Emerging Mechanisms of Pulmonary Vasoconstriction in SARS-CoV-2-Induced Acute Respiratory Distress Syndrome (ARDS) and Potential Therapeutic Targets
Abstract
:1. Introduction
2. COVID-19 in Patients with Pulmonary Hypertension (PH)
3. Pulmonary Vascular Sequela in SARS-CoV-2 Infection
4. Mechanisms of Vasoconstriction in COVID-19
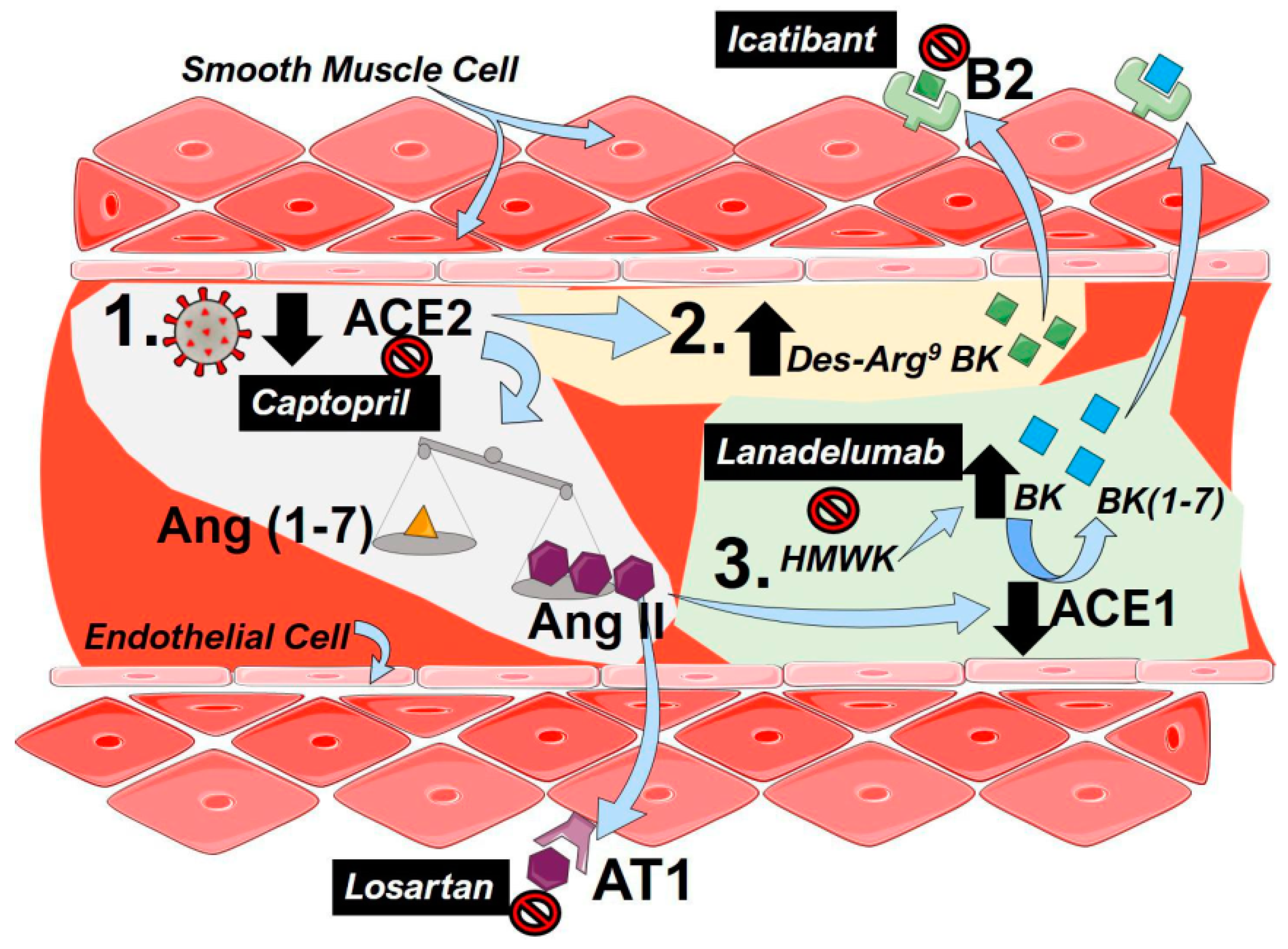
4.1. The Renin–Angiotensin System (RAS)
4.2. The Kallikrein–Kinin System (KKS)
5. Drugs Targeting the RAS
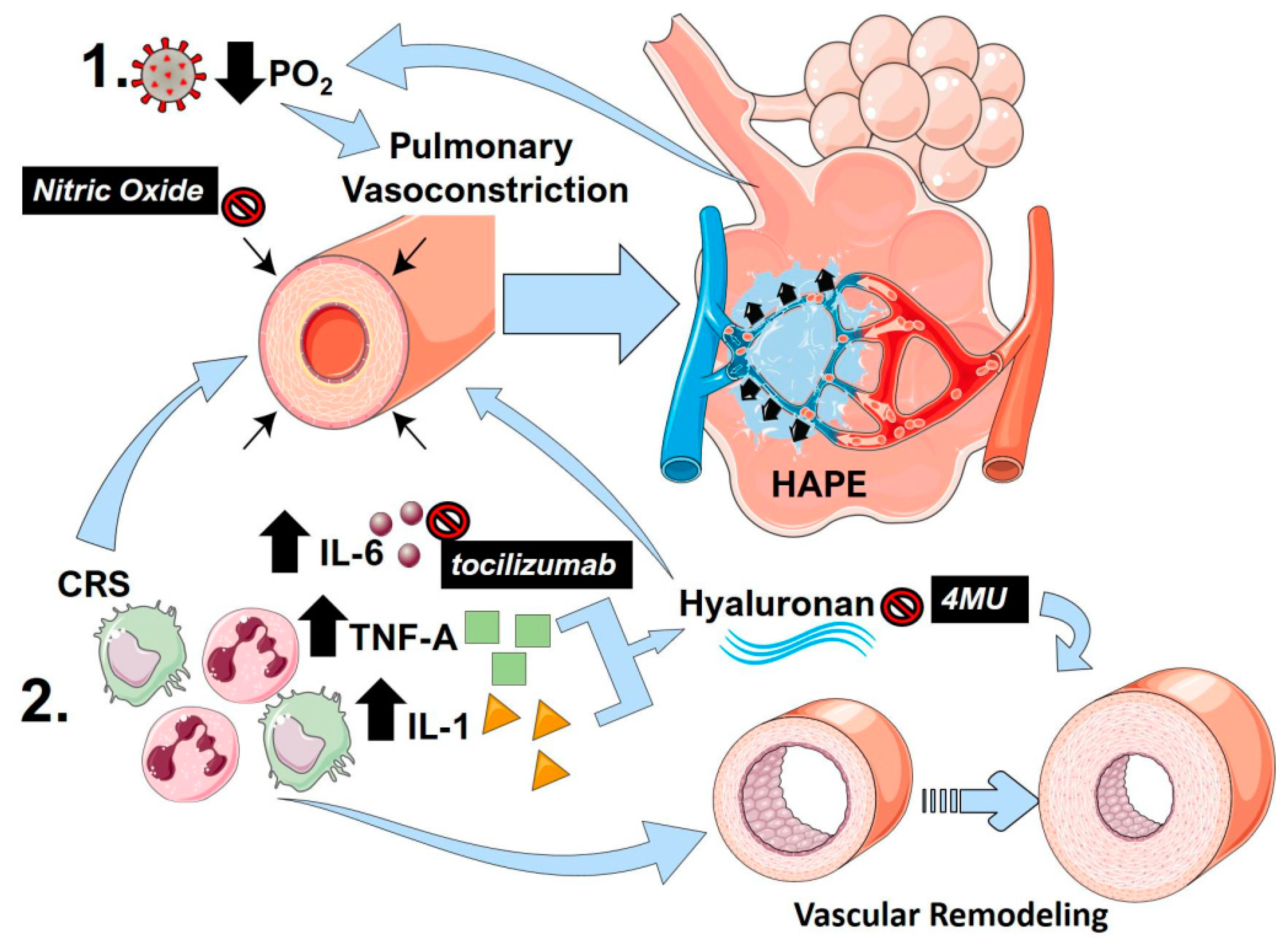
6. Hypoxic Pulmonary Vasoconstriction
7. High-Altitude Induced Pulmonary Edema
8. Nitric Oxide
9. The Cytokine Release Storm (CRS)
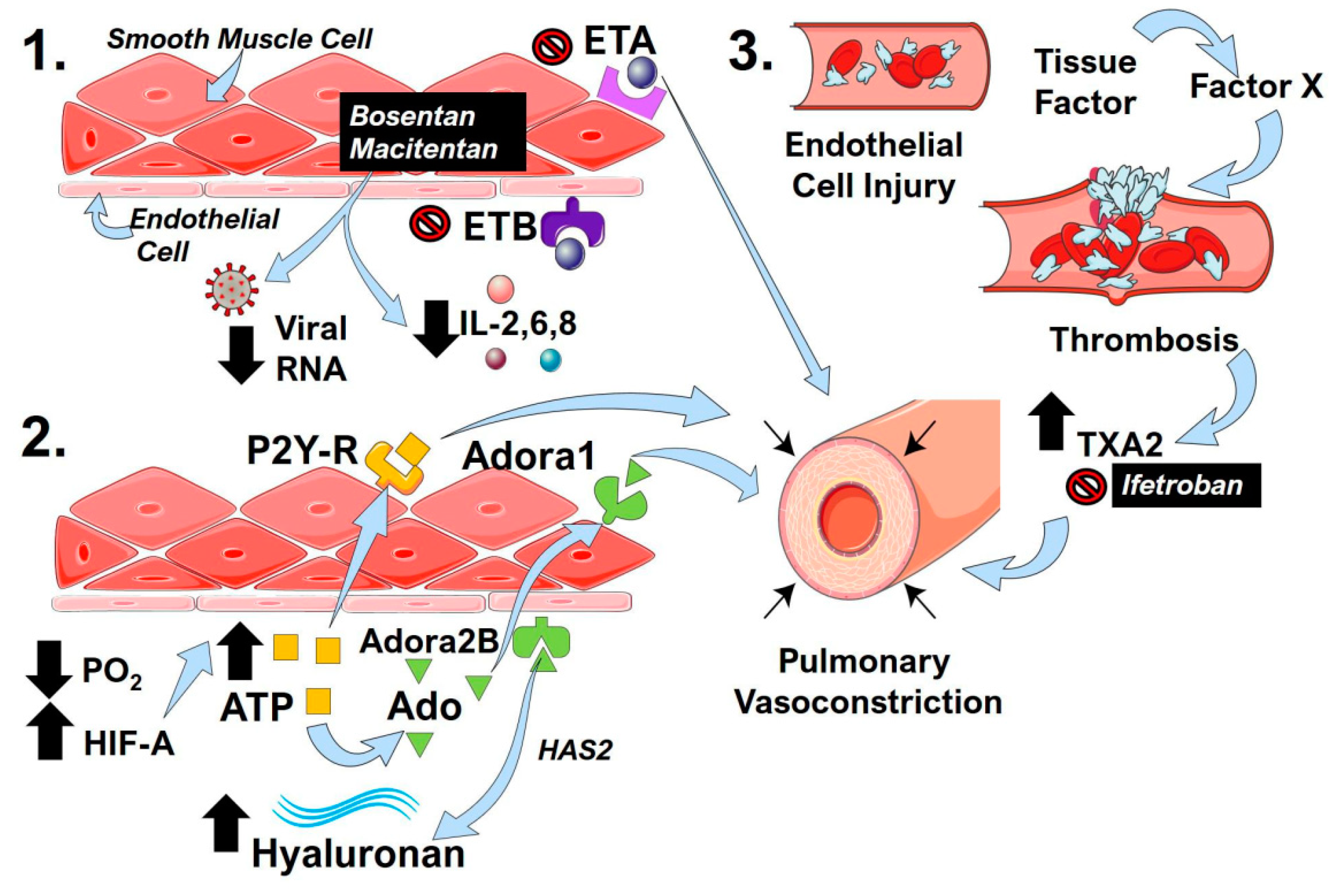
10. Endothelin
11. The Hypoxic-Adenosinergic Response
12. Clotting Cascade and Pulmonary Vascular Microthrombi
13. Conclusions
Funding
Conflicts of Interest
References
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L. The Acute Respiratory Distress Syndrome: Pathogenesis and Treatment. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 147–163. [Google Scholar] [CrossRef] [Green Version]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis Primers 2019, 5, 18. [Google Scholar] [CrossRef]
- Ashbaugh, D.G.; Bigelow, D.B.; Petty, T.L.; Levine, B.E. Acute respiratory distress in adults. Lancet 1967, 2, 319–323. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Archer, S.L.; Weir, E.K.; Wilkins, M.R. Basic Science of Pulmonary Arterial Hypertension for Clinicians. Circulation 2010, 121, 2045–2066. [Google Scholar] [CrossRef] [PubMed]
- Dweck, M.R.; Bularga, A.; Hahn, R.T.; Bing, R.; Lee, K.K.; Chapman, A.R.; White, A.; Di Salvo, G.; Sade, L.E.; Pearce, K.; et al. Global evaluation of echocardiography in patients with COVID-19. Eur. Hear. J. Cardiovasc. Imaging 2020, 21, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Lax, S.F.; Skok, K.; Zechner, P.; Kessler, H.H.; Kaufmann, N.; Koelblinger, C.; Vander, K.; Bargfrieder, U.; Trauner, M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome. Ann. Intern. Med. 2020, 173, 350–361. [Google Scholar] [CrossRef]
- Oudkerk, M.; Büller, H.R.; Kuijpers, D.; Van Es, N.; Oudkerk, S.F.; McLoud, T.C.; Gommers, D.; Van Dissel, J.; Cate, H.T.; Van Beek, E.J.R. Diagnosis, Prevention, and Treatment of Thromboembolic Complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology 2020, 297, E216–E222. [Google Scholar] [CrossRef]
- Han, H.; Yang, L.; Liu, R.; Liu, F.; Wu, K.-L.; Li, J.; Liu, X.-H.; Zhu, C.-L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin. Chem. Lab. Med. 2020, 58, 1116–1120. [Google Scholar] [CrossRef] [Green Version]
- Klok, F.; Kruip, M.; Van Der Meer, N.; Arbous, M.; Gommers, D.; Kant, K.; Kaptein, F.; Van Paassen, J.; Stals, M.; Huisman, M.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Buja, L.M.; Wolf, D.A.; Zhao, B.; Akkanti, B.; McDonald, M.; Lelenwa, L.; Reilly, N.; Ottaviani, G.; Elghetany, M.T.; Trujillo, D.O.; et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020, 48, 107233. [Google Scholar] [CrossRef]
- Barth, R.F.; Buja, L.M.; Parwani, A.V. The spectrum of pathological findings in coronavirus disease (COVID-19) and the pathogenesis of SARS-CoV-2. Diagn. Pathol. 2020, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzik, T.J.; Mohiddin, S.A.; DiMarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nature reviews. Cardiology 2020, 17, 259–260. [Google Scholar]
- Potus, F.; Mai, V.; Lebret, M.; Malenfant, S.; Breton-Gagnon, E.; Lajoie, A.C.; Boucherat, O.; Bonnet, S.; Provencher, S. Novel insights on the pulmonary vascular consequences of COVID-19. Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L277–L288. [Google Scholar] [CrossRef] [PubMed]
- Horn, E.M.; Chakinala, M.; Oudiz, R.; Joseloff, E.; Rosenzweig, E.B. Could pulmonary arterial hypertension patients be at a lower risk from severe COVID-19? Pulm. Circ. 2020, 10, 2045894020922799. [Google Scholar] [PubMed] [Green Version]
- Fernandes, T.M.; Papamatheakis, D.G.; Poch, D.S.; Kim, N.H. Letter to the Editor regarding “Could pulmonary arterial hypertension patients be at lower risk from severe COVID-19?”. Pulm. Circ. 2020, 10, 2045894020925761. [Google Scholar] [CrossRef]
- Ryan, J.J.; Melendres-Groves, L.; Zamanian, R.T.; Oudiz, R.J.; Chakinala, M.M.; Rosenzweig, E.B.; Gomberg-Maitland, M. Care of patients with pulmonary arterial hypertension during the coronavirus (COVID-19) pandemic. Pulm. Circ. 2020, 10, 2045894020920153. [Google Scholar] [CrossRef] [Green Version]
- Fletcher-Sandersjöö, A.; Bellander, B.-M. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb. Res. 2020, 194, 36–41. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- SahayMD, S.; Farber, H.W. Management of hospitalized patients with pulmonary arterial hypertension and COVID-19 infection. Pulm. Circ. 2020, 10, 2045894020933480. [Google Scholar] [CrossRef]
- Pinto, V.M.; Derchi, G.E.; Bacigalupo, L.; Pontali, E.; Forni, G.L. COVID-19 in a Patient with β-Thalassemia Major and Severe Pulmonary Arterial Hypertension. Hemoglobin 2020, 44, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Burri, P.H.; Djonov, V. Intussusceptive angiogenesis—The alternative to capillary sprouting. Mol. Asp. Med. 2002, 23, 1–27. [Google Scholar] [CrossRef]
- Tuder, R.M.; Voelkel, N.F. Angiogenesis and Pulmonary Hypertension: A Unique Process in a Unique Disease. Antioxid. Redox Signal. 2002, 4, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, M.; Ruffenach, G.; Meloche, J.; Bonnet, S. Adaptation and Remodelling of the Pulmonary Circulation in Pulmonary Hypertension. Can. J. Cardiol. 2015, 31, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.J.; Santos, R.A.; Bradford, C.N.; Mecca, A.P.; Sumners, C.; Katovich, M.J.; Raizada, M.K. Therapeutic Implications of the Vasoprotective Axis of the Renin-Angiotensin System in Cardiovascular Diseases. Hypertension 2010, 55, 207–213. [Google Scholar] [CrossRef]
- Kalra, J.; Prakash, A.; Kumar, P.; Majeed, A.B.A. Cerebroprotective effects of RAS inhibitors: Beyond their cardio-renal actions. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 459–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passos-Silva, D.G.; Brandan, E.; Santos, R.A.S. Angiotensins as therapeutic targets beyond heart disease. Trends Pharm. Sci. 2015, 36, 310–320. [Google Scholar] [CrossRef]
- Iwai, M.; Horiuchi, M. Devil and angel in the renin–angiotensin system: ACE–angiotensin II–AT1 receptor axis vs. ACE2–angiotensin-(1–7)–Mas receptor axis. Hypertens. Res. 2009, 32, 533–536. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 1–5. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.C.; Lely, A.T.; Navis, G.J.; Van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Glowacka, I.; Bertram, S.; Herzog, P.; Pfefferle, S.; Steffen, I.; Muench, M.O.; Simmons, G.; Hofmann, H.; Kuri, T.; Weber, F.; et al. Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. J. Virol. 2009, 84, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Insel, P. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Authorea 2020. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, V.; Veeraveedu, P.T.; Gurusamy, N.; Lakshmanan, A.P.; Yamaguchi, K.; Ma, M.; Suzuki, K.; Kodama, M.; Watanabe, K. Telmisartan acts through the modulation of ACE-2/ANG 1-7/mas receptor in rats with dilated cardiomyopathy induced by experimental autoimmune myocarditis. Life Sci. 2012, 90, 289–300. [Google Scholar] [CrossRef]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Kai, H.; Kai, M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—Lessons from available evidence and insights into COVID-19. Hypertens. Res. 2020, 43, 648–654. [Google Scholar] [CrossRef]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: The cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit. Care 2020, 24, 1–8. [Google Scholar] [CrossRef]
- Shenoy, V.; Qi, Y.; Katovich, M.J.; Raizada, M.K. ACE2, a promising therapeutic target for pulmonary hypertension. Curr. Opin. Pharm. 2011, 11, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, J.; Martin, M.; He, M.; Gongol, B.; Marin, T.L.; Chen, L.; Shi, X.; Yin, Y.; Shang, F.; et al. AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 509–520. [Google Scholar] [CrossRef]
- Rathinasabapathy, A.; Bryant, A.J.; Suzuki, T.; Moore, C.; Shay, S.; Gladson, S.; West, J.D.; Carrier, E.J. rhACE2 Therapy Modifies Bleomycin-Induced Pulmonary Hypertension via Rescue of Vascular Remodeling. Front. Physiol. 2018, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Herizi, A.; Jover, B.; Bouriquet, N.; Mimran, A. Prevention of the cardiovascular and renal effects of angiotensin II by endothelin blockade. Hypertens 1998, 31, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Funke, C.; Farr, M.; Werner, B.; Dittmann, S.; Überla, K.; Piper, C.; Niehaus, K.; Horstkotte, D. Antiviral effect of Bosentan and Valsartan during coxsackievirus B3 infection of human endothelial cells. J. Gen. Virol. 2010, 91, 1959–1970. [Google Scholar] [CrossRef]
- Rhaleb, N.-E.; Yang, X.-P.; Carretero, O.A. The Kallikrein-Kinin System as a Regulator of Cardiovascular and Renal Function. Compr. Physiol. 2011, 1, 971–993. [Google Scholar] [CrossRef] [Green Version]
- Van De Veerdonk, F.L.; Netea, M.G.; Van Deuren, M.; Van Der Meer, J.W.; De Mast, Q.; Brüggemann, R.J.; Van Der Hoeven, H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 2020, 9. [Google Scholar] [CrossRef]
- Roche, J.A.; Roche, R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. Faseb J. 2020, 34, 7265–7269. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Wohlford-Lenane, C.; Yamaguchi, Y.; Prindle, T.; Fulton, W.B.; Wang, S.; McCray, P.B.; Chappell, M.; Hackam, D.J.; Jia, H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Cell. Mol. Physiol. 2018, 314, L17–L31. [Google Scholar] [CrossRef] [PubMed]
- Duchêne, J.; Ahluwalia, A. The kinin B1 receptor and inflammation: New therapeutic target for cardiovascular disease. Curr. Opin. Pharm. 2009, 9, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Charignon, D.; Späth, P.; Martin, L.; Drouet, C. Icatibant, the bradykinin B2 receptor antagonist with target to the interconnected kinin systems. Expert Opin. Pharm. 2012, 13, 2233–2247. [Google Scholar] [CrossRef]
- Cabrini, D.A.; Calixto, J.B. Characterization of des-Arg9-bradykinin-induced contraction in guinea-pig gallbladder in vitro. Eur. J. Pharm. 1997, 331, 31–38. [Google Scholar] [CrossRef]
- Griesbacher, T.; Sametz, W.; Legat, F.J.; Diethart, S.; Hammer, S.; Juan, H. Effects of the non-peptide B2 antagonist FR173657 on kinin-induced smooth muscle contraction and relaxation, vasoconstriction and prostaglandin release. Br. J. Pharm. 1997, 121, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasciolo, J.C.; Vargas, L.; Lama, M.C.; Nolly, H. Bradykinin-induced vasoconstriction of rat mesenteric arteries precontracted with noradrenaline. Br. J. Pharm. 1990, 101, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Colarusso, C.; Terlizzi, M.; Pinto, A.; Sorrentino, R. A lesson from a saboteur: High-MW kininogen impact in coronavirus-induced disease 2019. Br. J. Pharm. 2020, 177, 4866–4872. [Google Scholar] [CrossRef]
- Ghahestani, S.M.; Mahmoudi, J.; Hajebrahimi, S.; Sioofy-Khojine, A.-B.; Salehi-Pourmehr, H.; Sadeghi-Ghyassi, F.; Mostafaei, H. Bradykinin as a Probable Aspect in SARS-Cov-2 Scenarios: Is Bradykinin Sneaking out of our Sight? Iran. J. Allergy Asthma Immunol. 2020, 19, 13–17. [Google Scholar] [CrossRef]
- Lauer, S.; Fischer, L.G.; Van Aken, H.K.; Nofer, J.-R.; Freise, H. Gadolinium chloride modulates bradykinin-induced pulmonary vasoconstriction and hypoxic pulmonary vasoconstriction during polymicrobial abdominal sepsis in rats. Exp. Lung Res. 2015, 41, 270–282. [Google Scholar] [CrossRef]
- Fischer, L.G.; Hilpert, J.H.; Freise, H.; Wendholt, D.; Van Aken, H.; Sielenkämper, A.W. Bradykinin-Induced Pulmonary Vasoconstriction Is Time and Inducible Nitric Oxide Synthase Dependent in a Peritonitis Sepsis Model. Anesth. Analg. 2004, 99, 864–871. [Google Scholar] [CrossRef]
- Taraseviciene-Stewart, L.; Scerbavicius, R.; Stewart, J.M.; Gera, L.; Demura, Y.; Cool, C.; Kasper, M.; Voelkel, N.F. Treatment of severe pulmonary hypertension: A bradykinin receptor 2 agonist B9972 causes reduction of pulmonary artery pressure and right ventricular hypertrophy. Peptides 2005, 26, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Rico-Mesa, J.S.; White, A.; Anderson, A.S. Outcomes in Patients with COVID-19 Infection Taking ACEI/ARB. Curr. Cardiol. Rep. 2020, 22, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.-J.; Xie, J.; Liu, Y.-M.; Zhao, Y.-C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients with Hypertension Hospitalized With COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- Onweni, C.L.; Zhang, Y.S.; Caulfield, T.; Hopkins, C.E.; Fairweather, D.L.; Freeman, W.D. ACEI/ARB therapy in COVID-19: The double-edged sword of ACE2 and SARS-CoV-2 viral docking. Crit. Care 2020, 24, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, Z.; Li, Y.; Huang, W.; Zhou, M.; Zhang, X.; Jiang, W. Angiotensin-Converting Enzyme Inhibition Attenuates Lipopolysaccharide-Induced Lung Injury by Regulating the Balance Between Angiotensin-Converting Enzyme and Angiotensin-Converting Enzyme 2 and Inhibiting Mitogen-Activated Protein Kinase Activation. Shock 2015, 43, 395–404. [Google Scholar] [CrossRef]
- He, X.; Han, B.; Mura, M.; Xia, S.; Wang, S.; Ma, T.; Liu, M.; Liu, Z. Angiotensin-Converting Enzyme Inhibitor Captopril Prevents Oleic Acid-Induced Severe Acute Lung Injury in Rats. Shock 2007, 28, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Mo, H.; Cai, L.; Kong, T.; Zheng, W.; Ye, J.; Qi, J.; Xiao, Z. Losartan Prevents Sepsis-Induced Acute Lung Injury and Decreases Activation of Nuclear Factorκb And Mitogen-Activated Protein Kinases. Shock 2009, 31, 500–506. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nat. Cell Biol. 2005, 436, 112–116. [Google Scholar] [CrossRef]
- Al-Shamlan, F.; El-Hashim, A.Z. Bradykinin sensitizes the cough reflex via a B2 receptor dependent activation of TRPV1 and TRPA1 channels through metabolites of cyclooxygenase and 12-lipoxygenase. Respir. Res. 2019, 20, 1–15. [Google Scholar] [CrossRef]
- Lumpuy-Castillo, J.; Lorenzo-Almorós, A.; Pello-Lázaro, A.M.; Sánchez-Ferrer, C.F.; Egido, J.; Tuñón, J.; Peiró, C.; Lorenzo, Ó. Cardiovascular Damage in COVID-19: Therapeutic Approaches Targeting the Renin-Angiotensin-Aldosterone System. Int. J. Mol. Sci. 2020, 21, 6471. [Google Scholar] [CrossRef]
- Young, J.M.; Williams, D.R.; Thompson, A.A.R. Thin Air, Thick Vessels: Historical and Current Perspectives on Hypoxic Pulmonary Hypertension. Front. Med. 2019, 6, 93. [Google Scholar] [CrossRef] [Green Version]
- Lumb, A.B.; Slinger, P. Hypoxic Pulmonary Vasoconstriction. Surv. Anesth. 2015, 59, 188. [Google Scholar] [CrossRef]
- Archer, S.L.; Sharp, W.W.; Weir, E.K. Differentiating COVID-19 Pneumonia from Acute Respiratory Distress Syndrome (ARDS) and High Altitude Pulmonary Edema (HAPE): Therapeutic Implications. Circulation 2020, 142, 101–104. [Google Scholar] [CrossRef]
- Kylhammar, D.; Rådegran, G. The principal pathways involved in thein vivomodulation of hypoxic pulmonary vasoconstriction, pulmonary arterial remodelling and pulmonary hypertension. Acta Physiol. 2016, 219, 728–756. [Google Scholar] [CrossRef]
- Strielkov, I.; Krause, N.C.; Sommer, N.; Schermuly, R.T.; Ghofrani, H.-A.; Grimminger, F.; Gudermann, T.; Dietrich, A.; Weissmann, N. Hypoxic pulmonary vasoconstriction in isolated mouse pulmonary arterial vessels. Exp. Physiol. 2018, 103, 1185–1191. [Google Scholar] [CrossRef]
- Talbot, N.P.; Balanos, G.M.; Dorrington, K.L.; Robbins, P.A. Two temporal components within the human pulmonary vascular response to approximately 2 h of isocapnic hypoxia. J. Appl. Physiol. 2005, 98, 1125–1139. [Google Scholar] [CrossRef]
- Dorrington, K.L.; Clar, C.; Young, J.D.; Jonas, M.; Tansley, J.G.; Robbins, P.A. Time course of the human pulmonary vascular response to 8 hours of isocapnic hypoxia. Am. J. Physiol. Content 1997, 273, H1126–H1134. [Google Scholar] [CrossRef]
- McLoughlin, P. Hypoxic pulmonary vasoconstriction: Building a solid base. Exp. Physiol. 2018, 103, 1181–1182. [Google Scholar] [CrossRef]
- Rowan, S.C.; Keane, M.P.; Gaine, S.; McLoughlin, P. Hypoxic pulmonary hypertension in chronic lung diseases: Novel vasoconstrictor pathways. Lancet Respir. Med. 2016, 4, 225–236. [Google Scholar] [CrossRef]
- Price, L.C.; Wort, S.J. Pulmonary hypertension in ARDS: Inflammation matters! Thorax 2017, 72, 396–397. [Google Scholar] [CrossRef]
- Ryan, D.; Frohlich, S.; McLoughlin, P. Pulmonary vascular dysfunction in ARDS. Ann. Intensiv. Care 2014, 4, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zochios, V.; Parhar, K.; Tunnicliffe, W.; Roscoe, A.; Gao, F. The Right Ventricle in ARDS. Chest 2017, 152, 181–193. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P.T. Hypoxic Pulmonary Vasoconstriction. Physiol. Rev. 2012, 92, 367–520. [Google Scholar] [CrossRef] [PubMed]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in Critically Ill Patients in the Seattle Region—Case Series. New Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Ullah, W.; Saeed, R.; Sarwar, U.; Patel, R.; Fischman, D.L. COVID-19 Complicated by Acute Pulmonary Embolism and Right-Sided Heart Failure. JACC Case Rep. 2020, 2, 1379–1382. [Google Scholar] [CrossRef]
- Bartsch, P. High altitude pulmonary edema. Med. Sci. Sports Ex. 1999, 31, S23–S27. [Google Scholar] [CrossRef]
- Solaimanzadeh, I. Acetazolamide, Nifedipine and Phosphodiesterase Inhibitors: Rationale for Their Utilization as Adjunctive Countermeasures in the Treatment of Coronavirus Disease 2019 (COVID-19). Cureus 2020, 12, e7343. [Google Scholar] [CrossRef] [Green Version]
- Luks, A.M.; Freer, L.; Grissom, C.K.; McIntosh, S.E.; Schoene, R.B.; Swenson, E.R.; Hackett, P.H. COVID-19 Lung Injury is Not High Altitude Pulmonary Edema. High. Alt. Med. Biol. 2020, 21, 192–193. [Google Scholar] [CrossRef] [Green Version]
- Strapazzon, G.; Hilty, M.P.; Bouzat, P.; Pratali, L.; Brugger, H.; Rauch, S. To compare the incomparable: COVID-19 pneumonia and high-altitude disease. Eur. Respir. J. 2020, 55, 2001362. [Google Scholar] [CrossRef]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensiv. Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef]
- Dunham-Snary, K.J.; Wu, D.; Sykes, E.A.; Thakrar, A.; Parlow, L.R.; Mewburn, J.D.; Parlow, J.L.; Archer, S.L. Hypoxic Pulmonary Vasoconstriction. Chest 2017, 151, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akmal, A.H.; Hasan, M. Role of nitric oxide in management of acute respiratory distress syndrome. Ann. Thorac. Med. 2008, 3, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Garg, A.; Vignesh, C.; Singh, V.K. Acute right heart syndrome: Rescue treatment with inhaled nitric oxide. Indian J. Crit. Care Med. 2014, 18, 40–42. [Google Scholar] [CrossRef] [Green Version]
- Keyaerts, E.; Vijgen, L.; Chen, L.; Maes, P.; Hedenstierna, G.; Van Ranst, M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004, 8, 223–226. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, P.; Gao, H.; Sun, B.; Chao, D.; Wang, F.; Zhu, Y.; Hedenstierna, G.; Wang, C.G. Inhalation of Nitric Oxide in the Treatment of Severe Acute Respiratory Syndrome: A Rescue Trial in Beijing. Clin. Infect. Dis. 2004, 39, 1531–1535. [Google Scholar] [CrossRef] [Green Version]
- Carvajal, J.A.; Germain, A.M.; Huidobro-Toro, J.P.; Weiner, C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 2000, 184, 409–420. [Google Scholar] [CrossRef]
- Thomas, M.K.; Francis, S.H.; Corbin, J.D. Characterization of a purified bovine lung cGMP-binding cGMP phosphodiesterase. J. Biol. Chem. 1990, 265, 14964–14970. [Google Scholar]
- Frostell, C.G.; Blomqvist, H.; Hedenstierna, G.; Lundberg, J.; Zapol, W.M. Inhaled Nitric Oxide Selectively Reverses Human Hypoxic Pulmonary Vasoconstriction without Causing Systemic Vasodilation. Anesthesiology 1993, 78, 427–435. [Google Scholar] [CrossRef]
- Scherrer, U.; Rexhaj, E.; Jayet, P.-Y.; Allemann, Y.; Sartori, C. New Insights in the Pathogenesis of High-Altitude Pulmonary Edema. Prog. Cardiovasc. Dis. 2010, 52, 485–492. [Google Scholar] [CrossRef]
- Hirakawa, A.; Sakamoto, H.; Misumi, K.; Kamimura, T.; Shimizu, R. Effects of Inhaled Nitric Oxide on Hypoxic Pulmonary Vasoconstriction in Dogs and a Case Report of Venae Cavae Syndrome. J. Veter. Med. Sci. 1996, 58, 551–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghofrani, H.-A.; Reichenberger, F.; Kohstall, M.G.; Mrosek, E.H.; Seeger, W.; Olschewski, H.; Seeger, W.; Grimminger, F. Sildenafil Increased Exercise Capacity during Hypoxia at Low Altitudes and at Mount Everest Base Camp. Ann. Intern. Med. 2004, 141, 169–177. [Google Scholar] [CrossRef]
- Maggiorini, M.; Rocca, H.-P.B.-L.; Peth, S.; Fischler, M.; Böhm, T.; Bernheim, A.; Kiencke, S.; Bloch, K.E.; Dehnert, C.; Naeije, R.; et al. Both Tadalafil and Dexamethasone May Reduce the Incidence of High-Altitude Pulmonary Edema. Ann. Intern. Med. 2006, 145, 497–506. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intens. Care Med. 2020, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Ragab, D.; Eldin, H.S.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Wang, G.-Q. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020, 92, 814–818. [Google Scholar] [CrossRef]
- Liu, B.; Li, M.; Zhou, Z.; Guan, X.; Xiang, Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020, 111, 102452. [Google Scholar] [CrossRef]
- Steiner, M.K.; Syrkina, O.L.; Kolliputi, N.; Mark, E.J.; Hales, C.A.; Waxman, A.B. Interleukin-6 overexpression induces pulmonary hypertension. Circ. Res. 2008, 104, 236–244. [Google Scholar] [CrossRef]
- Tamura, Y.; Phan, C.; Tu, L.; Le Hiress, M.; Thuillet, R.; Jutant, E.-M.; Fadel, E.; Savale, L.; Huertas, A.; Humbert, M.; et al. Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J. Clin. Investig. 2018, 128, 1956–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.-Y.; Collum, S.; Luo, F.; Weng, T.; Le, T.-T.; Hernandez, A.M.; Philip, K.; Molina, J.G.; Garcia-Morales, L.J.; Cao, Y.; et al. Macrophage bone morphogenic protein receptor 2 depletion in idiopathic pulmonary fibrosis and Group III pulmonary hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2016, 311, L238–L254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pullamsetti, S.S.; Seeger, W.; Savai, R. Classical IL-6 signaling: A promising therapeutic target for pulmonary arterial hypertension. J. Clin. Investig. 2018, 128, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.A.; Dunmore, B.J.; Long, L.; Crosby, A.; Al-Lamki, R.; Deighton, J.; Southwood, M.; Yang, X.; Nikolic, M.Z.; Herrera, B.; et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat. Commun. 2017, 8, 14079. [Google Scholar] [CrossRef]
- Wagner, E.M. TNF-α induced bronchial vasoconstriction. Am. J. Physiol. Circ. Physiol. 2000, 279, H946–H951. [Google Scholar] [CrossRef]
- Bell, T.J.; Brand, O.J.; Morgan, D.J.; Salek-Ardakani, S.; Jagger, C.; Fujimori, T.; Cholewa, L.; Tilakaratna, V.; Östling, J.; Thomas, M.; et al. Defective lung function following influenza virus is due to prolonged, reversible hyaluronan synthesis. Matrix Biol. 2019, 80, 14–28. [Google Scholar] [CrossRef]
- Aytekin, M.; Comhair, S.A.A.; De La Motte, C.; Bandyopadhyay, S.K.; Farver, C.F.; Hascall, V.C.; Erzurum, S.C.; Dweik, R.A. High levels of hyaluronan in idiopathic pulmonary arterial hypertension. Am. J. Physiol. Cell. Mol. Physiol. 2008, 295, L789–L799. [Google Scholar] [CrossRef] [Green Version]
- Papakonstantinou, E.; Kouri, F.M.; Karakiulakis, G.; Klagas, I.; Eickelberg, O. Increased hyaluronic acid content in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2008, 32, 1504–1512. [Google Scholar] [CrossRef] [Green Version]
- Collum, S.; Chen, N.; Hernandez, A.M.; Hanmandlu, A.; Sweeney, H.; Mertens, T.C.J.; Weng, T.; Luo, F.; Molina, J.G.; Davies, J.; et al. Inhibition of hyaluronan synthesis attenuates pulmonary hypertension associated with lung fibrosis. Br. J. Pharm. 2017, 174, 3284–3301. [Google Scholar] [CrossRef] [Green Version]
- Collum, S.; Molina, J.G.; Hanmandlu, A.; Bi, W.; Pedroza, M.; Mertens, T.C.J.; Wareing, N.; Wei, W.; Wilson, C.; Sun, W.; et al. Adenosine and hyaluronan promote lung fibrosis and pulmonary hypertension in combined pulmonary fibrosis and emphysema. Dis. Model. Mech. 2019, 12, dmm038711. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andonegui-Elguera, S.; Taniguchi-Ponciano, K.; Gonzalez-Bonilla, C.R.; Torres, J.; Mayani, H.; Herrera, L.A.; Peña-Martínez, E.; Silva-Román, G.; Vela-Patiño, S.; Ferreira-Hermosillo, A.; et al. Molecular Alterations Prompted by SARS-CoV-2 Infection: Induction of Hyaluronan, Glycosaminoglycan and Mucopolysaccharide Metabolism. Arch. Med. Res. 2020. [Google Scholar] [CrossRef]
- Correale, M.; Ferraretti, A.; Monaco, I.; Grazioli, D.; Di Biase, M.; Brunetti, N.D. Endothelin-receptor antagonists in the management of pulmonary arterial hypertension: Where do we stand? Vasc. Health Risk Manag. 2018, 14, 253–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglarz, M.; Steiner, P.; Wanner, D.; Rey, M.; Hess, P.; Clozel, M. Vascular Effects of Endothelin Receptor Antagonists Depends on Their Selectivity for ETA Versus ETB Receptors and on the Functionality of Endothelial ETB Receptors. J. Cardiovasc. Pharm. 2015, 66, 332–337. [Google Scholar] [CrossRef] [Green Version]
- Bellisai, F.; Morozzi, G.; Scaccia, F.; Chellini, F.; Simpatico, A.; Pecetti, G.; Galeazzi, M. Evaluation of the Effect of Bosentan Treatment on Proinflammatory Cytokine Serum Levels in Patients Affected by Systemic Sclerosis. Int. J. Immunopathol. Pharm. 2011, 24, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Tcherakian, C.; Rivaud, E.; Catherinot, E.; Zucman, D.; Metivier, A.C.; Couderc, L.J. Pulmonary arterial hypertension related to HIV: Is inflammation related to IL-6 the cornerstone? Rev. Pneumol. Clin. 2011, 67, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Huang, J.-A.; Fraidenburg, D.R. Bosentan as Rescue Treatment in Refractory Hypoxemia and Pulmonary Hypertension in a Patient with ARDS and H7N9 Influenza Virus Infection. Lung 2014, 192, 635–636. [Google Scholar] [CrossRef]
- Dötsch, A.; Eisele, L.; Rabeling, M.; Rump, K.; Walstein, K.; Bick, A.; Cox, L.; Engler, A.; Bachmann, H.S.; Jöckel, K.-H.; et al. Hypoxia Inducible Factor-2 Alpha and Prolinhydroxylase 2 Polymorphisms in Patients with Acute Respiratory Distress Syndrome (ARDS). Int. J. Mol. Sci. 2017, 18, 1266. [Google Scholar] [CrossRef] [Green Version]
- Colgan, S.P.; Eltzschig, H.K.; Eckle, T.; Thompson, L.F. Physiological roles for ecto-5′-nucleotidase (CD73). Purinerg. Signall. 2006, 2, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Ellsworth, M.L. The red blood cell as an oxygen sensor: What is the evidence? Acta Physiol. Scand. 2000, 168, 551–559. [Google Scholar] [CrossRef]
- Karmouty-Quintana, H.; Xia, Y.; Blackburn, M.R. Adenosine signaling during acute and chronic disease states. J. Mol. Med. 2013, 91, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, M.R. Too much of a good thing: Adenosine overload in adenosine-deaminase-deficient mice. Trends Pharm. Sci. 2003, 24, 66–70. [Google Scholar] [CrossRef]
- Rajagopal, K.; Bryant, A.J.; Sahay, S.; Wareing, N.; Zhou, Y.; Pandit, L.M.; Karmouty-Quintana, H. Idiopathic Pulmonary Fibrosis (IPF) and pulmonary hypertension: Heracles meets the Hydra. Br. J. Pharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kylhammar, D.; Bune, L.T.; Rådegran, G. P2Y1 and P2Y12 receptors in hypoxia- and adenosine diphosphate-induced pulmonary vasoconstriction in vivo in the pig. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 114, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Alencar, A.K.N.; Montes, G.C.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. Adenosine Receptors as Drug Targets for Treatment of Pulmonary Arterial Hypertension. Front. Pharm. 2017, 8, 858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, F.; Le, N.; Mills, T.; Chen, N.; Karmouty-Quintana, H.; Molina, J.G.; Davies, J.; Philip, K.; Volcik, K.A.; Liu, H.; et al. Extracellular adenosine levels are associated with the progression and exacerbation of pulmonary fibrosis. FASEB J. 2015, 30, 874–883. [Google Scholar] [CrossRef] [Green Version]
- Pedroza, M.; Schneider, D.J.; Karmouty-Quintana, H.; Coote, J.; Shaw, S.; Corrigan, R.; Molina, J.G.; Alcorn, J.L.; Galas, D.; Gelinas, R.; et al. Interleukin-6 Contributes to Inflammation and Remodeling in a Model of Adenosine Mediated Lung Injury. PLoS ONE 2011, 6, e22667. [Google Scholar] [CrossRef] [Green Version]
- Mertens, T.C.J.; Hanmandlu, A.; Tu, L.; Phan, C.; Collum, S.D.; Chen, N.-Y.; Weng, T.; Davies, J.; Liu, C.; Eltzschig, H.K.; et al. Switching-Off Adora2b in Vascular Smooth Muscle Cells Halts the Development of Pulmonary Hypertension. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef]
- Morris, C.R. Mechanisms of Vasculopathy in Sickle Cell Disease and Thalassemia. Hematology 2008, 2008, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Pietra, G.G.; Edwards, W.D.; Kay, J.M.; Rich, S.; Kernis, J.; Schloo, B.; Ayres, S.M.; Bergofsky, E.H.; Brundage, B.H.; Detre, K.M. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 1989, 80, 1198–1206. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.-R.; Zhu, Y.; Halushka, P.V.; Lincoln, T.M.; Mendelsohn, M.E. Mechanism of platelet inhibition by nitric oxide: In vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 4888–4893. [Google Scholar]
- Neuhof, H.; Seeger, W.; Wolf, H. Generation of mediators by limited proteolysis during blood coagulation and fibrinolysis—Its pathogenetic role in the adult respiratory distress syndrome (ARDS). Resuscitation 1986, 14, 23–32. [Google Scholar] [CrossRef]
- White, T.A.; Witt, T.A.; Pan, S.; Mueske, C.S.; Kleppe, L.S.; Holroyd, E.W.; Champion, H.C.; Simari, R.D. Tissue Factor Pathway Inhibitor Overexpression Inhibits Hypoxia-Induced Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol. 2010, 43, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, J.P.; Ellery, P.E.R.; Maroney, S.A.; Mast, A.E. Biology of tissue factor pathway inhibitor. Blood 2014, 123, 2934–2943. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, L.; Grover, G.; Stier, C.T., Jr. Ifetroban Sodium: An Effective TxA2/PGH2 Receptor Antagonist. Cardiovasc. Drug Rev. 2001, 19, 97–115. [Google Scholar] [CrossRef] [Green Version]
- High Dose Inhaled Nitric Oxide for COVID-19 (ICU Patients). Available online: https://ClinicalTrials.gov/show/NCT04383002 (accessed on 20 October 2020).
- Nitric Oxide Inhalation Therapy for COVID-19 Infections in the ED. Available online: https://ClinicalTrials.gov/show/NCT04338828 (accessed on 20 October 2020).
- NO Prevention of COVID-19 for Healthcare Providers. Available online: https://ClinicalTrials.gov/show/NCT04312243 (accessed on 20 October 2020).
- Nitric Oxide Gas Inhalation in Severe Acute Respiratory Syndrome in COVID-19. Available online: https://ClinicalTrials.gov/show/NCT04306393 (accessed on 20 October 2020).
- Goh, K.J.; Choong, M.C.; Cheong, E.H.; Kalimuddin, S.; Wen, S.D.; Phua, G.C.; Chan, K.S.; Mohideen, S.H. Rapid Progression to Acute Respiratory Distress Syndrome: Review of Current Understanding of Critical Illness from COVID-19 Infection. Ann. Acad. Med. 2020, 49, 1–9. [Google Scholar]
- Inhaled Nitric Oxide for Preventing Progression in COVID-19. Available online: https://ClinicalTrials.gov/show/NCT04388683 (accessed on 20 October 2020).
- RLF-100 (Aviptadil) Intermediate Population Expanded Access Protocol (SAMICARE). Available online: https://ClinicalTrials.gov/show/NCT04453839 (accessed on 20 October 2020).
- Intravenous Aviptadil for Critical COVID-19 With Respiratory Failure. Available online: https://ClinicalTrials.gov/show/NCT04311697 (accessed on 20 October 2020).
- Inhaled Aviptadil for the Treatment of Moderate and Severe COVID-19. Available online: https://ClinicalTrials.gov/show/NCT04360096 (accessed on 20 October 2020).
- R-107 Shows Promise in Early Study for PAH Linked to COVID-19. Available online: https://pulmonaryhypertensionnews.com/2020/06/17/kalyteras-r-107-liquid-nitric-oxide-shows-promise-early-study-pah-linked-covid-19/ (accessed on 20 October 2020).
- mulTi-Arm Therapeutic Study in Pre-ICu Patients Admitted with Covid-19 - Experimental Drugs and Mechanisms. Available online: https://ClinicalTrials.gov/show/NCT04393246 (accessed on 20 October 2020).
- Losartan for Patients With COVID-19 Requiring Hospitalization. Available online: https://ClinicalTrials.gov/show/NCT04312009 (accessed on 20 October 2020).
| Drug Name | Clinical Trial Registration | Molecular Target | Phase Level | Reference |
|---|---|---|---|---|
| Nitric Oxide Gas Inhalation | NCT04305457, NCT04290871, NCT0433882, NCT04312243, NCT04306393, NCT03331445, NCT04388683, NCT04383002 | Nitric Oxide | Phase 2 | [82,90,156,157,158,159,160,161] |
| RLF-100 (Aviptadil) | NCT04453839 | Protects alveolar type II cells from the SARS-CoV-2 virus | Phase 2,3 | [162] |
| Intravenous Aviptadil | NCT04311697 | Protects alveolar type II cells from the SARS-CoV-2 virus | Phase 2 | [163] |
| Inhaled Aviptadil | NCT04360096 | Protects alveolar type II cells from the SARS-CoV-2 virus | Phase 2,3 | [164] |
| R-107 | Waiting for registration | Steadily releases NO into lung tissues | Not Applicable | [165] |
| Sildenafil | NCT04304313 | Inhibitor of cGMP-specific phosphodiesterase (PDE-5) | Phase 3 | [85] |
| Ambrisentan | NCT04393246 | Endothelin receptor antagonist, and is selective for the type A endothelin receptor (ETA) | Phase 2 | [166] |
| Losartan | NCT0431117 and NCT04312009 | An angiotensin II receptor blocker (ARB) | Phase 2 | [167] |
| Remdesivir | NCT04292899 | RNA polymerase inhibitor | Phase 3 | [89] |
| Drug Name | Target | Other Beneficial Effects | References |
|---|---|---|---|
| 4-methylumbelliferone (4MU) | Hyaluronan inhibitor | [129,130] | |
| Captopril | ACE inhibitors | [75,76] | |
| Icatibant | Selective antagonist for the B2 receptor | Reduction of cough symptoms and edema | [63,64] |
| Ifetroban | Thromboxane A2 antagonist | Inhibition of thrombotic processes | [155] |
| Lanadelumab | A monoclonal antibody (class IgG1 kappa) against high-molecular-weight kininogen that yields bradykinin | Reduction of cough symptoms and edema | [59,67] |
| Losartan | An angiotensin II receptor blocker (ARB) | Reduction of inflammation | [77] |
| Macitentan (ACT-064992), Bosentan | Prevents the binding of ET-1 to both endothelin A (ETA) and endothelin B (ETB) receptors (endothelin receptor antagonist) | Inhibition of inflammation, Reduction of viral RNA counts | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmouty-Quintana, H.; Thandavarayan, R.A.; Keller, S.P.; Sahay, S.; Pandit, L.M.; Akkanti, B. Emerging Mechanisms of Pulmonary Vasoconstriction in SARS-CoV-2-Induced Acute Respiratory Distress Syndrome (ARDS) and Potential Therapeutic Targets. Int. J. Mol. Sci. 2020, 21, 8081. https://doi.org/10.3390/ijms21218081
Karmouty-Quintana H, Thandavarayan RA, Keller SP, Sahay S, Pandit LM, Akkanti B. Emerging Mechanisms of Pulmonary Vasoconstriction in SARS-CoV-2-Induced Acute Respiratory Distress Syndrome (ARDS) and Potential Therapeutic Targets. International Journal of Molecular Sciences. 2020; 21(21):8081. https://doi.org/10.3390/ijms21218081
Chicago/Turabian StyleKarmouty-Quintana, Harry, Rajarajan A. Thandavarayan, Steven P. Keller, Sandeep Sahay, Lavannya M. Pandit, and Bindu Akkanti. 2020. "Emerging Mechanisms of Pulmonary Vasoconstriction in SARS-CoV-2-Induced Acute Respiratory Distress Syndrome (ARDS) and Potential Therapeutic Targets" International Journal of Molecular Sciences 21, no. 21: 8081. https://doi.org/10.3390/ijms21218081
APA StyleKarmouty-Quintana, H., Thandavarayan, R. A., Keller, S. P., Sahay, S., Pandit, L. M., & Akkanti, B. (2020). Emerging Mechanisms of Pulmonary Vasoconstriction in SARS-CoV-2-Induced Acute Respiratory Distress Syndrome (ARDS) and Potential Therapeutic Targets. International Journal of Molecular Sciences, 21(21), 8081. https://doi.org/10.3390/ijms21218081







