Pulsed Electromagnetic Fields Stimulate HIF-1α-Independent VEGF Release in 1321N1 Human Astrocytes Protecting Neuron-like SH-SY5Y Cells from Oxygen-Glucose Deprivation
Abstract
:1. Introduction
2. Results
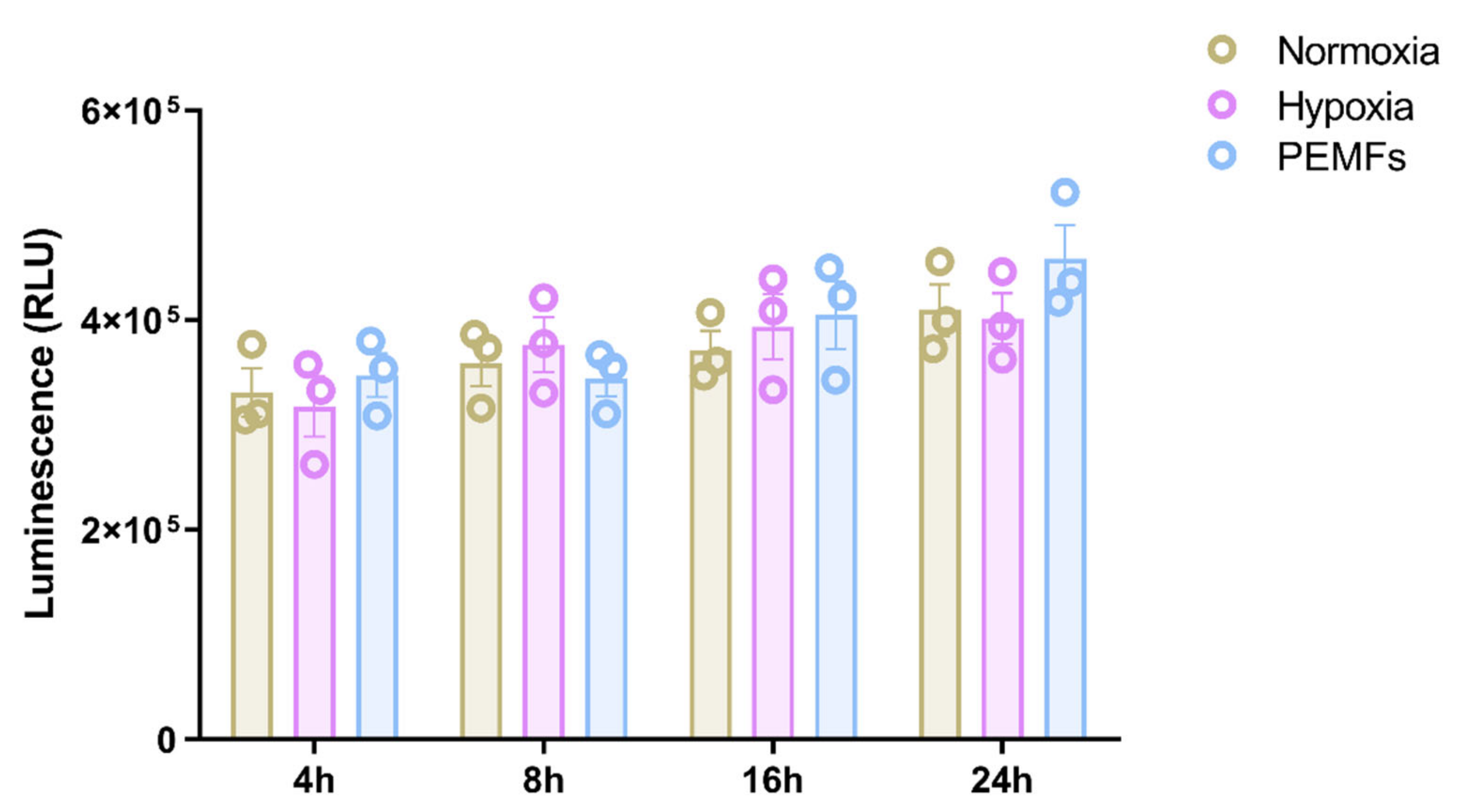
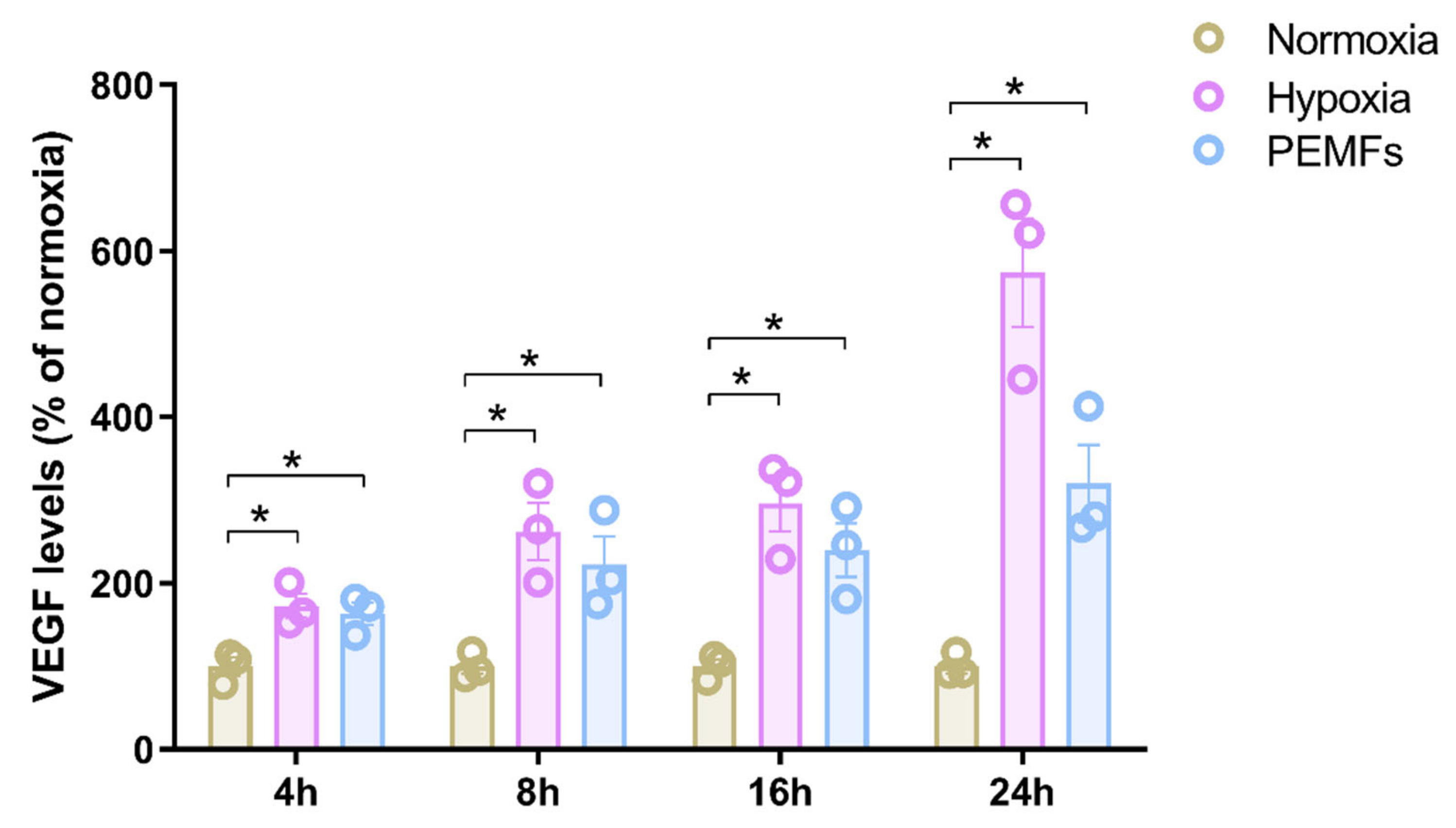
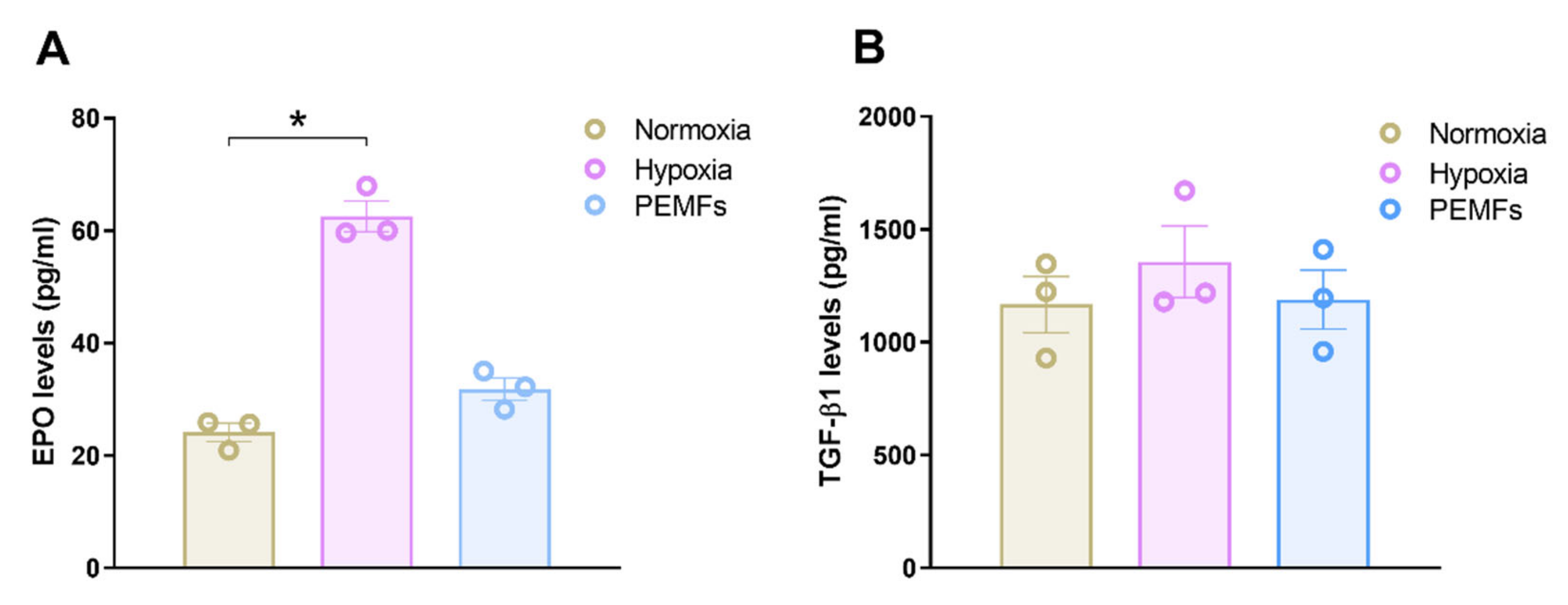
2.1. Effect of Pulsed Electromagnetic Fields on Cells Viability and Neurotrophic Factor Production in 1321N1 Astrocytes
2.2. The PEMF-Induced Release of VEGF in 1321N1 Cells Is Not Mediated by HIF-1α
2.3. Astrocyte Conditioned Medium Derived from PEMF-Exposed 1321N1 Cells Protects SH-SY5Y Cells from Oxygen-Glucose Deprivation
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Pulsed Electromagnetic Field Exposure System
4.3. Cell Viability Assessment
4.4. Cell Proliferation Assay
4.5. AlphaLISA Assays
4.6. Flow Cytometry Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PEMFs | Pulsed Electromagnetic Fields |
| VEGF | Vascular endothelial growth factor |
| EPO | Erythropoietin |
| TGF-β1 | Transforming growth factor-beta 1 |
| ACM | Astrocyte conditioned medium |
| OGD | Oxygen-glucose deprivation |
| BBB | Blood-brain barrier |
References
- Varani, K.; Vincenzi, F.; Ravani, A.; Pasquini, S.; Merighi, S.; Gessi, S.; Setti, S.; Cadossi, M.; Borea, P.A.; Cadossi, R. Adenosine Receptors as a Biological Pathway for the Anti-Inflammatory and Beneficial Effects of Low Frequency Low Energy Pulsed Electromagnetic Fields. Mediat. Inflamm. 2017, 2017, 2740963. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.R.; Malling, A.S.B.; Morberg, B.M.; Gredal, O.; Bech, P.; Wermuth, L. Effects of Long-Term Treatment with T-PEMF on Forearm Muscle Activation and Motor Function in Parkinson’s Disease. Case Rep. Neurol. 2018, 10, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Malling, A.S.B.; Morberg, B.M.; Wermuth, L.; Gredal, O.; Bech, P.; Jensen, B.R. Effect of transcranial pulsed electromagnetic fields (T-PEMF) on functional rate of force development and movement speed in persons with Parkinson’s disease: A randomized clinical trial. PLoS ONE 2018, 13, e0204478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arendash, G.W.; Sanchez-Ramos, J.; Mori, T.; Mamcarz, M.; Lin, X.; Runfeldt, M.; Wang, L.; Zhang, G.; Sava, V.; Tan, J.; et al. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer’s disease mice. J. Alzheimers Dis. 2010, 19, 191–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhou, J.; Gu, L.; Zuo, Y. The change of HCN1/HCN2 mRNA expression in peripheral nerve after chronic constriction injury induced neuropathy followed by pulsed electromagnetic field therapy. Oncotarget 2017, 8, 1110–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, G.; Cadossi, R.; Steinberg, G. Protection against focal cerebral ischemia following exposure to a pulsed electromagnetic field. Bioelectromagnetics 1994, 15, 205–216. [Google Scholar] [CrossRef]
- Pena-Philippides, J.C.; Yang, Y.; Bragina, O.; Hagberg, S.; Nemoto, E.; Roitbak, T. Effect of pulsed electromagnetic field (PEMF) on infarct size and inflammation after cerebral ischemia in mice. Transl. Stroke Res. 2014, 5, 491–500. [Google Scholar] [CrossRef]
- Urnukhsaikhan, E.; Mishig-Ochir, T.; Kim, S.-C.; Park, J.-K.; Seo, Y.-K. Neuroprotective Effect of Low Frequency-Pulsed Electromagnetic Fields in Ischemic Stroke. Appl. Biochem. Biotechnol. 2017, 181, 1360–1371. [Google Scholar] [CrossRef]
- Vincenzi, F.; Ravani, A.; Pasquini, S.; Merighi, S.; Gessi, S.; Setti, S.; Cadossi, R.; Borea, P.A.; Varani, K. Pulsed Electromagnetic Field Exposure Reduces Hypoxia and Inflammation Damage in Neuron-Like and Microglial Cells. J. Cell. Physiol. 2017, 232, 1200–1208. [Google Scholar] [CrossRef]
- Gessi, S.; Merighi, S.; Bencivenni, S.; Battistello, E.; Vincenzi, F.; Setti, S.; Cadossi, M.; Borea, P.A.; Cadossi, R.; Varani, K. Pulsed electromagnetic field and relief of hypoxia-induced neuronal cell death: The signaling pathway. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Merighi, S.; Gessi, S.; Bencivenni, S.; Battistello, E.; Vincenzi, F.; Setti, S.; Cadossi, M.; Borea, P.A.; Cadossi, R.; Varani, K. Signaling pathways involved in anti-inflammatory effects of Pulsed Electromagnetic Field in microglial cells. Cytokine 2020, 125, 154777. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.-B.; Xu, L.; Lu, Y.; Sun, X.; Yue, S.; Xiong, X.-X.; Giffard, R.G. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia 2013, 61, 1784–1794. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Fernandez, S.; Almeida, A.; Bolaños, J.P. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem. J. 2012, 443, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Trendelenburg, G.; Dirnagl, U. Neuroprotective role of astrocytes in cerebral ischemia: Focus on ischemic preconditioning. Glia 2005, 50, 307–320. [Google Scholar] [CrossRef]
- Liu, Z.; Chopp, M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog. Neurobiol. 2016, 144, 103–120. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, S.L.B.; Pillat, M.M.; Cheffer, A.; Lameu, C.; Schwindt, T.T.; Ulrich, H. Functions of neurotrophins and growth factors in neurogenesis and brain repair. Cytom. A 2013, 83, 76–89. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hong, G.; Lee, K.S.S.; Hammock, B.D.; Gebremedhin, D.; Harder, D.R.; Koehler, R.C.; Sapirstein, A. Inhibition of soluble epoxide hydrolase augments astrocyte release of vascular endothelial growth factor and neuronal recovery after oxygen-glucose deprivation. J. Neurochem. 2017, 140, 814–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, A.; Wadhwani, S.; Grammas, P. Multiple neurotrophic effects of VEGF on cultured neurons. Neuropeptides 2010, 44, 323–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Abe, K.; Itoyama, Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J. Cereb. Blood Flow Metab. 1998, 18, 887–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Jin, K.; Xie, L.; Childs, J.; Mao, X.O.; Logvinova, A.; Greenberg, D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Investig. 2003, 111, 1843–1851. [Google Scholar] [CrossRef]
- Souvenir, R.; Doycheva, D.; Zhang, J.H.; Tang, J. Erythropoietin in stroke therapy: Friend or foe. Curr. Med. Chem. 2015, 22, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhou, C.; Du, F.; Lu, Y.; Peng, B.; Chen, L.; Zhu, L. Ginkgolide B preconditioning on astrocytes promotes neuronal survival in ischemic injury via up-regulating erythropoietin secretion. Neurochem. Int. 2013, 62, 157–164. [Google Scholar] [CrossRef]
- Lin, C.-H.; Cheng, F.-C.; Lu, Y.-Z.; Chu, L.-F.; Wang, C.-H.; Hsueh, C.-M. Protection of ischemic brain cells is dependent on astrocyte-derived growth factors and their receptors. Exp. Neurol. 2006, 201, 225–233. [Google Scholar] [CrossRef]
- Dhandapani, K.M.; Hadman, M.; De Sevilla, L.; Wade, M.F.; Mahesh, V.B.; Brann, D.W. Astrocyte protection of neurons: Role of transforming growth factor-beta signaling via a c-Jun-AP-1 protective pathway. J. Biol. Chem. 2003, 278, 43329–43339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Xu, H.; Lei, T.; Yang, Y.; Jing, D.; Dai, S.; Luo, P.; Xu, Q. A Pulsed Electromagnetic Field Protects against Glutamate-Induced Excitotoxicity by Modulating the Endocannabinoid System in HT22 Cells. Front. Neurosci. 2017, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Capone, F.; Liberti, M.; Apollonio, F.; Camera, F.; Setti, S.; Cadossi, R.; Quattrocchi, C.C.; Di Lazzaro, V. An open-label, one-arm, dose-escalation study to evaluate safety and tolerability of extremely low frequency magnetic fields in acute ischemic stroke. Sci. Rep. 2017, 7, 12145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cekanaviciute, E.; Buckwalter, M.S. Astrocytes: Integrative Regulators of Neuroinflammation in Stroke and Other Neurological Diseases. Neurotherapeutics 2016, 13, 685–701. [Google Scholar] [CrossRef]
- Dzietko, M.; Derugin, N.; Wendland, M.F.; Vexler, Z.S.; Ferriero, D.M. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl. Stroke Res. 2013, 4, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-P.; Liu, H.-J.; Liu, X.-F. VEGF promotes angiogenesis and functional recovery in stroke rats. J. Invest. Surg. 2010, 23, 149–155. [Google Scholar] [CrossRef]
- Geiseler, S.J.; Morland, C. The Janus Face of VEGF in Stroke. Int. J. Mol. Sci. 2018, 19, 1362. [Google Scholar] [CrossRef] [Green Version]
- Kaya, D.; Gürsoy-Ozdemir, Y.; Yemisci, M.; Tuncer, N.; Aktan, S.; Dalkara, T. VEGF protects brain against focal ischemia without increasing blood--brain permeability when administered intracerebroventricularly. J. Cereb. Blood Flow Metab. 2005, 25, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.J.; Love, C.; Spector, M.; Cool, S.M.; Nurcombe, V.; Lo, E.H. Endogenous regeneration: Engineering growth factors for stroke. Neurochem. Int. 2017, 107, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amalia, L.; Sadeli, H.A.; Parwati, I.; Rizal, A.; Panigoro, R. Hypoxia-inducible factor-1α in acute ischemic stroke: Neuroprotection for better clinical outcome. Heliyon 2020, 6, e04286. [Google Scholar] [CrossRef] [PubMed]
- Shi, H. Hypoxia inducible factor 1 as a therapeutic target in ischemic stroke. Curr. Med. Chem. 2009, 16, 4593–4600. [Google Scholar] [CrossRef] [Green Version]
- Hyvärinen, T.; Hagman, S.; Ristola, M.; Sukki, L.; Veijula, K.; Kreutzer, J.; Kallio, P.; Narkilahti, S. Co-stimulation with IL-1β and TNF-α induces an inflammatory reactive astrocyte phenotype with neurosupportive characteristics in a human pluripotent stem cell model system. Sci. Rep. 2019, 9, 16944. [Google Scholar] [CrossRef]
- Yan, J.; Tan, T.; Huang, Q. Protective effect of astrocyte-conditioned medium on neurons following hypoxia and mechanical injury. Chin. J. Traumatol. 2013, 16, 3–9. [Google Scholar]
- Lamarche, F.; Signorini-Allibe, N.; Gonthier, B.; Barret, L. Influence of vitamin E, sodium selenite, and astrocyte-conditioned medium on neuronal survival after chronic exposure to ethanol. Alcohol 2004, 33, 127–138. [Google Scholar] [CrossRef]
- Song, C.; Wu, Y.-S.; Yang, Z.-Y.; Kalueff, A.V.; Tsao, Y.-Y.; Dong, Y.; Su, K.-P. Astrocyte-Conditioned Medium Protects Prefrontal Cortical Neurons from Glutamate-Induced Cell Death by Inhibiting TNF-α Expression. Neuroimmunomodulation 2019, 26, 33–42. [Google Scholar] [CrossRef]
- Varani, K.; Vincenzi, F.; Targa, M.; Corciulo, C.; Fini, M.; Setti, S.; Cadossi, R.; Borea, P.A. Effect of pulsed electromagnetic field exposure on adenosine receptors in rat brain. Bioelectromagnetics 2012, 33, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, F.; Targa, M.; Corciulo, C.; Gessi, S.; Merighi, S.; Setti, S.; Cadossi, R.; Borea, P.A.; Varani, K. The anti-tumor effect of A3 adenosine receptors is potentiated by pulsed electromagnetic fields in cultured neural cancer cells. PLoS ONE 2012, 7, e39317. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vincenzi, F.; Pasquini, S.; Setti, S.; Salati, S.; Cadossi, R.; Borea, P.A.; Varani, K. Pulsed Electromagnetic Fields Stimulate HIF-1α-Independent VEGF Release in 1321N1 Human Astrocytes Protecting Neuron-like SH-SY5Y Cells from Oxygen-Glucose Deprivation. Int. J. Mol. Sci. 2020, 21, 8053. https://doi.org/10.3390/ijms21218053
Vincenzi F, Pasquini S, Setti S, Salati S, Cadossi R, Borea PA, Varani K. Pulsed Electromagnetic Fields Stimulate HIF-1α-Independent VEGF Release in 1321N1 Human Astrocytes Protecting Neuron-like SH-SY5Y Cells from Oxygen-Glucose Deprivation. International Journal of Molecular Sciences. 2020; 21(21):8053. https://doi.org/10.3390/ijms21218053
Chicago/Turabian StyleVincenzi, Fabrizio, Silvia Pasquini, Stefania Setti, Simona Salati, Ruggero Cadossi, Pier Andrea Borea, and Katia Varani. 2020. "Pulsed Electromagnetic Fields Stimulate HIF-1α-Independent VEGF Release in 1321N1 Human Astrocytes Protecting Neuron-like SH-SY5Y Cells from Oxygen-Glucose Deprivation" International Journal of Molecular Sciences 21, no. 21: 8053. https://doi.org/10.3390/ijms21218053
APA StyleVincenzi, F., Pasquini, S., Setti, S., Salati, S., Cadossi, R., Borea, P. A., & Varani, K. (2020). Pulsed Electromagnetic Fields Stimulate HIF-1α-Independent VEGF Release in 1321N1 Human Astrocytes Protecting Neuron-like SH-SY5Y Cells from Oxygen-Glucose Deprivation. International Journal of Molecular Sciences, 21(21), 8053. https://doi.org/10.3390/ijms21218053







