Nonanal Stimulates Growth Factors via Cyclic Adenosine Monophosphate (cAMP) Signaling in Human Hair Follicle Dermal Papilla Cells
Abstract
1. Introduction
2. Results
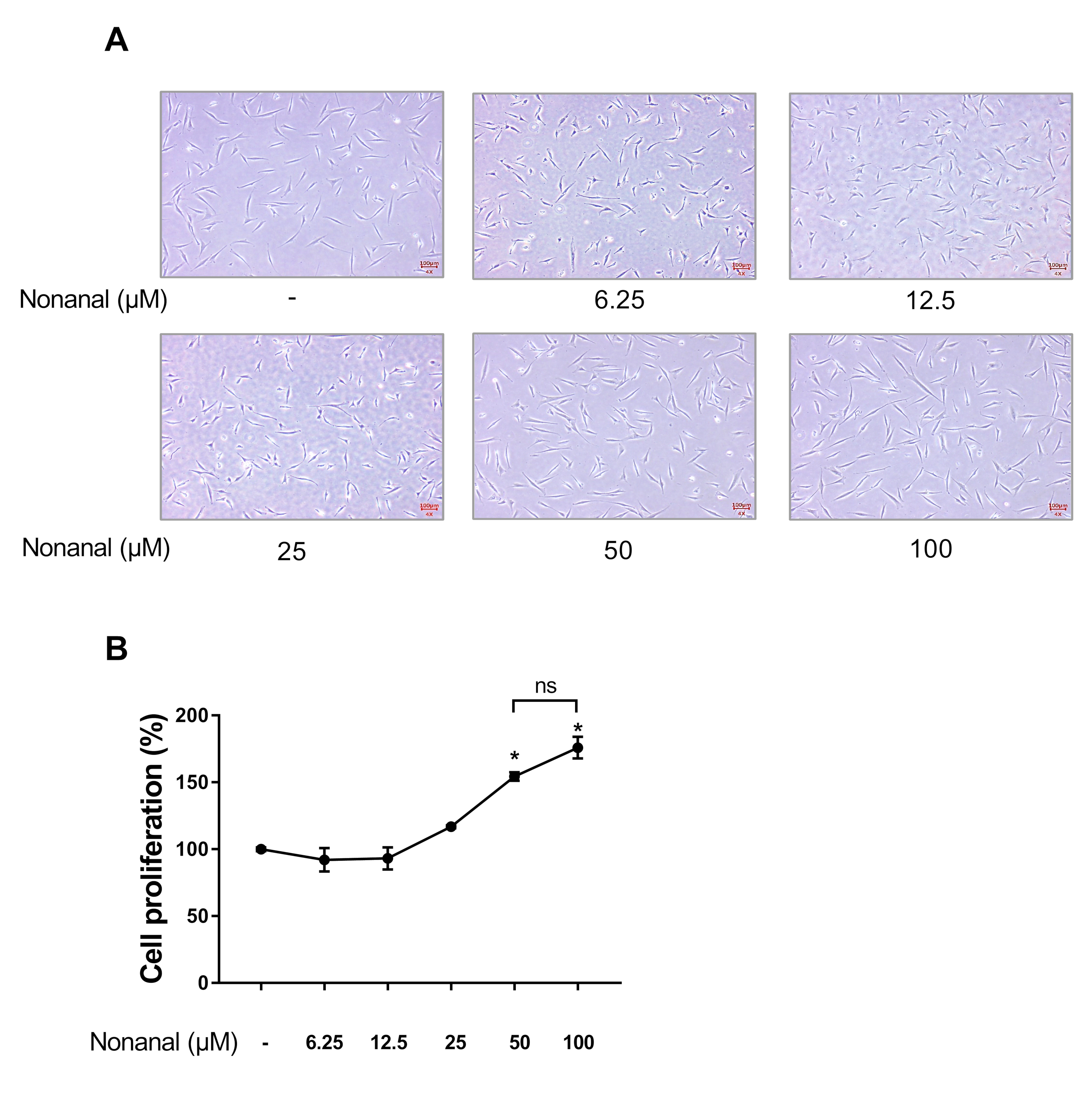
2.1. Nonanal Increases Cell Proliferation
2.2. Nonanal Enhances Gene Expression of Growth Factors
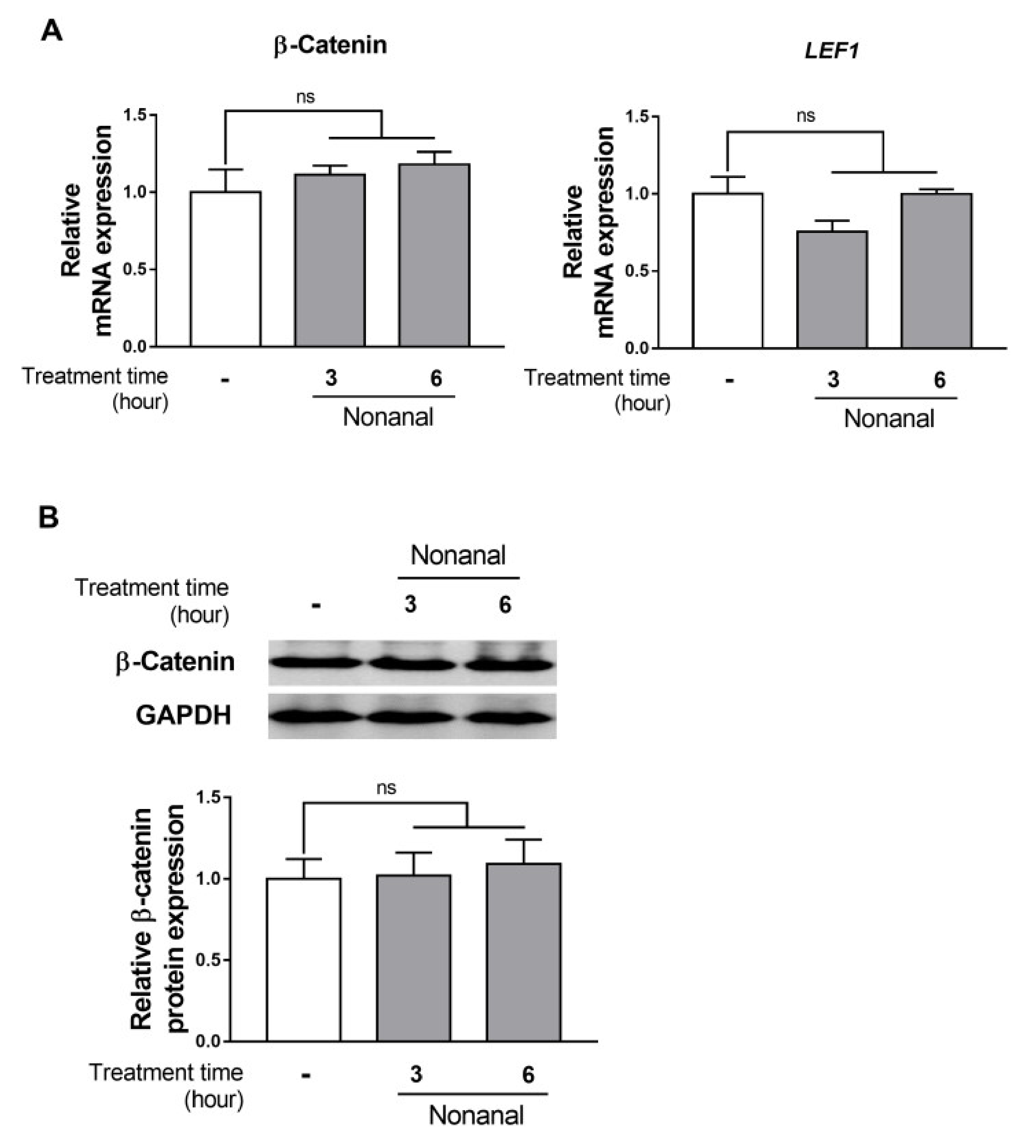
2.3. Nonanal Does Not Affect the β-Catenin Signaling Pathway
2.4. Nonanal Activates the cAMP/PKA Signaling Pathway
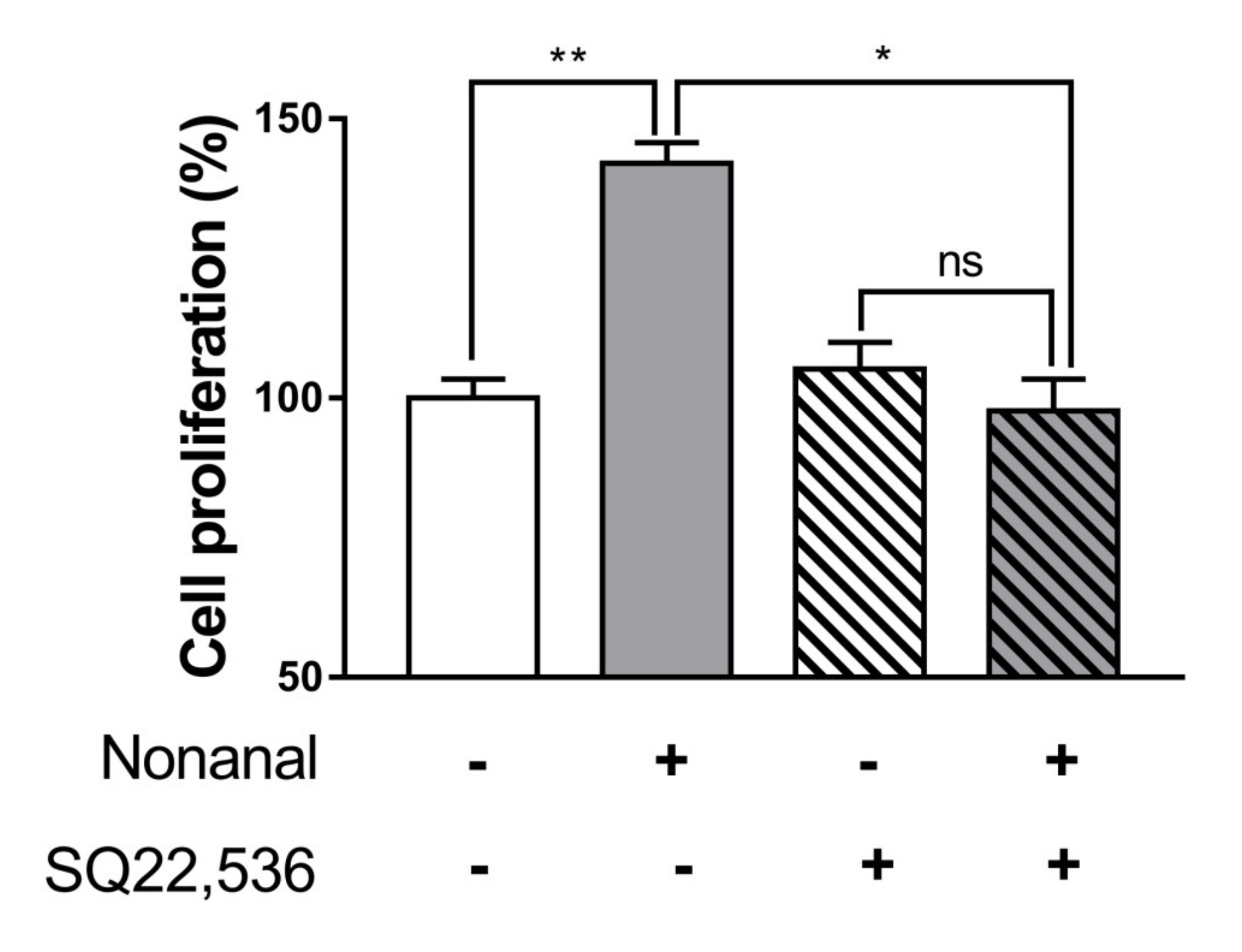
2.5. cAMP Is Involved in Nonanal-Induced DPC Proliferation
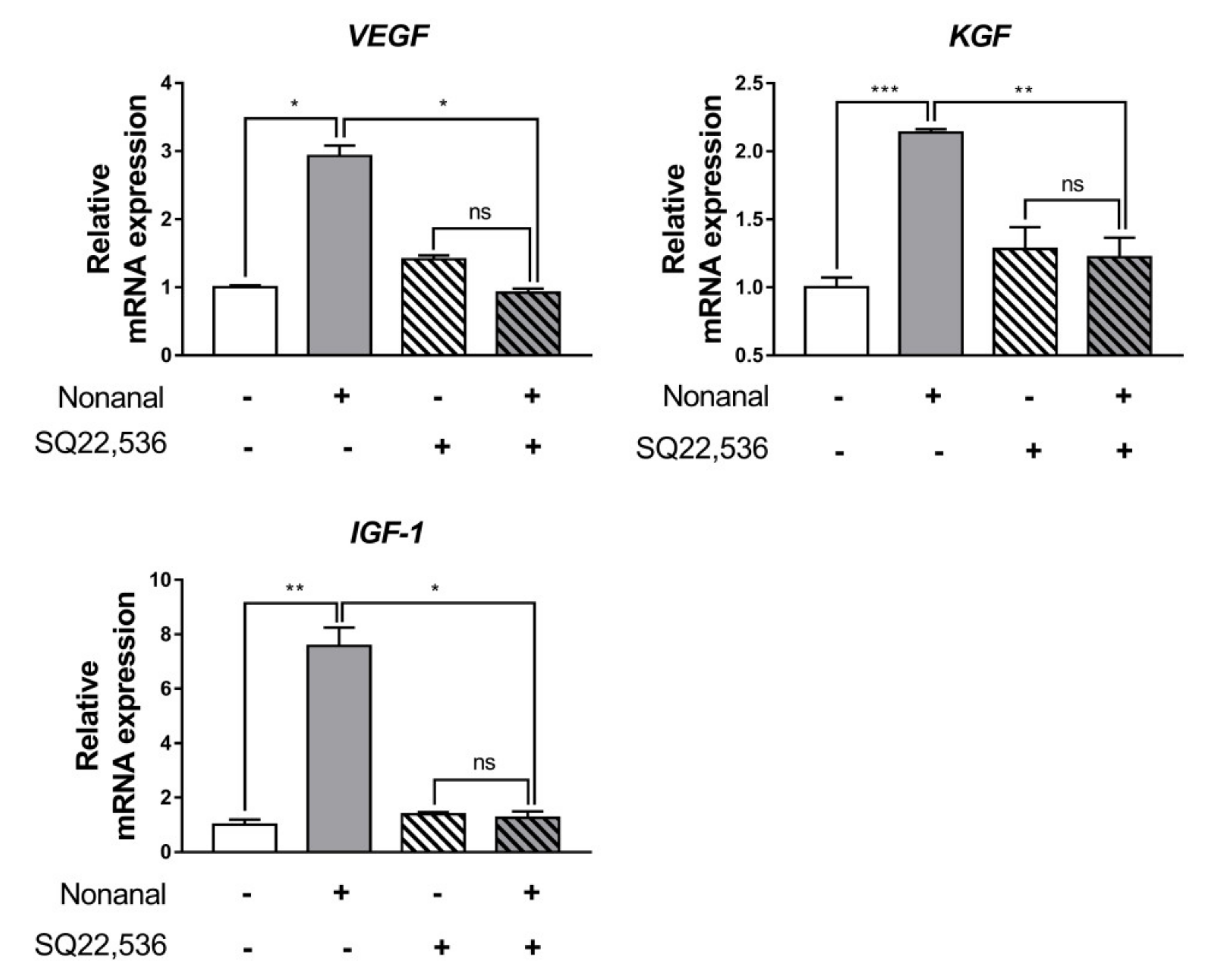
2.6. cAMP Is Implicated in Nonanal-Induced Gene Expression of Growth Factors
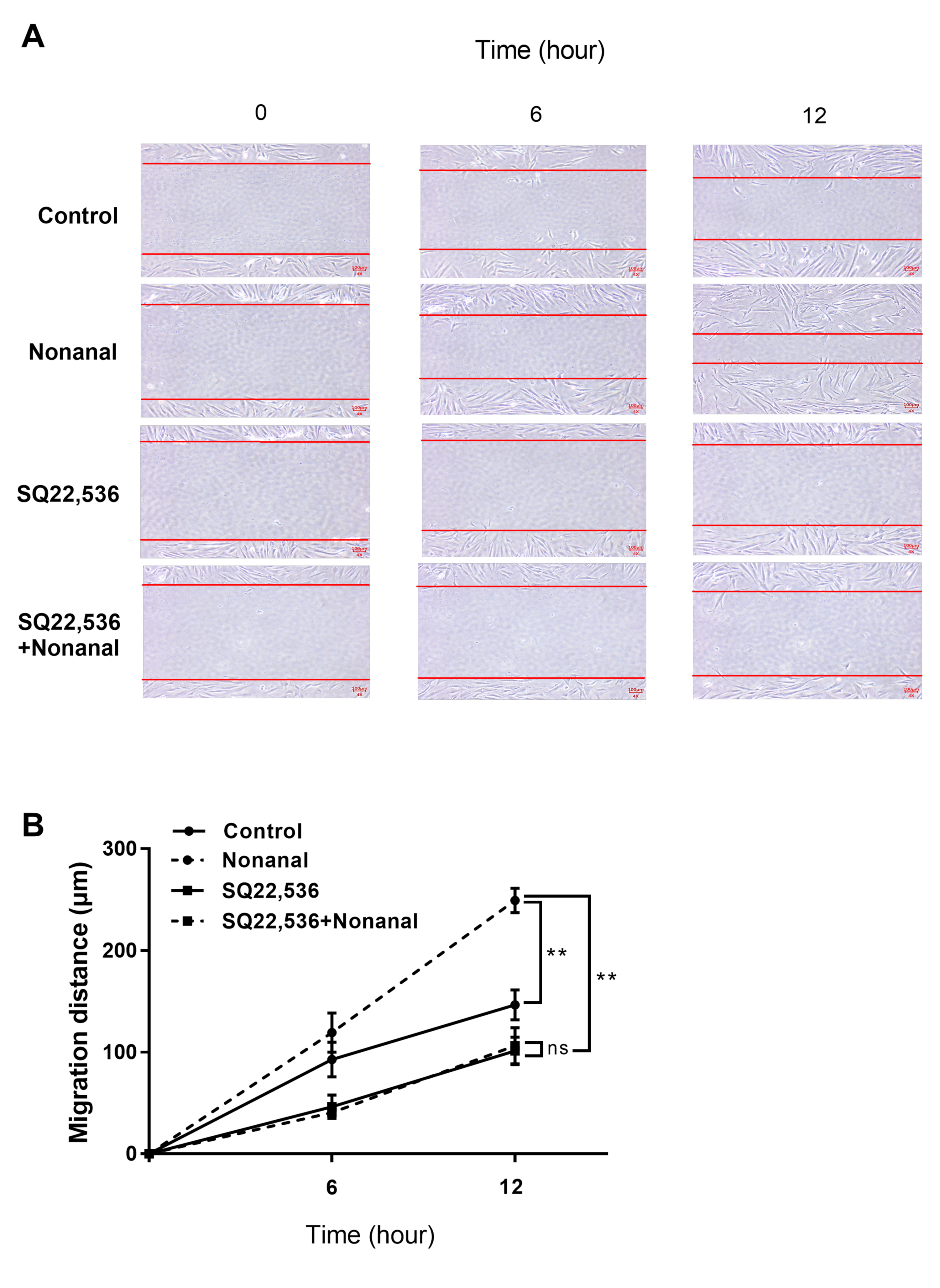
2.7. Nonanal Promotes Cell Migration via cAMP
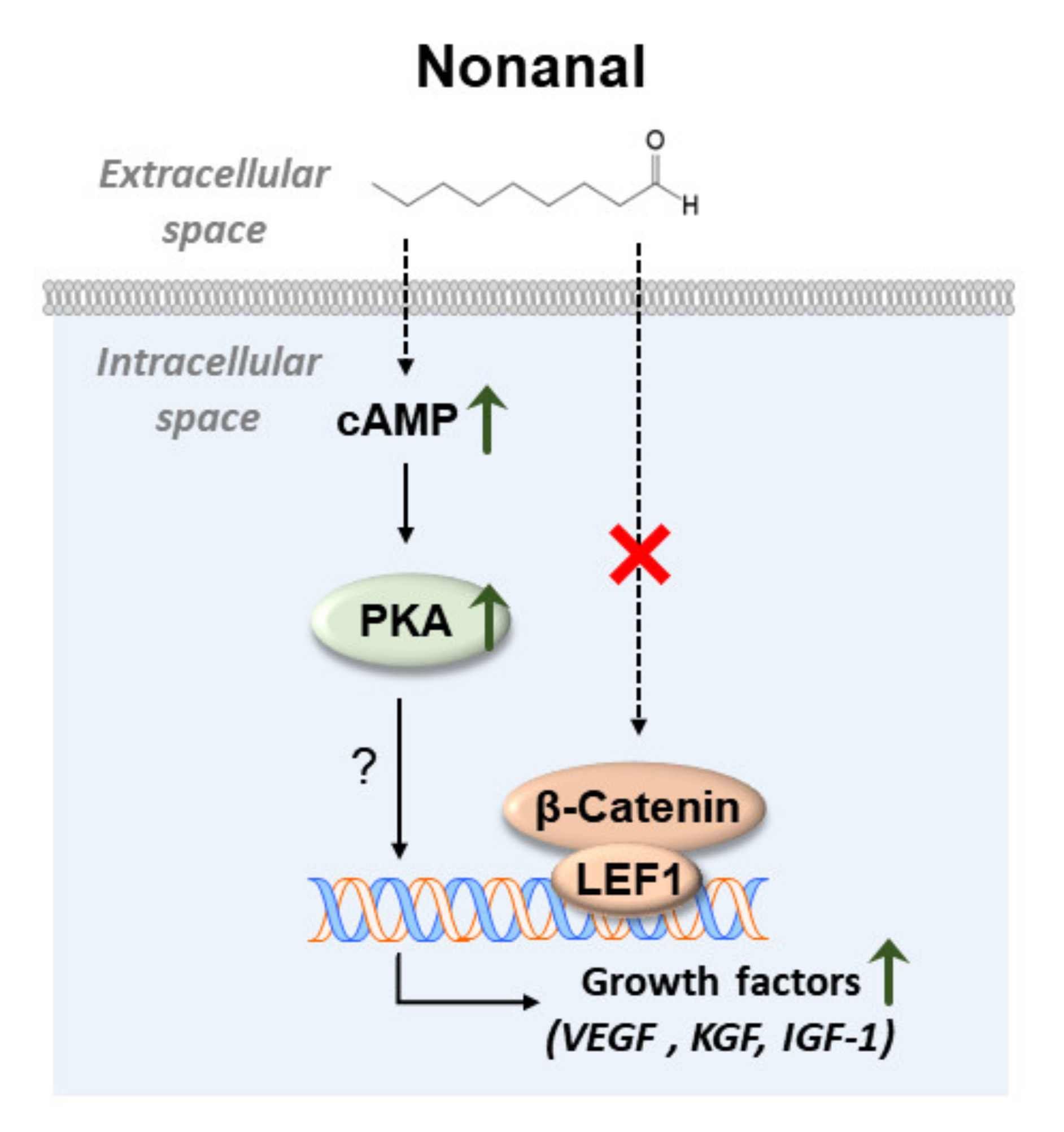
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. WST-1 Assay
4.3. cAMP Measurements
4.4. Real-Time PCR
4.5. Western Blotting
4.6. Migration Assay
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Danilenko, D.M.; Ring, B.D.; Pierce, G.F. Growth factors and cytokines in hair follicle development and cycling: Recent insights from animal models and the potentials for clinical therapy. Mol. Med. Today 1996, 2, 460–467. [Google Scholar] [CrossRef]
- Shirakata, Y. Regulation of epidermal keratinocytes by growth factors. J. Dermatol. Sci. 2010, 59, 73–80. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Gao, Y.; Liu, X.; Bai, T.; Li, M.; Li, L.; Chi, G.; Xu, H.; Liu, F. Maintenance of high proliferation and multipotent potential of human hair follicle-derived mesenchymal stem cells by growth factors. Int. J. Mol. Med. 2013, 31, 913–921. [Google Scholar] [CrossRef]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef]
- Rahmani, W.; Abbasi, S.; Hagner, A.; Raharjo, E.; Kumar, R.; Hotta, A.; Magness, S.; Metzger, D.; Biernaskie, J. Hair follicle dermal stem cells regenerate the dermal sheath, repopulate the dermal papilla, and modulate hair type. Dev. Cell 2014, 31, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.G.; Slomiany, W.P.; Allison, R. Hair loss: Common causes and treatment. Am. Fam. Physician 2017, 96, 371–378. [Google Scholar] [PubMed]
- Navarro, M.; Asín, M.; Martínez, M.; Martínez, A.; Molina, C.; Moscoso, L.; Pino, A.; Orive, G.; Anitua, E. Management of androgenetic alopecia: A comparative clinical study between plasma rich in growth factors and topical minoxidil. Eur. J. Plast. Surg. 2016, 39, 173–180. [Google Scholar] [CrossRef]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef]
- Leirós, G.J.; Ceruti, J.M.; Castellanos, M.L.; Kusinsky, A.G.; Balañá, M.E. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol. Cell. Endocrinol. 2017, 439, 26–34. [Google Scholar] [CrossRef]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef]
- Myung, P.S.; Takeo, M.; Ito, M.; Atit, R.P. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Investig. Dermatol. 2013, 133, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Chéret, J.; Bertolini, M.; Ponce, L.; Lehmann, J.; Tsai, T.; Alam, M.; Hatt, H.; Paus, R. Olfactory receptor OR2AT4 regulates human hair growth. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-A.; Hwang, Y.-L.; Lee, M.-H.; Kim, N.-R.; Roh, S.-S.; Lee, Y.; Kim, C.D.; Lee, J.-H.; Choi, K.-C. Adenosine stimulates growth of dermal papilla and lengthens the anagen phase by increasing the cysteine level via fibroblast growth factors 2 and 7 in an organ culture of mouse vibrissae hair follicles. Int. J. Mol. Med. 2012, 29, 195–201. [Google Scholar] [PubMed]
- Choi, M.; Choi, S.-J.; Jang, S.; Choi, H.-I.; Kang, B.-M.; Hwang, S.T.; Kwon, O. Shikimic acid, a mannose bioisostere, promotes hair growth with the induction of anagen hair cycle. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Stevens, R.C.; Cherezov, V.; Katritch, V.; Abagyan, R.; Kuhn, P.; Rosen, H.; Wüthrich, K. The GPCR Network: A large-scale collaboration to determine human GPCR structure and function. Nat. Rev. Drug Discov. 2013, 12, 25–34. [Google Scholar] [CrossRef]
- Sun, Y.N.; Cui, L.; Li, W.; Yan, X.T.; Yang, S.Y.; Kang, J.I.; Kang, H.K.; Kim, Y.H. Promotion effect of constituents from the root of Polygonum multiflorum on hair growth. Bioorganic Med. Chem. Lett. 2013, 23, 4801–4805. [Google Scholar] [CrossRef]
- Gasparri, F.; Mariani, M.; Sola, F.; Galvani, A. Quantification of the proliferation index of human dermal fibroblast cultures with the ArrayScan™ high-content screening reader. J. Biomol. Screen. 2004, 9, 232–243. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef]
- Guenther, E.; Althausen, D. The Essential Oils; Van Nostrand: New York, NY, USA, 1948; Volume 1. [Google Scholar]
- Omonov, T.S.; Kharraz, E.; Foley, P.; Curtis, J.M. The production of biobased nonanal by ozonolysis of fatty acids. RSC Adv. 2014, 4, 53617–53627. [Google Scholar] [CrossRef]
- Heyneman, C.A.; Lawless-Liday, C.; Wall, G.C. Oral versus topical NSAIDs in rheumatic diseases. Drugs 2000, 60, 555–574. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Liu, P.-F.; Huang, C.-M. Decreasing systemic toxicity via transdermal delivery of anticancer drugs. Curr. Drug Metab. 2008, 9, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. Viewp. 2000, 9, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Gary, M.; Monakhova, Y.B.; Kuballa, T.; Mildau, G. Rapid NMR screening of total aldehydes to detect oxidative rancidity in vegetable oils and decorative cosmetics. Spectrosc. Eur. 2010, 22, 11. [Google Scholar]
- Ruzsanyi, V.; Mochalski, P.; Schmid, A.; Wiesenhofer, H.; Klieber, M.; Hinterhuber, H.; Amann, A. Ion mobility spectrometry for detection of skin volatiles. J. Chromatogr. B 2012, 911, 84–92. [Google Scholar] [CrossRef]
- Panzella, L. Natural Phenolic Compounds for Health, Food and Cosmetic Applications. Antioxidants 2020, 9, 427. [Google Scholar] [CrossRef]
- Rubin, J.S.; Bottaro, D.P.; Chedid, M.; Miki, T.; Ron, D.; Cheon, H.-G.; Taylor, W.G.; Fortney, E.; Sakata, H.; Finch, P.W. Keratinocyte growth factor. Cell Biol. Int. 1995, 19, 399–412. [Google Scholar] [CrossRef]
- Gibbs, S.; Silva Pinto, A.N.; Murli, S.; Huber, M.; Hohl, D.; Ponec, M. Epidermal growth factor and keratinocyte growth factor differentially regulate epidermal migration, growth, and differentiation. Wound Repair Regen. 2000, 8, 192–203. [Google Scholar] [CrossRef]
- Messenger, A.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef]
- Wise, L.M.; Inder, M.K.; Real, N.C.; Stuart, G.S.; Fleming, S.B.; Mercer, A.A. The vascular endothelial growth factor (VEGF)-E encoded by orf virus regulates keratinocyte proliferation and migration and promotes epidermal regeneration. Cell. Microbiol. 2012, 14, 1376–1390. [Google Scholar] [CrossRef]
- Shigematsu, S.; Yamauchi, K.; Nakajima, K.; Iijima, S.; Aizawa, T.; Hashizume, K. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr. J. 1999, 46, S59–S62. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.; Chang, C.; Chen, J.; Jee, S. Human hair follicle dermal papilla cell, dermal sheath cell and interstitial dermal fibroblast characteristics. J. Formos. Med. Assoc. Taiwan Yi Zhi 1996, 95, 667–674. [Google Scholar] [PubMed]
- Itami, S.; Kurata, S.; Takayasu, S. Androgen induction of follicular epithelial cell growth is mediated via insulin-like growth factor-I from dermal papilla cells. Biochem. Biophys. Res. Commun. 1995, 212, 988–994. [Google Scholar] [CrossRef]
- Guvakova, M.A. Insulin-like growth factors control cell migration in health and disease. Int. J. Biochem. Cell Biol. 2007, 39, 890–909. [Google Scholar] [CrossRef]
- Seeger, M.A.; Paller, A.S. The roles of growth factors in keratinocyte migration. Adv. Wound Care 2015, 4, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef]
- Lei, M.; Guo, H.; Qiu, W.; Lai, X.; Yang, T.; Widelitz, R.B.; Chuong, C.M.; Lian, X.; Yang, L. Modulating hair follicle size with W nt10b/DKK 1 during hair regeneration. Exp. Dermatol. 2014, 23, 407–413. [Google Scholar] [CrossRef]
- Elliott, K.; Messenger, A.G.; Stephenson, T.J. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: Implications for the control of hair follicle size and androgen responses. J. Investig. Dermatol. 1999, 113, 873–877. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem. Biophys. Res. Commun. 2018, 500, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative stress–associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Schmiedeberg, K.; Shirokova, E.; Weber, H.-P.; Schilling, B.; Meyerhof, W.; Krautwurst, D. Structural determinants of odorant recognition by the human olfactory receptors OR1A1 and OR1A2. J. Struct. Biol. 2007, 159, 400–412. [Google Scholar] [CrossRef]
- Sanz, G.; Schlegel, C.; Pernollet, J.-C.; Briand, L. Comparison of odorant specificity of two human olfactory receptors from different phylogenetic classes and evidence for antagonism. Chem. Senses 2005, 30, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, W.; Frazer, J.; Unett, D. Functional assays for screening GPCR targets. Curr. Opin. Biotechnol. 2005, 16, 655–665. [Google Scholar] [CrossRef]
- Selbie, L.A.; Hill, S.J. G protein-coupled-receptor cross-talk: The fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol. Sci. 1998, 19, 87–93. [Google Scholar] [CrossRef]


| Gene Name | Primer Sequences (5′→ 3′) |
|---|---|
| Catenin beta 1 (CTNNB1) | F: CCGACACCAAGAAGCAGAGATG R: GTGGGATGGTGGGTGTAAGAG |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | F: AAATCAAGTGGGGCGATGC R: AGGGGGCAGAGATGATGACC |
| Insulin-like growth factor-1 (IGF-1) | F: TTGCTCTCAACATCTCCCATCT R: TGCATCTTCACCTTCAAGAAAT |
| Keratinocyte growth factor (KGF) | F: AGAGAAGGGATGCTGGAGGT R: CGTAAGGGGCACTGTTTTAGAG |
| Lymphoid enhancer factor 1 (LEF1) | F: CAACCCTACCCATCCTCACTG R: GGCTCCTGCTCCTTTCTCTGT |
| Vascular endothelial growth factor (VEGF) | F: CTTCTGAGTTGCCCAGGAGA R: GGATGGAGGAAGGTCAACCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Kang, W.; Choi, D.; Son, B.; Park, T. Nonanal Stimulates Growth Factors via Cyclic Adenosine Monophosphate (cAMP) Signaling in Human Hair Follicle Dermal Papilla Cells. Int. J. Mol. Sci. 2020, 21, 8054. https://doi.org/10.3390/ijms21218054
Park S, Kang W, Choi D, Son B, Park T. Nonanal Stimulates Growth Factors via Cyclic Adenosine Monophosphate (cAMP) Signaling in Human Hair Follicle Dermal Papilla Cells. International Journal of Molecular Sciences. 2020; 21(21):8054. https://doi.org/10.3390/ijms21218054
Chicago/Turabian StylePark, Soyoon, Wesuk Kang, Dabin Choi, Bomin Son, and Taesun Park. 2020. "Nonanal Stimulates Growth Factors via Cyclic Adenosine Monophosphate (cAMP) Signaling in Human Hair Follicle Dermal Papilla Cells" International Journal of Molecular Sciences 21, no. 21: 8054. https://doi.org/10.3390/ijms21218054
APA StylePark, S., Kang, W., Choi, D., Son, B., & Park, T. (2020). Nonanal Stimulates Growth Factors via Cyclic Adenosine Monophosphate (cAMP) Signaling in Human Hair Follicle Dermal Papilla Cells. International Journal of Molecular Sciences, 21(21), 8054. https://doi.org/10.3390/ijms21218054





