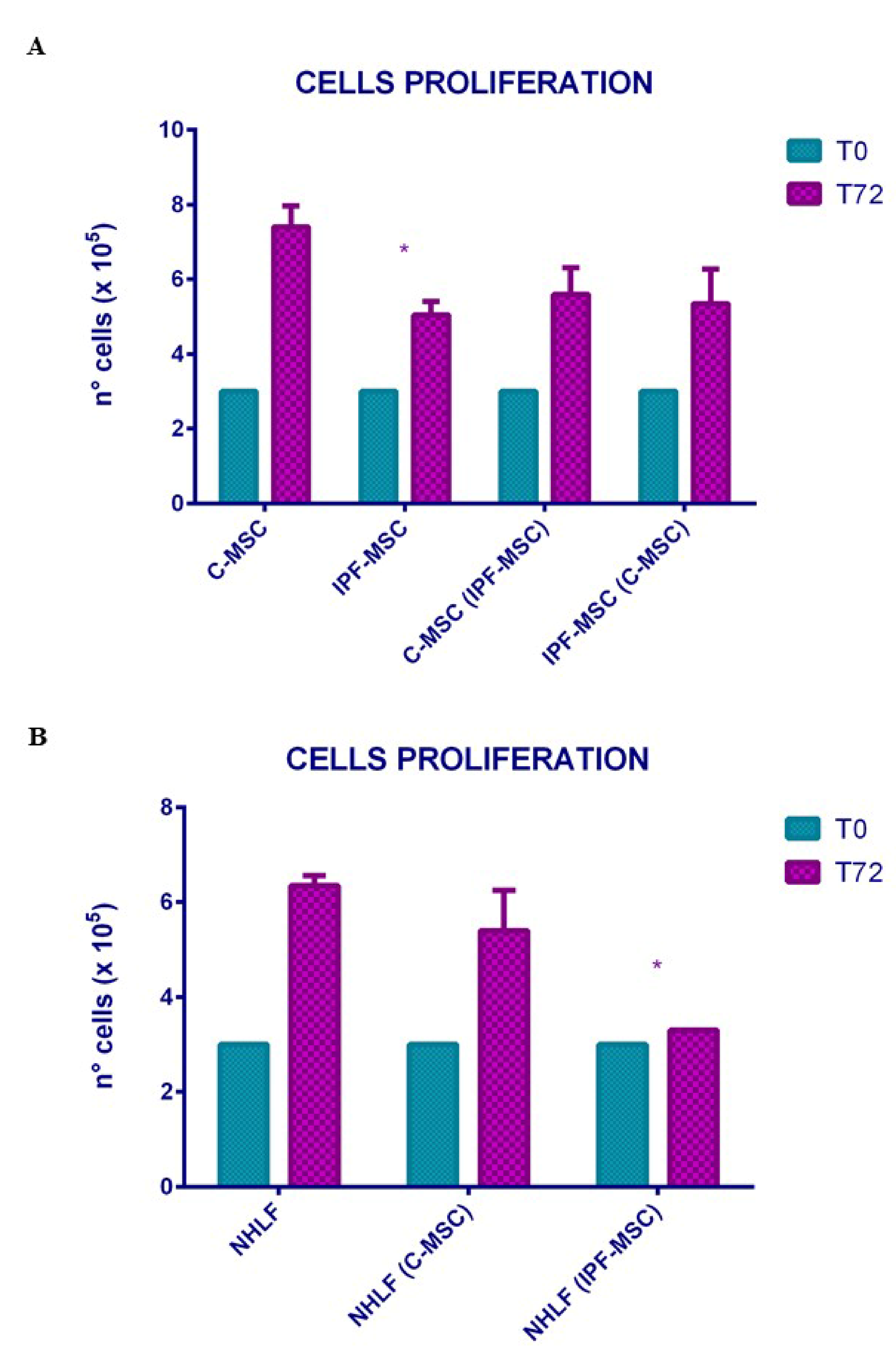
2.3. Cell Proliferation: Differences between Mock and Co-Cultured Cells
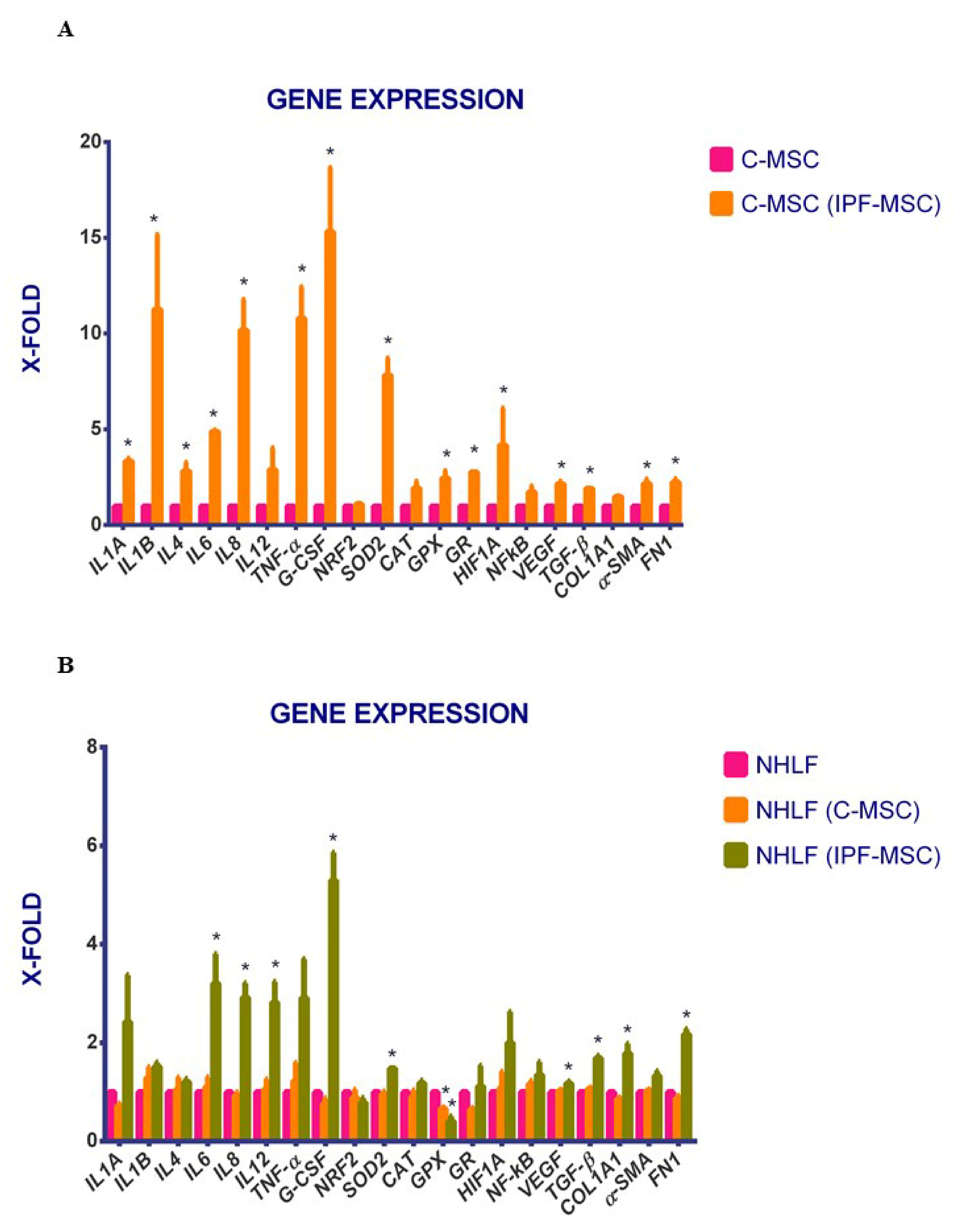
2.4. C-MSCs vs. IPF-MSCs: Expression of Genes Related to Inflammation
2.5. Expression of Genes Related to Inflammation after Co-Cultures
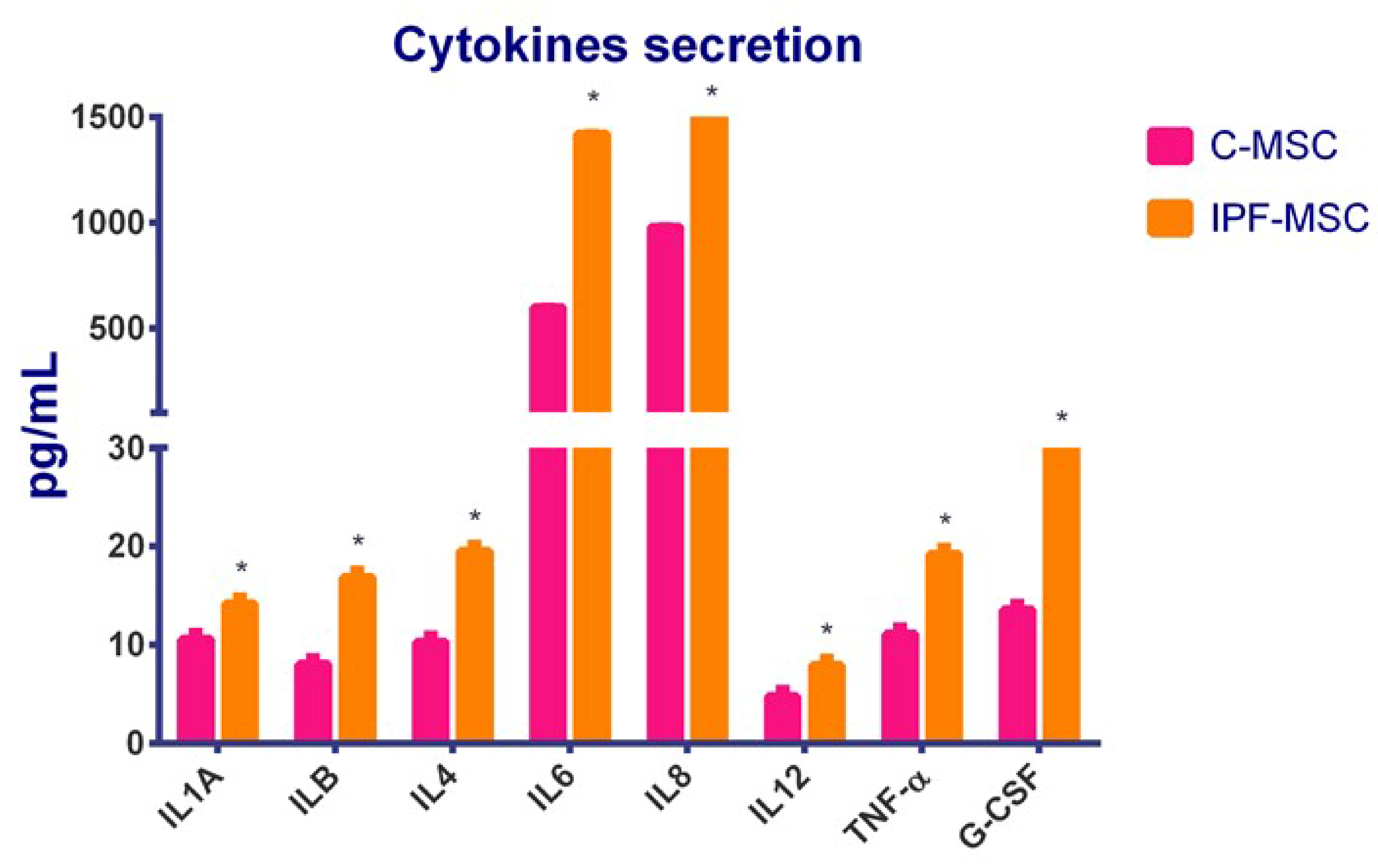
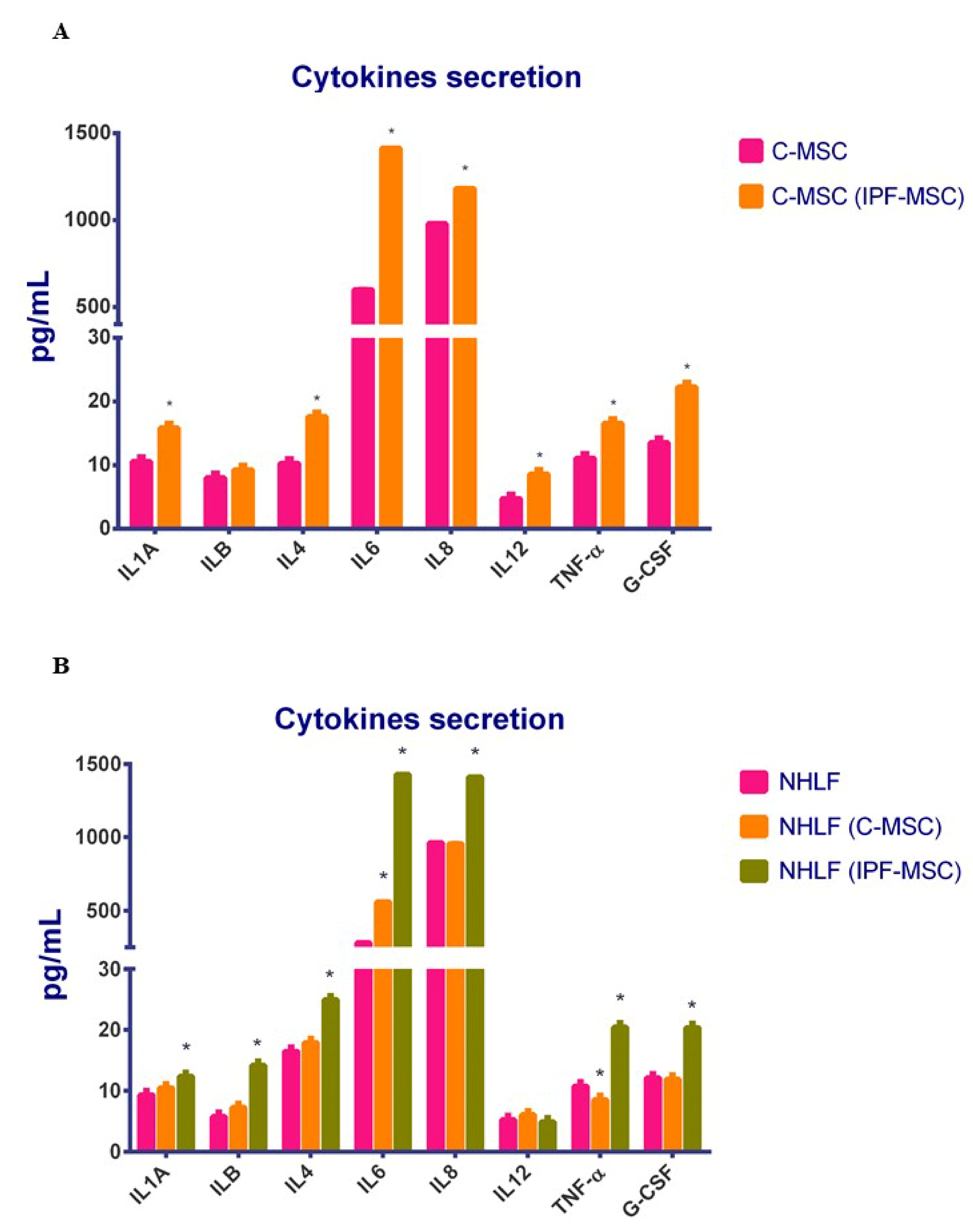
2.6. C-MSCs vs. IPF-MSCs: Cytokines Secretion
2.7. Cytokines Secretion after Co-Cultures
3. Discussion
4. Material and Methods
4.1. Patients Enrollment
4.2. Isolation and Characterization of Mesenchymal Stem Cells from Healthy and Fibrotic Lung
4.3. Production of α-SMA, Collagen Type 1, Fibronectin, and TGF-β1
4.4. NHLF Culture
4.5. MSCs Co-Cultures
4.6. Expression of Genes Related to Inflammation, Oxidative Stress, Hypoxya, and ECM Pathway
4.7. ELISA of Inflammation-Related Cytokines
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.M.; Nepali, K.; Liou, J.P. Idiopathic pulmonary fibrosis: Current status, recent progress, and emerging targets. J. Med. Chem. 2017, 60, 527–553. [Google Scholar] [CrossRef]
- Padilla, M. Idiopathic pulmonary fibrosis: The role of pathobiology in making a definitive diagnosis. Am. J. Manag. Care 2015, 21, s276–s283. [Google Scholar]
- King, T.E., Jr.; Tooze, J.A.; Schwarz, M.I.; Brown, K.R.; Cherniack, R.M. Predicting survival in idiopathic pulmonary fibrosis: Scoring system and survival model. Am. J. Respir. Crit. Care Med. 2001, 164, 1171–1181. [Google Scholar] [CrossRef]
- Tian, Y.; Li, H.; Gao, Y.; Liu, C.; Qiu, T.; Wu, H.; Cao, M.; Zhang, Y.; Ding, H.; Chen, J.; et al. Quantitative proteomic characterization of lung tissue in idiopathic pulmonary fibrosis. Clin. Proteom. 2019, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.P.; Schwartz, D.A. Molecular mechanisms in progressive idiopathic pulmonary fibrosis. Annu. Rev. Med. 2013, 64, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Desai, O.; Winkler, J.; Minasyan, M.; Herzog, E.L. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front. Med. 2018, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, T.S.; Tager, A.M.; Borok, Z.; Moore, B.B.; Schwartz, D.A.; Anstrom, K.J. Future directions in idiopathic pulmonary fibrosis research. An NHLBI workshop report. Am. J. Respir. Crit. Care. Med. 2014, 189, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Bringardner, B.D.; Baran, C.P.; Eubank, T.D.; Marsh, C.B. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid. Redox Signal. 2008, 10, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Bartels, K.; Grenz, A.; Eltzschig, H.K.P. Hypoxia and inflammation are two sides of the same coin. Proc. Natl. Acad. Sci. USA 2013, 110, 18351–18352. [Google Scholar] [CrossRef]
- Senavirathna, L.K.; Huang, C.; Yang, X.; Munteanu, M.C.; Sathiaseelan, R.; Xu, D.; Henke, C.A.; Liu, L. Hypoxia induces pulmonary fibroblast proliferation through NFAT signaling. Sci. Rep. 2018, 8, 2709. [Google Scholar] [CrossRef]
- Cheresh, P.; Kim, S.J.; Tulasiram, S.; Kamp, D.W. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta 2013, 1832, 1028–1040. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem. Cell 2013, 13, 392–402. [Google Scholar] [CrossRef]
- Campanati, A.; Orciani, M.; Sorgentoni, G.; Consales, V.; Offidani, A.; Di Primio, R. Pathogenetic characteristics of mesenchymal stem cells in hidradenitis suppurativa. JAMA Dermatol. 2018, 154, 1184–1190. [Google Scholar] [CrossRef]
- Orciani, M.; Caffarini, M.; Lazzarini, R.; Delli Carpini, G.; Tsiroglou, D.; Di Primio, R.; Ciavattini, A. Mesenchymal Stem Cells from Cervix and Age: New Insights into CIN Regression Rate. Oxid. Med. Cell. Longev. 2018, 2018, 1545784. [Google Scholar] [CrossRef]
- Martin, A.; Jahn, A.; Rio Bocos, C.; Montes, A.; Pons, P.J.; Mercader, J.; Velasco, J.; Gómez, C.; Villena, C.; Carvajal, Á.F.; et al. Characterization of Mesenchymal Stem Cells obtained from human lungs with and without Idiopathic Pulmonary Fibrosis. Eur. J. Respir. 2020, 6, 103. [Google Scholar]
- Hostettler, K.E.; Gazdhar, A.; Khan, P.; Savic, S.; Tamo, L.; Lardinois, D. Multipotent mesenchymal stem cells in lung fibrosis. PLoS ONE 2017, 12, e0181946. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Yue, S.; Luo, Z. Mesenchymal stem cells in idiopathic pulmonary fibrosis. Oncotarget 2017, 8, 102600. [Google Scholar] [CrossRef] [PubMed]
- Toonkel, R.L.; Hare, J.M.; Matthay, M.A.; Glassberg, M.K. Mesenchymal stem cells and idiopathic pulmonary fibrosis. Potential for clinical testing. Am. J. Respir. Crit. Care. Med. 2013, 188, 133–140. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Caffarini, M.; Biagini, A.; Lucarini, G.; Delli Carpini, G.; Berretta, A. Chronic inflammation may enhance leiomyoma development by the involvement of progenitor cells. Stem. Cells Int. 2018, 2018, 1716246. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, V.; Rezende, N.C.; Scotland, K.B.; Shaffer, S.M.; Persson, J.L.; Gudas, L.J.; Mongan, N.P. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem. Cells Dev. 2009, 18, 1093–1108. [Google Scholar] [CrossRef]
- Lin, L.; Han, Q.; Xiong, Y.; Li, T.; Liu, Z.; Xu, H. Krüpple-like-factor 4 attenuates lung fibrosis via inhibiting epithelial-mesenchymal transition. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Jiang, Y.; Wells, A.; Sylakowski, K.; Clark, A.M.; Ma, B. Adult stem cell functioning in the tumor micro-environment. Int. J. Mol. Sci. 2019, 20, 2566. [Google Scholar] [CrossRef]
- Fernandez, I.E.; Eickelberg, O. The impact of TGF-β on lung fibrosis: From targeting to biomarkers. Proc. Am. Thorac. Soc. 2012, 9, 111–116. [Google Scholar] [CrossRef]
- Meyer, K.C.; Danoff, S.K.; Lancaster, L.H.; Nathan, S.D. Management of idiopathic pulmonary fibrosis in the elderly patient. Chest 2015, 148, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; White, T.A.; Iijima, K.; Haak, A.J.; Ligresti, G.; Atkinson, E.J. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Schuliga, M.; Pechkovsky, D.V.; Read, J.; Waters, D.W.; Blokland, K.E.; Reid, A.T. Mitochondrial dysfunction contributes to the senescent phenotype of IPF lung fibroblasts. J. Cell. Mol. Med. 2018, 22, 5847–5861. [Google Scholar] [CrossRef]
- Blokland, K.E.; Waters, D.W.; Schuliga, M.; Read, J.; Pouwels, S.D.; Grainge, C.L. Senescence of IPF Lung Fibroblasts Disrupt Alveolar Epithelial Cell Proliferation and Promote Migration in Wound Healing. Phamaceutics 2020, 12, 389. [Google Scholar] [CrossRef]
- Raghu, G.; Anstrom, K.J.; King, T.E.; Lasky, J.A., Jr.; Martinez, F.J. Idiopathic Pulmonary Fibrosis Clinical Research Network. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 2012, 366, 1968–1977. [Google Scholar] [PubMed]
- Heukels, P.; Moor, C.C.; von der Thüsen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef]
- Contreras, R.A.; Figueroa, F.E.; Djouad, F.; Luz-Crawford, P. Mesenchymal stem cells regulate the innate and adaptive immune responses dampening arthritis progression. Stem. Cells Int. 2016, 2016, 3162743. [Google Scholar] [CrossRef]
- Saito, F.; Tasaka, S.; Inoue, K.; Miyamoto, K.; Nakano, Y.; Ogawa, Y. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am. J. Respir. Cell Mol. 2008, 38, 566–571. [Google Scholar] [CrossRef]
- Yang, L.; Herrera, J.; Gilbertsen, A.; Xia, H.; Smith, K.; Benyumov, A.; Bitterman, P.B.; Henke, C.A. IL-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L127–L136. [Google Scholar] [CrossRef]
- Francisco-Cruz, A.; Aguilar-Santelises, M.; Ramos-Espinosa, O.; Mata-Espinosa, D.; Marquina-Castillo, B.; Barrios-Payan, J.; Hernandez-Pando, R. Granulocyte–macrophage colony-stimulating factor: Not just another haematopoietic growth factor. Med. Oncol. 2014, 31, 774. [Google Scholar] [CrossRef]
- Reynolds, H.Y. Lung inflammation and fibrosis: An alveolar macrophage-centered perspective from the 1970s to 1980s. Am. J. Respir. Crit. Care Med. 2005, 171, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.; Choi, H.; Hsieh, M.H.; Neugent, M.L.; Ahn, J.M.; Hayenga, H.N. Targeting hypoxia-inducible factor-1α/pyruvate dehydrogenase kinase 1 axis by dichloroacetate suppresses bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2018, 58, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, N.; Ishii, Y.; Morishima, Y.; Yageta, Y.; Haraguchi, N.; Itoh, K.; Yamamoto, M.; Hizawa, N. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir. Res. 2010, 11, 31. [Google Scholar] [CrossRef]
- Lee, E.J.; Cárdenes, N.; Álvarez, D.; Sellarés, J.; Sembrat, J.; Aranda, P.; Peng, Y.; Bullock, J.; Nouraie, S.M.; Mora, A.L.; et al. Mesenchymal stem cells reduce ER stress via PERK-Nrf2 pathway in an aged mouse model. Respirology 2020, 25, 417–426. [Google Scholar] [CrossRef]
- Kliment, C.R.; Oury, T.D. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic. Biol. Med. 2010, 49, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Colella, S.; Haentschel, M.; Shah, P.; Poletti, V.; Hetzel, J. Transbronchial lung cryobiopsy in interstitial lung diseases: Best practice. Respiration 2018, 95, 383–391. [Google Scholar] [CrossRef]
- Campanati, A.; Orciani, M.; Sorgentoni, G.; Consales, V.; Mattioli Belmonte, M.; Di Primio, R.; Offidani, A. Indirect co-cultures of healthy mesenchymal stem cells restore the physiological phenotypical profile of psoriatic mesenchymal stem cells. Clin. Exp. Immunol. 2018, 193, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, R.; Olivieri, F.; Ferretti, C.; Mattioli-Belmonte, M.; Di Primio, R.; Orciani, M. mRNAs and miRNAs profiling of mesenchymal stem cells derived from amniotic fluid and skin: The double face of the coin. Cell Tissue Res. 2014, 355, 121–130. [Google Scholar] [CrossRef]
- Campanati, A.; Orciani, M.; Lazzarini, R.; Ganzetti, G.; Consales, V.; Sorgentoni, G. TNF-α inhibitors reduce the pathological Th1–Th17/Th2 imbalance in cutaneous mesenchymal stem cells of psoriasis patients. Exp. Dermatol. 2017, 26, 319–324. [Google Scholar] [CrossRef]
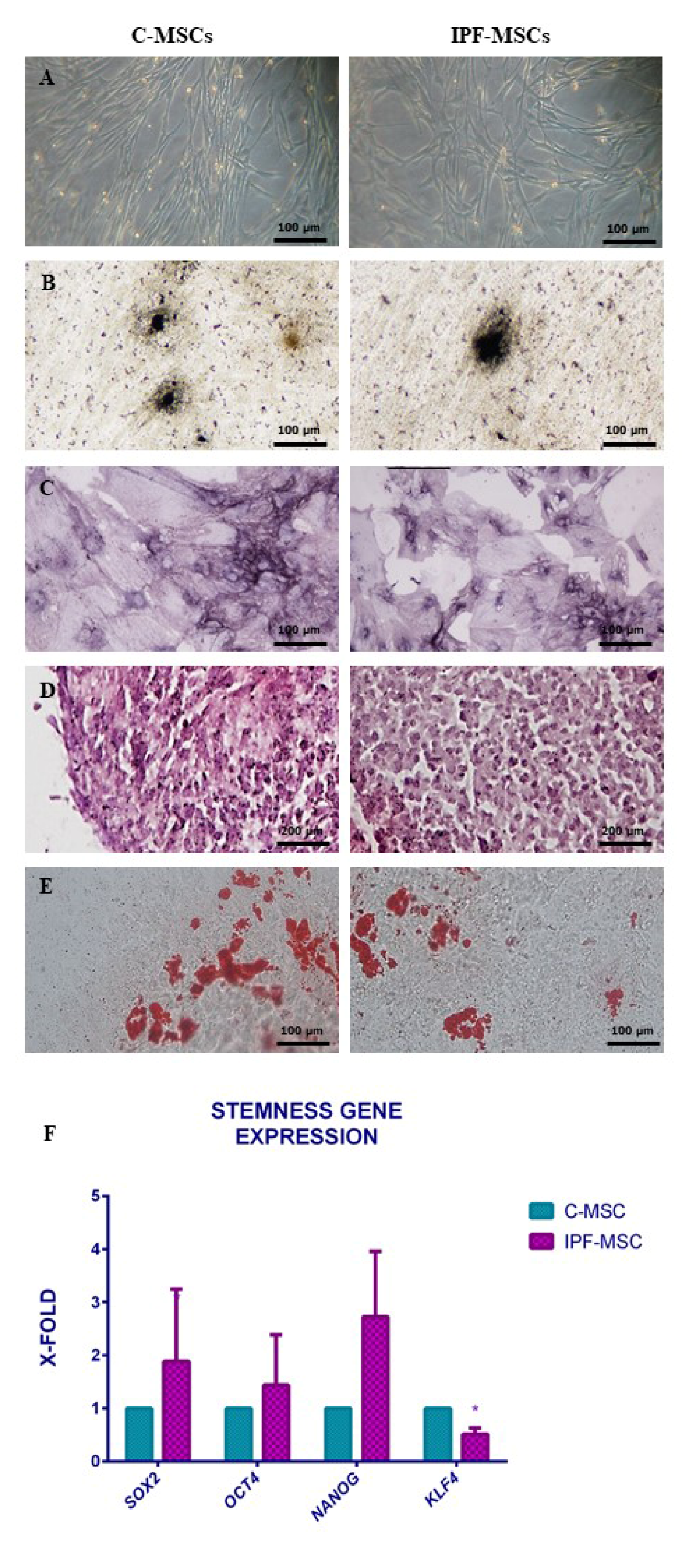
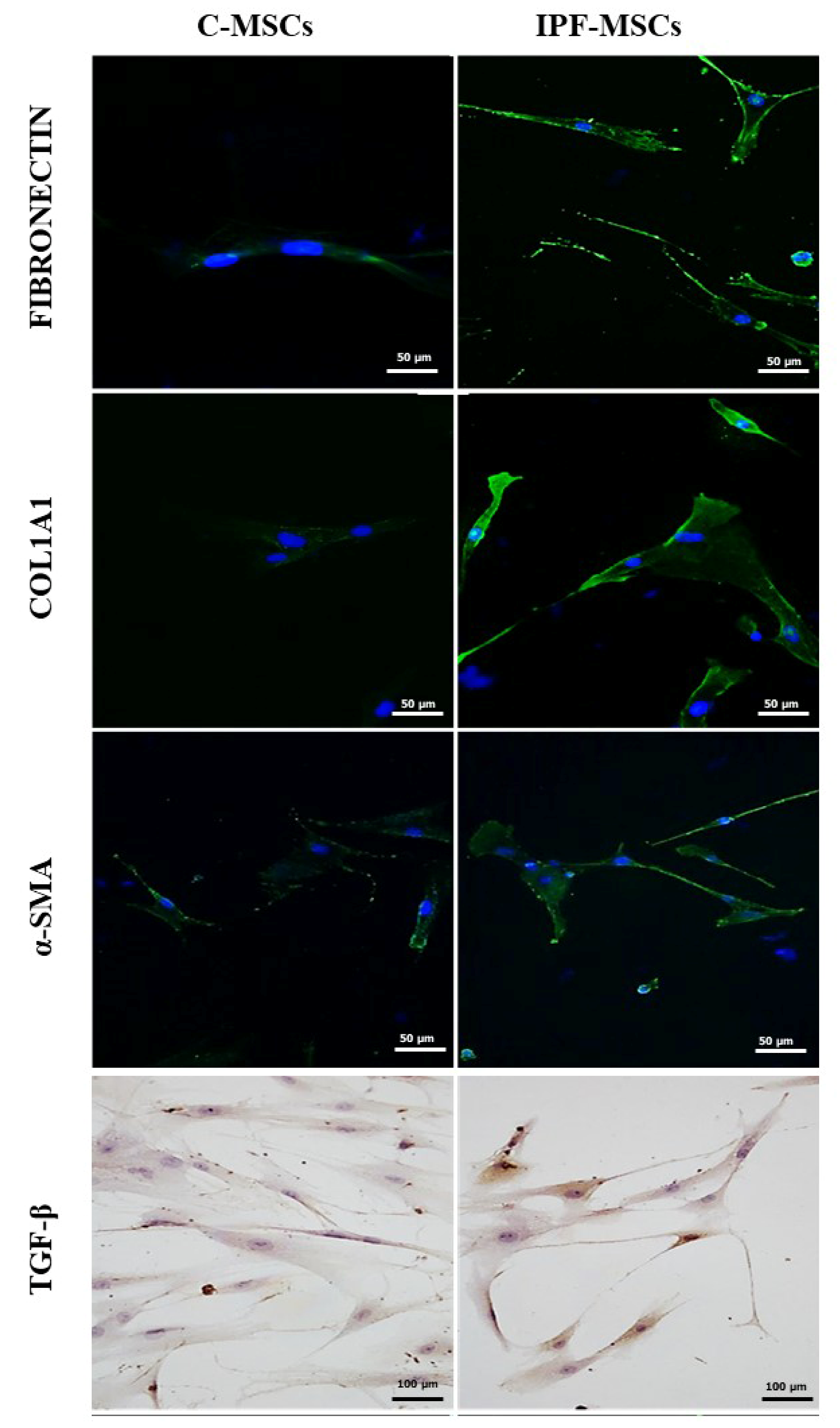

| C-MSCs | IPF-MSCs | |
|---|---|---|
| FIBRONECTIN % | 44.59 ± 9.596 | 89.98 ± 4.539 * |
| COL1A1 % | 12.47 ± 2.529 | 77.19 ± 4.440 * |
| α-SMA % | 16.09 ± 7.094 | 76.58 ± 4.690 * |
| TGF-β % | 4.757 ± 0.793 | 32.73 ± 5.046 * |
| Characteristics | IPF | CTRL |
|---|---|---|
| Age, years | 70.3 ± 6.4 | 61.7 ± 0.5 |
| Sex, no. male/no. female | 1/2 | 0/3 |
| Smoking status (no. Never/Ever) | 1/2 | 2/1 |
| Pulmonary function test at baseline, % predicted | ||
| FEV1 | 85.0 ± 31.0 | 89.5 ± 14 |
| FVC | 83.3 ± 38.3 | 98.5 ± 19.5 |
| DLco | 56.0 ± 4.0 | 68 ± 17 |
| GENE | FORWARD 5′–3′ | REVERSE 5′–3′ |
|---|---|---|
| GAPDH | CCCTTCATTGACCTCAACTACATG | TGGGATTTCCATTGATGACAAGC |
| SOX2 | ACACCAATCCCATCCACACT | GCAAACTTCCTGCAAAGCTC |
| OCT4 | AGCGAACCAGTATCGAGAAC | TTACAGAACCACACTCGGAC |
| NANOG | TGAACCTCAGCTACAAACAG | CTGGATGTTCTGGGTCTGGT |
| KLF4 | CCCACACAGGTGAGAAACCT | ATGTGTAAGGCGAGGTGGTC |
| HIF1A | GAAAGAGCCCGATGCCCT | TGATATGATCGTGTCCCCAGC |
| NFKB | AATGGTGGAGTCTGGGAAGG | TCTGACGTTTCCTCTGCACT |
| VEGF | CCTCCGAAACCATGAACTTT | ATGATTCTGCCCTCCTCCTTCT |
| SOD2 | CTGGACAAACCTCAGCCCTAAC | AACCTGAGCCTTGGACACCAAC |
| CAT | GTGCGGAGATTCAACACTGCCA | TTCTCACACACGCGGCAATG |
| GPX | GTGCTCGGCTTCCCGTGCAAC | CTCGAAGAGCATGAAGTTGGGC |
| GR | TATGTGAGCCGCCTGAATGCCA | CACTGACCTCTATTGTGGGCTTG |
| NRF2 | CAGCGACGGAAAGAGTATGA | TGGGCAACCTGGGAGTAG |
| IL1A | TCATTGGCGTTTGAGTCAGC | ACCACCATGCTCTCCTTGAA |
| IL1B | CGAATCTCCGACCACCACTA | AGCCTCGTTATCCCATGTGT |
| IL4 | TTTGCTGCCTCCAAGAACAC | GTCGAGCCGTTTCAGGAATC |
| IL6 | ATTCTGCGCAGCTTTAAGGA | AACAACAATCTGAGGTGCCC |
| IL8 | GTGTGGGTCTGTTGTAGGGT | TCGGATATTCTCTTGGCCCT |
| IL12 | AATGTTCCCATGCCTTCACC | CCAATGGTAAACAGGCCTCC |
| TNF-α | CGAGTCTGGGCAGGTCTACTTT | AAGCTGTAGGCCCCAGTGAGTT |
| G-CSF | GGACATGGTTTGACTCCCGA | CTTCCTTTCACACACAGGCC |
| TGF-β1 | CAAGTGGACATCAACGGGTTC | TGCGGAAGTCAATGTAGC |
| COL1A1 | GAGGGCCAAGACGACGAAGACATC | CAGATCACGTCATCGCACAAC |
| α-SMA | AGCCAAGCACTGTCAGGAATC | AGCCATTGTCACACACCAAGG |
| FN1 | CGGGAGGCATTAGAAGGGAT | TTGCTTTGACTGACAGCCAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonifazi, M.; Di Vincenzo, M.; Caffarini, M.; Mei, F.; Salati, M.; Zuccatosta, L.; Refai, M.; Mattioli-Belmonte, M.; Gasparini, S.; Orciani, M. How the Pathological Microenvironment Affects the Behavior of Mesenchymal Stem Cells in the Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 8140. https://doi.org/10.3390/ijms21218140
Bonifazi M, Di Vincenzo M, Caffarini M, Mei F, Salati M, Zuccatosta L, Refai M, Mattioli-Belmonte M, Gasparini S, Orciani M. How the Pathological Microenvironment Affects the Behavior of Mesenchymal Stem Cells in the Idiopathic Pulmonary Fibrosis. International Journal of Molecular Sciences. 2020; 21(21):8140. https://doi.org/10.3390/ijms21218140
Chicago/Turabian StyleBonifazi, Martina, Mariangela Di Vincenzo, Miriam Caffarini, Federico Mei, Michele Salati, Lina Zuccatosta, Majed Refai, Monica Mattioli-Belmonte, Stefano Gasparini, and Monia Orciani. 2020. "How the Pathological Microenvironment Affects the Behavior of Mesenchymal Stem Cells in the Idiopathic Pulmonary Fibrosis" International Journal of Molecular Sciences 21, no. 21: 8140. https://doi.org/10.3390/ijms21218140
APA StyleBonifazi, M., Di Vincenzo, M., Caffarini, M., Mei, F., Salati, M., Zuccatosta, L., Refai, M., Mattioli-Belmonte, M., Gasparini, S., & Orciani, M. (2020). How the Pathological Microenvironment Affects the Behavior of Mesenchymal Stem Cells in the Idiopathic Pulmonary Fibrosis. International Journal of Molecular Sciences, 21(21), 8140. https://doi.org/10.3390/ijms21218140







