Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review
Abstract
:1. Introduction
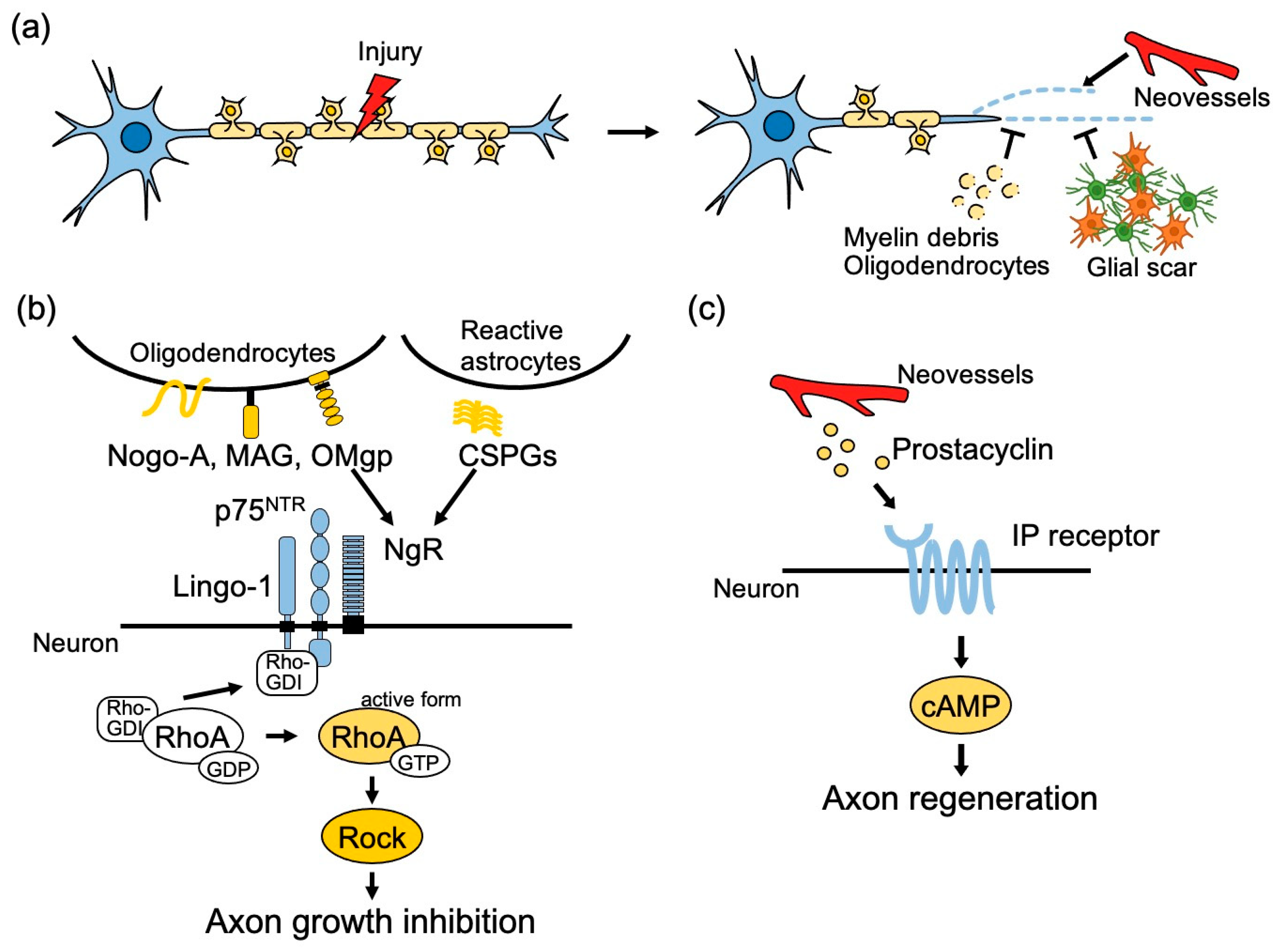
2. Molecular Mechanisms of Axonal Regeneration
2.1. Role of Extrinsic Factors
2.2. Role of Intrinsic Factors
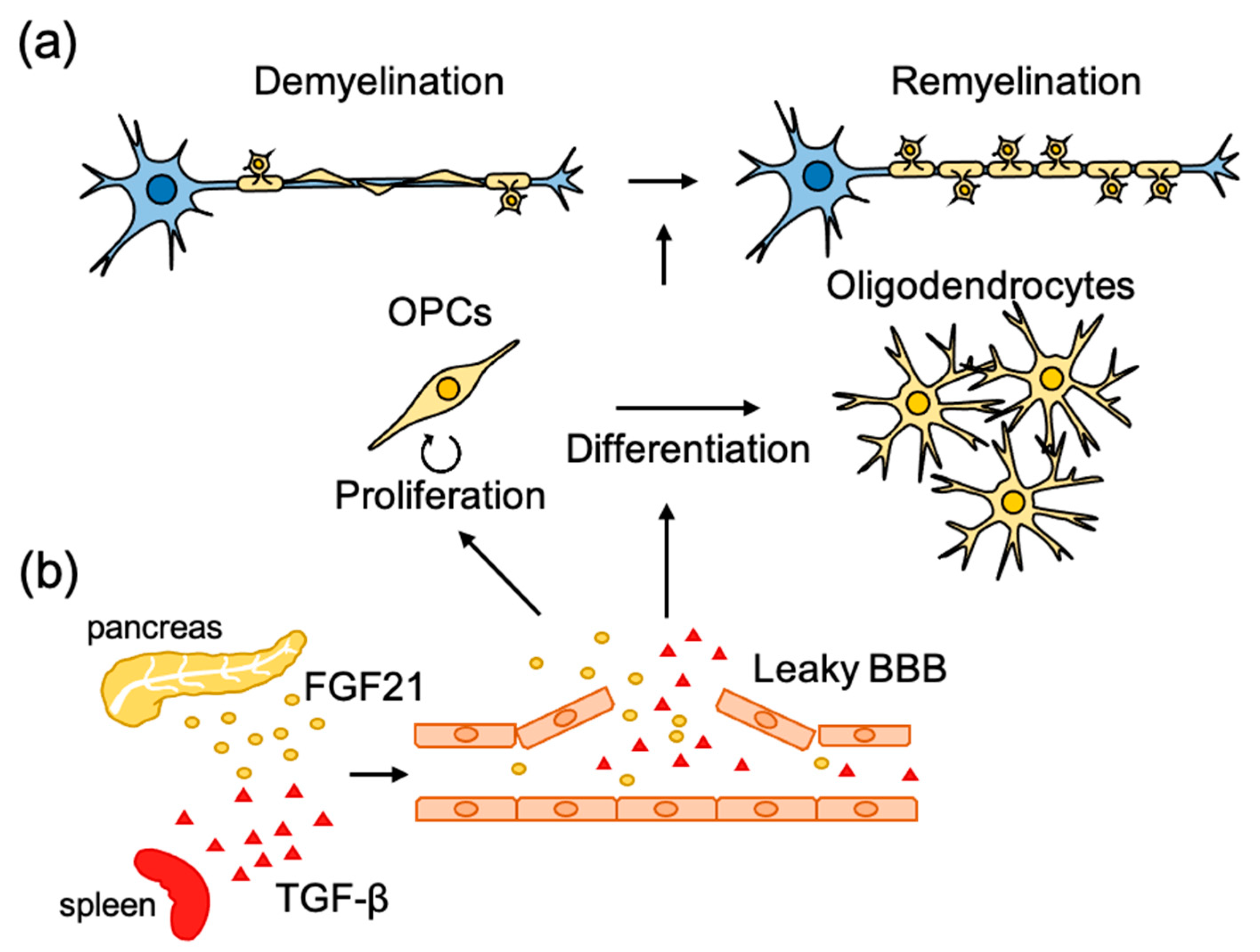
3. Molecular Mechanism of Remyelination
3.1. Role of Extrinsic Factors
3.2. Role of Systemic Factors
3.3. Role of Intrinsic Factors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 9cRA | RXR by 9-cis-retinoic acid |
| AAV | adeno-associated viral |
| aOPCs | adult oligodendrocyte progenitor cells |
| BBB | blood–brain barrier |
| cAMP | cyclic adenosine monophosphate |
| ChIP | chromatin immunoprecipitation |
| CNS | central nervous system |
| CSPGs | chondroitin sulfate proteoglycans |
| CST | corticospinal tract |
| DRG | dorsal root ganglion |
| EAE | autoimmune encephalomyelitis |
| ERβ | estrogen receptor-β |
| FGF21 | fibroblast growth factor 21 |
| HMGCR | 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase |
| HTT | huntingtin |
| IL-1β | interleukin-1β |
| Inpp5f | inositol polyphosphate-5-phosphatase f |
| IP | I type prostaglandin |
| KOR | κ-opioid receptor |
| LPA | lysophosphatidic acid signaling |
| LPAR1 | lysophosphatidic acid receptor 1 |
| LPC | lysophosphatidylcholine |
| LPPR1 | phospholipid phosphatase-related 1 |
| MAG | myelin-associated glycoprotein |
| MBP | myelin basic protein |
| miRNAs | micro RNAs |
| MS | multiple sclerosis |
| Ngr1 | Nogo receptor 1 |
| NPCs | neural progenitor cells |
| OLCs | oligodendrocyte linage cells |
| OMgp | oligodendrocyte-myelin glycoprotein |
| ONI | optic nerve injury |
| PDGFRα | platelet-derived growth factor receptor-α |
| PGIS | prostacyclin synthase |
| PTEN | phosphatase and tensin homolog |
| RGC | retinal ganglion cell |
| RGMa | repulsive guidance molecule a |
| SCI | spinal cord injury |
| shRNA | small hairpin RNA |
| siRNA | small interfering |
| TGF-β1 | transforming growth factor-β1 |
| VGCCs | potential-dependent calcium channels |
References
- Geoffroy, C.G.; Zheng, B. Myelin-associated inhibitors in axonal growth after CNS injury. Curr. Opin. Neurobiol. 2014, 27. [Google Scholar] [CrossRef] [Green Version]
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018, 21. [Google Scholar] [CrossRef]
- He, Z.; Jin, Y. Intrinsic control of axon regeneration. Neuron 2016, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weidner, N.; Ner, A.; Salimi, N.; Tuszynski, M.H. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc. Natl. Acad. Sci. USA 2001, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steward, O.; Zheng, B.; Tessier-Lavigne, M.; Hofstadter, M.; Sharp, K.; Yee, K.M. Regenerative growth of corticospinal tract axons via the ventral column after spinal cord injury in mice. J. Neurosci. 2008. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.S.Y.; Zdunek, S.; Bergmann, O.; Bernard, S.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Brundin, L.; et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 2014, 159. [Google Scholar] [CrossRef] [Green Version]
- Schwab, M.E.; Strittmatter, S.M. Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 2014, 27, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Dickendesher, T.L.; Baldwin, K.T.; Mironova, Y.A.; Koriyama, Y.; Raiker, S.J.; Askew, K.L.; Wood, A.; Geoffroy, C.G.; Zheng, B.; Liepmann, C.D.; et al. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat. Neurosci. 2012, 15, 703–712. [Google Scholar] [CrossRef]
- Shen, Y.; Tenney, A.P.; Busch, S.A.; Horn, K.P.; Cuascut, F.X.; Liu, K.; He, Z.; Silver, J.; Flanagan, J.G. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 2009, 326, 592–596. [Google Scholar] [CrossRef] [Green Version]
- Snow, D.M.; Smith, J.D.; Cunningham, A.T.; McFarlin, J.; Goshorn, E.C. Neurite elongation on chondroitin sulfate proteoglycans is characterized by axonal fasciculation. Exp. Neurol. 2003, 182, 310–321. [Google Scholar] [CrossRef]
- Yamashita, T.; Higuchi, H.; Tohyama, M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell. Biol. 2002, 157, 565–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.C.; Koprivica, V.; Kim, J.A.; Sivasankaran, R.; Guo, Y.; Neve, R.L.; He, Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature 2002, 417, 941–944. [Google Scholar] [CrossRef]
- Mi, S.; Lee, X.; Shao, Z.; Thill, G.; Ji, B.; Relton, J.; Levesque, M.; Allaire, N.; Perrin, S.; Sands, B.; et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004, 7, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Dergham, P.; Ellezam, B.; Essagian, C.; Avedissian, H.; Lubell, W.D.; McKerracher, L. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 2002, 22, 6570–6577. [Google Scholar] [CrossRef] [Green Version]
- Koch, J.C.; Tönges, L.; Michel, U.; Bähr, M.; Lingor, P. Viral vector-mediated downregulation of RhoA increases survival and axonal regeneration of retinal ganglion cells. Front. Cell. Neurosci. 2014, 8, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwak, S.J.; Macks, C.; Jeong, D.U.; Kindy, M.; Lynn, M.; Webb, K.; Lee, J.S. RhoA knockdown by cationic amphiphilic copolymer/siRhoA polyplexes enhances axonal regeneration in rat spinal cord injury model. Biomaterials 2017, 121, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Siebold, C.; Yamashita, T.; Monnier, P.P.; Mueller, B.K.; Pasterkamp, R.J. RGMs: Structural insights, molecular regulation, and downstream signaling. Trends Cell Biol. 2017, 27. [Google Scholar] [CrossRef] [Green Version]
- Hata, K.; Fujitani, M.; Yasuda, Y.; Doya, H.; Saito, T.; Yamagishi, S.; Mueller, B.K.; Yamashita, T. RGMa inhibition promotes axonal growth and recovery after spinal cord injury. J. Cell Biol. 2006, 173. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, S.; Yamashita, T. Repulsive guidance molecule-a is involved in Th17-cell-induced neurodegeneration in autoimmune encephalomyelitis. Cell Rep. 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, R.; Kubo, T.; Mori, M.; Nakamura, Y.; Fujita, Y.; Akutsu, T.; Okuno, T.; Taniguchi, J.; Kumanogoh, A.; Yoshida, M.; et al. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat. Med. 2011, 17. [Google Scholar] [CrossRef]
- Cheng, J.; Korte, N.; Nortley, R.; Sethi, H.; Tang, Y.; Attwell, D. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathol. 2018, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seabrook, T.J.; Littlewood-Evans, A.; Brinkmann, V.; Pöllinger, B.; Schnell, C.; Hiestand, P.C. Angiogenesis is present in experimental autoimmune encephalomyelitis and pro-angiogenic factors are increased in multiple sclerosis lesions. J. Neuroinflamm. 2010, 7, 95. [Google Scholar] [CrossRef] [Green Version]
- Roscoe, W.A.; Welsh, M.E.; Carter, D.E.; Karlik, S.J. VEGF and angiogenesis in acute and chronic MOG ((35-55)) peptide induced EAE. J. Neuroimmunol. 2009, 209, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, R.; Takahashi, C.; Miyake, S.; Fujimura, H.; Mochizuki, H.; Yamashita, T. Angiogenesis induced by CNS inflammation promotes neuronal remodeling through vessel-derived prostacyclin. Nat. Med. 2012, 18. [Google Scholar] [CrossRef] [PubMed]
- Kerschensteiner, M.; Bareyre, F.M.; Buddeberg, B.S.; Merkler, D.; Stadelmann, C.; Brück, W.; Misgeld, T.; Schwab, M.E. Remodeling of axonal connections contributes to recovery in an animal model of multiple sclerosis. J. Exp. Med. 2004, 200, 1027–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vane, J.R.; Botting, R.M. Pharmacodynamic profile of prostacyclin. Am. J. Cardiol. 1995, 75, 3A-10A. [Google Scholar] [CrossRef]
- Tedeschi, A.; Dupraz, S.; Laskowski, C.J.; Xue, J.; Ulas, T.; Beyer, M.; Schultze, J.L.; Bradke, F. The calcium channel subunit Alpha2delta2 suppresses axon regeneration in the adult CNS. Neuron 2016, 92. [Google Scholar] [CrossRef] [Green Version]
- Hoppa, M.B.; Lana, B.; Margas, W.; Dolphin, A.C.; Ryan, T.A. α2δ expression sets presynaptic calcium channel abundance and release probability. Nature 2012, 486, 122–125. [Google Scholar] [CrossRef] [Green Version]
- Fink, K.L.; López-Giráldez, F.; Kim, I.-J.; Strittmatter, S.M.; Cafferty, W.B.J. Identification of intrinsic axon growth modulators for intact cns neurons after injury. Cell Rep. 2017, 18. [Google Scholar] [CrossRef]
- Cafferty, W.B.J.; Strittmatter, S.M. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26. [Google Scholar] [CrossRef] [Green Version]
- Kadoya, K.; Lu, P.; Nguyen, K.; Lee-Kubli, C.; Kumamaru, H.; Yao, L.; Knackert, J.; Poplawski, G.; Dulin, J.N.; Strobl, H.; et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 2016, 22. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150. [Google Scholar] [CrossRef] [Green Version]
- Kumamaru, H.; Kadoya, K.; Adler, A.F.; Takashima, Y.; Graham, L.; Coppola, G.; Tuszynski, M.H.; Expand, A. Generation and post-injury integration of human spinal cord neural stem cells. Nat. Methods 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Poplawski, G.H.D.; Kawaguchi, R.; Niekerk, E.V.; Lu, P.; Mehta, N.; Canete, P.; Lie, R.; Dragatsis, I.; Meves, J.M.; Zheng, B.; et al. Injured adult neurons regress to an embryonic transcriptional growth state. Nature 2020, 581. [Google Scholar] [CrossRef]
- Zou, Y.; Stagi, M.; Wang, X.; Yigitkanli, K.; Siegel, C.S.; Nakatsu, F.; Cafferty, W.B.; Strittmatter, S.M. Gene-silencing screen for mammalian axon regeneration identifies Inpp5f (Sac2) as an endogenous suppressor of repair after spinal cord injury. J. Neurosci. 2015, 35, 10429–10439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekine, Y.; Lin-Moore, A.; Chenette, D.M.; Wang, X.; Jiang, Z.; Cafferty, W.B.; Hammarlund, M.; Strittmatter, S.M. Functional genome-wide screen identifies pathways restricting central nervous system axonal regeneration. Cell Rep. 2018, 23. [Google Scholar] [CrossRef] [Green Version]
- Van Battum, E.Y.; Verhagen, M.G.; Vangoor, V.R.; Fujita, Y.; Derijck, A.A.H.A.; O’Duibhir, E.; Giuliani, G.; de Gunst, T.; Adolfs, Y.; Lelieveld, D.; et al. An image-based miRNA screen identifies miRNA-135s as regulators of CNS axon growth and regeneration by targeting krüppel-like factor 4. J. Neurosci. 2018, 38, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Zou, Y.; Zhang, C.L. Cross-talk between KLF4 and STAT3 regulates axon regeneration. Nat. Commun. 2013, 4, 2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usher, L.C.; Johnstone, A.; Ertürk, A.; Hu, Y.; Strikis, D.; Wanner, I.B.; Moorman, S.; Lee, J.W.; Min, J.; Ha, H.H.; et al. A chemical screen identifies novel compounds that overcome glial-mediated inhibition of neuronal regeneration. J. Neurosci. 2010, 30, 4693–4706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.C.; Campana, A.; Lange, P.S.; Lee, H.H.; Banerjee, K.; Bryson, J.B.; Mahishi, L.; Alam, S.; Giger, R.J.; Barnes, S.; et al. A large-scale chemical screen for regulators of the arginase 1 promoter identifies the soy isoflavone daidzeinas a clinically approved small molecule that can promote neuronal protection or regeneration via a cAMP-independent pathway. J. Neurosci. 2010, 30, 739–748. [Google Scholar] [CrossRef]
- Koprivica, V.; Cho, K.S.; Park, J.B.; Yiu, G.; Atwal, J.; Gore, B.; Kim, J.A.; Lin, E.; Tessier-Lavigne, M.; Chen, D.F.; et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 2005, 310, 106–110. [Google Scholar] [CrossRef]
- Li, H.; Kuwajima, T.; Oakley, D.; Nikulina, E.; Hou, J.; Yang, W.S.; Lowry, E.R.; Lamas, N.J.; Amoroso, M.W.; Croft, G.F.; et al. Protein prenylation constitutes an endogenous brake on axonal growth. Cell Rep. 2016, 16, 545–558. [Google Scholar] [CrossRef] [Green Version]
- Wichterle, H.; Lieberam, I.; Porter, J.A.; Jessell, T.M. Directed differentiation of embryonic stem cells into motor neurons. Cell 2002, 110, 385–397. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.B.; Rivers, L.E.; Young, K.M.; Jamen, F.; Richardson, W.D. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J. Neurosci. 2010, 30, 16383–16390. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.M.; Reynolds, R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide-induced demyelination. Exp. Neurol. 1999, 160, 333–347. [Google Scholar] [CrossRef]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 2010, 6, 578–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moyon, S.; Dubessy, A.L.; Aigrot, M.S.; Trotter, M.; Huang, J.K.; Dauphinot, L.; Potier, M.C.; Kerninon, C.; Melik Parsadaniantz, S.; Franklin, R.J.; et al. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J. Neurosci. 2015, 35, 4–20. [Google Scholar] [CrossRef]
- Barateiro, A.; Fernandes, A. Temporal oligodendrocyte lineage progression: In vitro models of proliferation, differentiation and myelination. Biochim. Biophys. Acta 2014, 1843, 1917–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, M.A.; Macklin, W.B. Zebrafish as a model to investigate CNS myelination. Glia 2015, 63, 177–193. [Google Scholar] [CrossRef] [Green Version]
- Buckley, C.E.; Marguerie, A.; Roach, A.G.; Goldsmith, P.; Fleming, A.; Alderton, W.K.; Franklin, R.J. Drug reprofiling using zebrafish identifies novel compounds with potential pro-myelination effects. Neuropharmacology 2010, 59, 149–159. [Google Scholar] [CrossRef]
- Ashikawa, Y.; Nishimura, Y.; Okabe, S.; Sasagawa, S.; Murakami, S.; Yuge, M.; Kawaguchi, K.; Kawase, R.; Tanaka, T. Activation of sterol regulatory element binding factors by fenofibrate and gemfibrozil stimulates myelination in zebrafish. Front. Pharmacol. 2016, 7, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmukh, V.A.; Tardif, V.; Lyssiotis, C.A.; Green, C.C.; Kerman, B.; Kim, H.J.; Padmanabhan, K.; Swoboda, J.G.; Ahmad, I.; Kondo, T.; et al. A regenerative approach to the treatment of multiple sclerosis. Nature 2013, 502, 327–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, F.; Fancy, S.P.J.; Shen, Y.A.; Niu, J.; Zhao, C.; Presley, B.; Miao, E.; Lee, S.; Mayoral, S.R.; Redmond, S.A.; et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 2014, 20, 954–960. [Google Scholar] [CrossRef] [Green Version]
- Najm, F.J.; Madhavan, M.; Zaremba, A.; Shick, E.; Karl, R.T.; Factor, D.C.; Miller, T.E.; Nevin, Z.S.; Kantor, C.; Sargent, A.; et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature 2015, 522, 216–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melchor, G.S.; Khan, T.; Reger, J.F.; Huang, J.K. Remyelination pharmacotherapy investigations highlight diverse mechanisms underlying multiple sclerosis progression. ACS Pharmacol. Transl. Sci. 2019, 2, 372–386. [Google Scholar] [CrossRef]
- Lariosa-Willingham, K.D.; Rosler, E.S.; Tung, J.S.; Dugas, J.C.; Collins, T.L.; Leonoudakis, D. Development of a central nervous system axonal myelination assay for high throughput screening. BMC Neurosci. 2016, 17, 16. [Google Scholar] [CrossRef] [Green Version]
- Porcu, G.; Serone, E.; De Nardis, V.; Di Giandomenico, D.; Lucisano, G.; Scardapane, M.; Poma, A.; Ragnini-Wilson, A. Clobetasol and halcinonide act as smoothened agonists to promote myelin gene expression and rxrγ receptor activation. PLoS ONE 2015, 10, e0144550. [Google Scholar] [CrossRef]
- Yao, X.; Su, T.; Verkman, A.S. Clobetasol promotes remyelination in a mouse model of neuromyelitis optica. Acta Neuropathol. Commun. 2016, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; He, Y.; Fan, S.; Sun, B. Clemastine rescues behavioral changes and enhances remyelination in the cuprizone mouse model of demyelination. Neurosci. Bull. 2015, 31, 617–625. [Google Scholar] [CrossRef]
- Mei, F.; Mayoral, S.R.; Nobuta, H.; Wang, F.; Desponts, C.; Lorrain, D.S.; Xiao, L.; Green, A.J.; Rowitch, D.; Whistler, J.; et al. Identification of the kappa-opioid receptor as a therapeutic target for oligodendrocyte remyelination. J. Neurosci. 2016, 36, 7925–7935. [Google Scholar] [CrossRef]
- Du, C.; Duan, Y.; Wei, W.; Cai, Y.; Chai, H.; Lv, J.; Du, X.; Zhu, J.; Xie, X. Kappa opioid receptor activation alleviates experimental autoimmune encephalomyelitis and promotes oligodendrocyte-mediated remyelination. Nat. Commun. 2016, 7, 11120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, J.L.; Alley, J.F.; Wellman, L.; Beitz, A.J. Decreased spinal cord opioid receptor mRNA expression and antinociception in a Theiler’s murine encephalomyelitis virus model of multiple sclerosis. Brain Res. 2008, 1191, 180–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, R. Mechanisms of disease: Neuropathic pain--a clinical perspective. Nat. Clin. Pract. Neurol. 2006, 2, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Muramatsu, R.; Maedera, N.; Koyama, Y.; Hamaguchi, M.; Fujimura, H.; Yoshida, M.; Konishi, M.; Itoh, N.; Mochizuki, H.; et al. Peripherally derived FGF21 promotes remyelination in the central nervous system. J. Clin. Investig. 2017, 127, 3496–3509. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, M.; Muramatsu, R.; Fujimura, H.; Mochizuki, H.; Kataoka, H.; Yamashita, T. Circulating transforming growth factor-β1 facilitates remyelination in the adult central nervous system. Elife 2019, 8. [Google Scholar] [CrossRef]
- Muramatsu, R.; Kuroda, M.; Matoba, K.; Lin, H.; Takahashi, C.; Koyama, Y.; Yamashita, T. Prostacyclin prevents pericyte loss and demyelination induced by lysophosphatidylcholine in the central nervous system. J. Biol. Chem. 2015, 290, 11515–11525. [Google Scholar] [CrossRef] [Green Version]
- Grainger, D.J.; Mosedale, D.E.; Metcalfe, J.C. TGF-beta in blood: A complex problem. Cytokine Growth Factor Rev. 2000, 11, 133–145. [Google Scholar] [CrossRef]
- Assoian, R.K.; Komoriya, A.; Meyers, C.A.; Miller, D.M.; Sporn, M.B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 1983, 258, 7155–7160. [Google Scholar]
- Ghafoory, S.; Varshney, R.; Robison, T.; Kouzbari, K.; Woolington, S.; Murphy, B.; Xia, L.; Ahamed, J. Platelet TGF-β1 deficiency decreases liver fibrosis in a mouse model of liver injury. Blood Adv. 2018, 2, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.K.; Jarjour, A.A.; Nait Oumesmar, B.; Kerninon, C.; Williams, A.; Krezel, W.; Kagechika, H.; Bauer, J.; Zhao, C.; Baron-Van Evercooren, A.; et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat. Neurosci. 2011, 14, 45–53. [Google Scholar] [CrossRef]
- Voskuhl, R.R.; Itoh, N.; Tassoni, A.; Matsukawa, M.A.; Ren, E.; Tse, V.; Jang, E.; Suen, T.T.; Itoh, Y. Gene expression in oligodendrocytes during remyelination reveals cholesterol homeostasis as a therapeutic target in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 116, 10130–10139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, E.; Yang, L.; Su, T.; Morris, D.R.; McKnight, G.S.; Amieux, P.S. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. USA 2009, 106, 13939–13944. [Google Scholar] [CrossRef] [Green Version]
- Saher, G.; Brügger, B.; Lappe-Siefke, C.; Möbius, W.; Tozawa, R.; Wehr, M.C.; Wieland, F.; Ishibashi, S.; Nave, K.A. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005, 8, 468–475. [Google Scholar] [CrossRef]
- Berghoff, S.A.; Gerndt, N.; Winchenbach, J.; Stumpf, S.K.; Hosang, L.; Odoardi, F.; Ruhwedel, T.; Böhler, C.; Barrette, B.; Stassart, R.; et al. Dietary cholesterol promotes repair of demyelinated lesions in the adult brain. Nat. Commun. 2017, 8, 14241. [Google Scholar] [CrossRef]
| Gene | Identify Method | In Vivo Manipulation/Outcomes | Reference |
|---|---|---|---|
| Cacna2d2 | Transcriptome analysis of DRG neurons during development and regeneration | α2δ2 blockade by pregabalin/enhanced axon regeneration after SCI | [27] |
| Lpar1 | Transcriptome analysis of CST sprouting neurons | LPAR1 blockage of LPAR1 by AM095/enhanced CST sprouting and functional recovery after pyramidotomy | [29] |
| Lppr1 | Transcriptome analysis of CST sprouting neurons | AAV-mediated overexpression of LPPR1/enhanced CST sprouting and functional recovery after pyramidotomy | [29] |
| huntingtin | Transcriptome analysis of CST neurons after SCI with/without NPC grafts | HTT cKO in CST neurons/diminished CST axon regeneration after SCI with NPC grafts | [34] |
| Inpp5f | Functional genomic screening on cortical neurons | Inpp5f KO mice/enhanced CST sprouting and functional recovery after SCI | [35] |
| Rab27 | Genome-wide functional genomic screening on cortical neurons | Rab27 KO mice/enhanced RGC regeneration after ONC, and enhanced RpST axon sprouting and functional recovery after SCI | [36] |
| Compound | Proposed Mechanism | Screening Model | In Vivo Model | Reference |
|---|---|---|---|---|
| Benztropine | Muscarinic receptor | OPCs from rat optic nerve | EAE and Cuprizone | [52] |
| Clemastine | Muscarinic receptor | OPCs from rat or mouse cortices | LPC, EAE, and Cuprizone | [53,59] |
| Miconazole | MAP kinase | Mouse ES-derived OPCs | LPC, EAE | [54] |
| Clobetasol | glucocorticoid receptor | Mouse ES-derived OPCs and mouse immortalized OL cell line | LPC, EAE, and NMO | [54,57,58] |
| U-50488 | κ-opioid receptor | Mouse ESC-derived OPCs | LPC, EAE, and cuprizone | [60,61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uyeda, A.; Muramatsu, R. Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review. Int. J. Mol. Sci. 2020, 21, 8116. https://doi.org/10.3390/ijms21218116
Uyeda A, Muramatsu R. Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review. International Journal of Molecular Sciences. 2020; 21(21):8116. https://doi.org/10.3390/ijms21218116
Chicago/Turabian StyleUyeda, Akiko, and Rieko Muramatsu. 2020. "Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review" International Journal of Molecular Sciences 21, no. 21: 8116. https://doi.org/10.3390/ijms21218116
APA StyleUyeda, A., & Muramatsu, R. (2020). Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review. International Journal of Molecular Sciences, 21(21), 8116. https://doi.org/10.3390/ijms21218116





