Co-Culture of Primary Human Coronary Artery and Internal Thoracic Artery Endothelial Cells Results in Mutually Beneficial Paracrine Interactions
Abstract
:1. Introduction
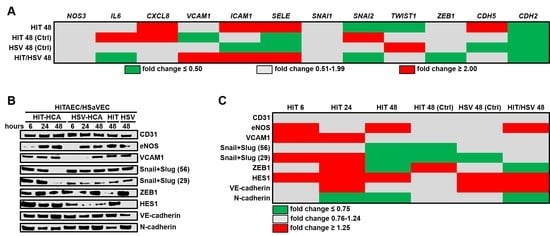
2. Results
3. Discussion
4. Materials and Methods
4.1. Co-Culture Model
4.2. RT-qPCR
4.3. Western Blotting
4.4. Secretome Profiling
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CABG | Coronary artery bypass grafting |
| SV | Saphenous vein |
| ITA | Internal thoracic artery |
| ECs | Endothelial cells |
| HITAEC | Human internal thoracic artery endothelial cells |
| eNOS | Endothelial nitric oxide synthase |
| VEGF | Vascular endothelial growth factor |
| HSaVEC | Human saphenous vein endothelial cells |
| HCAEC | Human coronary artery endothelial cells |
| EndoMT | Endothelial-to-mesenchymal transition |
| RNA | Ribonucleic acid |
| IL | Interleukin |
| CXCL | Chemokine (C-X-C motif) ligand |
| VCAM | Vascular cell adhesion molecule |
| ICAM | Intercellular cell adhesion molecule |
| SELE | E-selectin |
| SNAI | Snail family transcriptional repressor |
| TWIST | Twist family basic helix-loop-helix transcription factor |
| ZEB | Zinc finger E-box binding homeobox |
| CDH | Cadherin |
| VE-cadherin | Vascular endothelial cadherin |
| N-cadherin | Neural cadherin |
| HEY | Hairy enhancer of split-related family basic helix-loop-helix transcription factor with YRPW motif |
| HES | Hairy enhancer of split family helix-loop-helix transcription factor |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| CD | Cluster of differentiation |
| LAD | Left anterior descending coronary artery |
| PBS | Phosphate-buffered saline |
| RIPA | Radioimmunoprecipitation assay |
| cDNA | Complementary DNA |
| PECAM | Platelet endothelial cell adhesion molecule |
References
- Thielmann, M.; Sharma, V.; Al-Attar, N.; Bulluck, H.; Bisleri, G.; Bunge, J.J.; Czerny, M.; Ferdinandy, P.; Frey, U.H.; Heusch, G.; et al. ESC Joint Working Groups on Cardiovascular Surgery and the Cellular Biology of the Heart Position Paper: Perioperative myocardial injury and infarction in patients undergoing coronary artery bypass graft surgery. Eur. Heart J. 2017, 38, 2392–2407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrel, T.; Winkler, B. Current trends in selection of conduits for coronary artery bypass grafting. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Taggart, D.; Suma, H.; Puskas, J.D.; Crea, F.; Massetti, M. The choice of conduits in coronary artery bypass surgery. J. Am. Coll. Cardiol. 2015, 66, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Fajadet, J.; Chieffo, A. Current management of left main coronary artery disease. Eur. Heart J. 2012, 33, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Hillis, L.D.; Smith, P.K.; Anderson, J.L.; Bittl, J.A.; Bridges, C.R.; Byrne, J.G.; Cigarroa, J.E.; Disesa, V.J.; Hiratzka, L.F.; Hutter, A.M., Jr. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation 2011, 124, e652–e735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetto, U.; Raja, S.G.; Albanese, A.; Amrani, M.; Biondi-Zoccai, G.; Frati, G. Searching for the second best graft for coronary artery bypass surgery: A network meta-analysis of randomized controlled trials. Eur. J. Cardiothorac. Surg. 2014, 47, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J.; et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of Veterans Affairs Cooperative Study. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- FitzGibbon, G.M.; Kafka, H.P.; Leach, A.J.; Keon, W.J.; Hooper, G.; Burton, J.R. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5065 grafts related to survival and reoperation in 1388 patients during 25 years. J. Am. Coll. Cardiol. 1996, 28, 616–626. [Google Scholar] [CrossRef] [Green Version]
- Van Domburg, R.T.; Kappetein, A.P.; Bogers, A.J. The clinical outcome after coronary bypass surgery: A 30-year follow-up study. Eur. Heart J. 2009, 30, 453–458. [Google Scholar] [CrossRef]
- Harskamp, R.E.; Lopes, R.D.; Baisden, C.E.; De Winter, R.J.; Alexander, J.H. Saphenous vein graft failure after coronary artery bypass surgery: Pathophysiology, management, and future directions. Ann. Surg. 2013, 257, 824–833. [Google Scholar] [CrossRef]
- Otsuka, F.; Yahagi, K.; Sakakura, K.; Virmani, R. Why is the mammary artery so special and what protects it from atherosclerosis? Ann. Cardiothorac. Surg. 2013, 2, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, E.; De Souza, D.R.; Böning, A.; Liakopoulos, O.J.; Choi, Y.H.; Pepper, J.; Gibson, C.M.; Perrault, L.P.; Wolf, R.K.; Kim, K.B.; et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol. 2020, 17, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Bakaeen, F.; Benedetto, U.; Rahouma, M.; Di Franco, A.; Tam, D.Y.; Iannaccone, M.; Schwann, T.A.; Habib, R.; Ruel, M.; et al. Use rate and outcome in bilateral internal thoracic artery grafting: Insights from a systematic review and meta-analysis. J. Am. Heart Assoc. 2018, 7, e009361. [Google Scholar] [CrossRef] [Green Version]
- Taggart, D.P.; Gaudino, M.; Gerry, S.; Gray, A.; Lees, B.; Dimagli, A.; Puskas, J.D.; Zamvar, V.; Pawlaczyk, R.; Royse, A.G.; et al. Effect of total arterial grafting in the arterial revascularization trial. J. Thorac. Cardiovasc. Surg. 2020, S0022–S5223, 30591–30592. [Google Scholar] [CrossRef] [PubMed]
- Saran, N.; Locker, C.; Said, S.M.; Daly, R.C.; Maltais, S.; Stulak, J.M.; Greason, K.L.; Pochettino, A.; Schaff, H.V.; Dearani, J.A.; et al. Current trends in bilateral internal thoracic artery use for coronary revascularization: Extending benefit to high-risk patients. J. Thorac. Cardiovasc. Surg. 2018, 155, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.R.; Black, M.C.; Whited, W.M.; Sell-Dottin, K.; Alwair, H.; Ganzel, B.L.; Slaughter, M. Is the internal mammary artery graft beneficial in emergent coronary artery bypass surgery? A Society of Thoracic Surgeons National Database analysis. J. Cardiovasc. Surg. 2020. [Google Scholar] [CrossRef]
- Rafii, S.; Butler, J.M.; Ding, B.S. Angiocrine functions of organ-specific endothelial cells. Nature 2016, 529, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Roumenina, L.T.; Rayes, J.; Frimat, M.; Fremeaux-Bacchi, V. Endothelial cells: Source, barrier, and target of defensive mediators. Immunol. Rev. 2016, 274, 307–329. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Kusumbe, A.P.; Adams, R.H. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015, 25, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.; Jung, F.; Pietzsch, J. Human endothelial cell models in biomaterial research. Trends Biotechnol. 2017, 35, 265–277. [Google Scholar] [CrossRef]
- Potente, M.; Mäkinen, T. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aird, W.C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2012, 2, a006429. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Toesca, A.; Maggiano, N.; Pragliola, C.; Possati, G. Localization of nitric oxide synthase type III in the internal thoracic and radial arteries and the great saphenous vein: A comparative immunohistochemical study. J. Thorac. Cardiovasc. Surg. 2003, 125, 1510–1515. [Google Scholar] [CrossRef]
- Broeders, M.A.; Doevendans, P.A.; Maessen, J.G.; Van Gorsel, E.; Egbrink, M.G.; Daemen, M.J.; Tangelder, G.; Reneman, R.S.; Van Der Zee, R. The human internal thoracic artery releases more nitric oxide in response to vascular endothelial growth factor than the human saphenous vein. J. Thorac. Cardiovasc. Surg. 2001, 122, 305–309. [Google Scholar] [CrossRef] [Green Version]
- Tadjkarimi, S.; O’Neil, G.S.; Luu, T.N.; Allen, S.P.; Schyns, C.J.; Chester, A.H.; Yacoub, M.H. Comparison of cyclic GMP in human internal mammary artery and saphenous vein: Implications for coronary artery bypass graft patency. Cardiovasc. Res. 1992, 26, 297–300. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Jensen, H.K.; Mehta, J.L. Endothelial cell dysfunction as a novel therapeutic target in atherosclerosis. Expert Rev. Cardiovasc. Ther. 2016, 14, 1021–1033. [Google Scholar] [CrossRef]
- Cahill, P.A.; Redmond, E.M. Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.Y.; Schwartz, M.A.; Simons, M. Endothelial-to-mesenchymal transition, vascular inflammation, and atherosclerosis. Front. Cardiovasc. Med. 2020, 7, 53. [Google Scholar] [CrossRef]
- Cho, J.G.; Lee, A.; Chang, W.; Lee, M.-S.; Kim, J. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front. Immunol. 2018, 9, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lui, K.O.; Zhou, B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 2018, 15, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Shu, B.; Zhang, Y.; Wang, M. Endothelial response to pathophysiological stress. Arter. Thromb. Vasc. Biol. 2019, 39, e233–e243. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [CrossRef] [Green Version]
- Tyrrell, D.J.; Goldstein, D.R. Ageing and atherosclerosis: Vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 2020, 1–11. [Google Scholar] [CrossRef]
- Ridker, P.M. Anticytokine agents: Targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ. Res. 2019, 124, 437–450. [Google Scholar] [CrossRef]
- Apostolakis, S.; Vogiatzi, K.; Amanatidou, V.; Spandidos, D.A. Interleukin 8 and cardiovascular disease. Cardiovasc. Res. 2009, 84, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Schnoor, M.; Alcaide, P.; Voisin, M.-B.; Van Buul, J.D. Crossing the vascular wall: Common and unique mechanisms exploited by different leukocyte subsets during extravasation. Mediat. Inflamm. 2015, 2015, 946509. [Google Scholar] [CrossRef] [Green Version]
- Galkina, E.; Ley, K. Vascular adhesion molecules in atherosclerosis. Arter. Thromb. Vasc. Biol. 2007, 27, 2292–2301. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to mesenchymal transition: Role in physiology and in the pathogenesis of human diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to mesenchymal transition in cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Joh, J.H.; Park, H.C. The cutoff value of saphenous vein diameter to predict reflux. J. Korean Surg. Soc. 2013, 85, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Spivack, D.E.; Kelly, P.; Gaughan, J.P.; Van Bemmelen, P.S. Mapping of superficial extremity veins: Normal diameters and trends in a vascular patient-population. Ultrasound Med. Biol. 2012, 38, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Canham, P.B.; Finlay, H.M.; Boughner, D.R. Contrasting structure of the saphenous vein and internal mammary artery used as coronary bypass vessels. Cardiovasc. Res. 1997, 34, 557–567. [Google Scholar] [CrossRef]
- Dodge, J.T., Jr.; Brown, B.G.; Bolson, E.L.; Dodge, H.T. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation 1992, 86, 232–246. [Google Scholar] [CrossRef] [Green Version]
- Waller, B.F.; Orr, C.M.; Slack, J.D.; Pinkerton, C.A.; Van Tassel, J.; Peters, T. Anatomy, histology, and pathology of coronary arteries: A review relevant to new interventional and imaging techniques-Part I. Clin. Cardiol. 1992, 15, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Canver, C.C.; Ricotta, J.J.; Bhayana, J.N.; Fiedler, R.C.; Mentzer, R.M., Jr. Use of duplex imaging to assess suitability of the internal mammary artery for coronary artery surgery. J. Vasc. Surg. 1991, 13, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Ahn, B.H.; Kim, G.S.; Jeong, I.S.; Lee, K.S.; Song, S.Y.; Na, K.J.; Oh, S.G. Change in luminal diameter of the left internal thoracic artery anastomosed to the totally occluded left anterior descending coronary artery. J. Cardiothorac. Surg. 2016, 11, 157. [Google Scholar] [CrossRef] [Green Version]
- Gopal, D.; Singh, N.; Jagadeesh, A.; Ture, A.; Thimmarayappa, A. Comparison of left internal mammary artery diameter before and after left stellate ganglion block. Ann. Card. Anaesth. 2013, 16, 238–242. [Google Scholar] [CrossRef]
- Mirkhani, H.; Shafa, M.; Khazraei, H. Comparison of the effects of levosimendan and papaverine on human internal mammary artery and saphenous vein. Cardiovasc. Drugs Ther. 2009, 23, 355–359. [Google Scholar] [CrossRef]
- Nguyen, H.G.; Korach, A.; Collura, C.; Eskenazi, B.R.; Vita, J.A.; Shapira, O.M. Differential effects of natriuretic peptides on arterial and venous coronary artery bypass conduits. Ann. Thorac. Surg. 2009, 87, 748–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerer-Lercher, A.; Fersterer, J.; Holzmann, S.; Bonatti, J.; Ruttmann, E.; Hoefer, D.; Mair, J.; Puschendorf, B. Direct comparison of relaxation and cGMP production in human coronary by-pass grafts in response to stimulation with natriuretic peptides and a nitric oxide donor. Clin. Sci. 2006, 111, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkovic, I.; Wong, R.; LeMarchand, E.; Rivers-Auty, J.; Rajkovic, O.; Garlanda, C.; Allan, S.M.; Pinteaux, E. Pentraxin 3 promotes long-term cerebral blood flow recovery, angiogenesis, and neuronal survival after stroke. J. Mol. Med. 2018, 96, 1319–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Grande, B.; Varghese, L.; Molina-Holgado, F.; Rajkovic, O.; Garlanda, C.; Denes, A.; Pinteaux, E. Pentraxin 3 mediates neurogenesis and angiogenesis after cerebral ischaemia. J. Neuroinflamm. 2015, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Bhutia, S.K.; Azab, B.; Kegelman, T.P.; Peachy, L.; Santhekadur, P.K.; Dasgupta, S.; Dash, R.; Dent, P.; Grant, S.; et al. MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res. 2013, 73, 844–854. [Google Scholar] [CrossRef] [Green Version]
- Azar, W.J.; Azar, S.H.X.; Higgins, S.; Hu, J.F.; Hoffman, A.R.; Newgreen, D.F.; Werther, G.A.; Russo, V.C. IGFBP-2 enhances VEGF gene promoter activity and consequent promotion of angiogenesis by neuroblastoma cells. Endocrinology 2011, 152, 3332–3342. [Google Scholar] [CrossRef] [Green Version]
- Lefere, S.; Van De Velde, F.; Hoorens, A.; Raevens, S.; Van Campenhout, S.; Vandierendonck, A.; Neyt, S.; Vandeghinste, B.; Vanhove, C.; Debbaut, C.; et al. Angiopoietin-2 promotes pathological angiogenesis and is a therapeutic target in murine nonalcoholic fatty liver disease. Hepatology 2019, 69, 1087–1104. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Nakamura, T.; Kanetake, H.; Kanda, S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J. Cell Sci. 2002, 115, 175–183. [Google Scholar]
- Litwin, M.; Radwańska, A.; Paprocka, M.; Kieda, C.; Dobosz, T.; Witkiewicz, W.; Baczyńska, D. The role of FGF2 in migration and tubulogenesis of endothelial progenitor cells in relation to pro-angiogenic growth factor production. Mol. Cell. Biochem. 2015, 410, 131–142. [Google Scholar] [CrossRef]
- Esser, J.S.; Rahner, S.; Deckler, M.; Bode, C.; Patterson, C.; Moser, M. Fibroblast growth factor signaling pathway in endothelial cells is activated by BMPER to promote angiogenesis. Arter. Thromb. Vasc. Biol. 2015, 35, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Mao, W.; Chen, Q.; Huang, Z.; Wang, L.; Dai, J.; Wang, H. Endostar, a modified recombinant human endostatin, suppresses angiogenesis through inhibition of Wnt/β-catenin signaling pathway. PLoS ONE 2014, 9, e107463. [Google Scholar] [CrossRef]
- Ergün, S.; Kilic, N.; Wurmbach, J.; Ebrahimnejad, A.; Fernando, M.; Sevinc, S.; Kilic, E.; Chalajour, F.; Fiedler, W.; Lauke, H.; et al. Endostatin inhibits angiogenesis by stabilization of newly formed endothelial tubes. Angiogenesis 2001, 4, 193–206. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Qi, F.; Jia, L.; Wang, S.; Wang, C.; Song, N.; Fu, Y.; Li, L.; Luo, Y. Tumor cell-secreted angiogenin induces angiogenic activity of endothelial cells by suppressing miR-542-3p. Cancer Lett. 2015, 368, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Liu, S.; Tsuji, T.; Olson, K.A.; Hu, G.F. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene 2005, 24, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.W.; Chen, W.L.; Huang, S.M.; Chan, J.Y.H. Platelet-derived growth factor-AA is a substantial factor in the ability of adipose-derived stem cells and endothelial progenitor cells to enhance wound healing. FASEB J. 2019, 33, 2388–2395. [Google Scholar] [CrossRef] [Green Version]
- Shikada, Y.; Yonemitsu, Y.; Koga, T.; Onimaru, M.; Nakano, T.; Okano, S.; Sata, S.; Nakagawa, K.; Yoshino, I.; Maehara, Y.; et al. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non–small cell lung carcinomas. Cancer Res. 2005, 65, 7241–7248. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Zhao, R.; Lin, S.; Bai, X.; Zhang, L.; Yuan, S.; Sun, L. CXCL16 induces angiogenesis in autocrine signaling pathway involving hypoxia-inducible factor 1α in human umbilical vein endothelial cells. Oncol. Rep. 2015, 35, 1557–1565. [Google Scholar] [CrossRef]
- Zhuge, X.; Murayama, T.; Arai, H.; Yamauchi, R.; Tanaka, M.; Shimaoka, T.; Yonehara, S.; Kume, N.; Yokode, M.; Kita, T. CXCL16 is a novel angiogenic factor for human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2005, 331, 1295–1300. [Google Scholar] [CrossRef] [Green Version]
- Ghersi, G.; Chen, W.-T.; Lee, E.W.; Zukowska, Z. Critical role of dipeptidyl peptidase IV in neuropeptide Y-mediated endothelial cell migration in response to wounding. Peptides 2001, 22, 453–458. [Google Scholar] [CrossRef]
- Zukowska-Grojec, Z.; Karwatowska-Prokopczuk, E.; Rose, W.; Rone, J.; Movafagh, S.; Ji, H.; Yeh, Y.; Chen, W.T.; Kleinman, H.K.; Grouzmann, E.; et al. Neuropeptide Y: A novel angiogenic factor from the sympathetic nerves and endothelium. Circ. Res. 1998, 83, 187–195. [Google Scholar] [CrossRef]
- Xin, X.; Yang, S.; Ingle, G.; Zlot, C.; Rangell, L.; Kowalski, J.; Schwall, R.; Ferrara, N.; Gerritsen, M.E. Hepatocyte growth factor enhances vascular endothelial growth factor-induced angiogenesis in vitro and in vivo. Am. J. Pathol. 2001, 158, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Bussolino, F.; Di Renzo, M.F.; Ziche, M.; Bocchietto, E.; Olivero, M.; Naldini, L.; Gaudino, G.; Tamagnone, L.; Coffer, A.; Comoglio, P.M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992, 119, 629–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browne, S.; Jha, A.K.; Ameri, K.; Marcus, S.G.; Yeghiazarians, Y.; Healy, K.E. TGF-β1/CD105 signaling controls vascular network formation within growth factor sequestering hyaluronic acid hydrogels. PLoS ONE 2018, 13, e0194679. [Google Scholar] [CrossRef] [PubMed]
- Duff, S.E.; Li, C.; Garland, J.M.; Kumar, S. CD105 is important for angiogenesis: Evidence and potential applications. FASEB J. 2003, 17, 984–992. [Google Scholar] [CrossRef]
- Dallinga, M.G.; Habani, Y.I.; Kayser, R.P.; Van Noorden, C.J.F.; Klaassen, I.; Schlingemann, R.O. IGF-binding proteins 3 and 4 are regulators of sprouting angiogenesis. Mol. Biol. Rep. 2020, 47, 2561–2572. [Google Scholar] [CrossRef] [Green Version]
- Granata, R.; Trovato, L.; Lupia, E.; Sala, G.; Settanni, F.; Camussi, G.; Ghidoni, R.; Ghigo, E. Insulin-like growth factor binding protein-3 induces angiogenesis through IGF-I- and SphK1-dependent mechanisms. J. Thromb. Haemost. 2007, 5, 835–845. [Google Scholar] [CrossRef]
- Ferrari, G.; Cook, B.D.; Terushkin, V.; Pintucci, G.; Mignatti, P. Transforming growth factor-beta 1 (TGF-β1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J. Cell. Physiol. 2009, 219, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Budi, E.H.; Mamaï, O.; Hoffman, S.; Akhurst, R.J.; Derynck, R. Enhanced TGF-β signaling contributes to the insulin-induced angiogenic responses of endothelial cells. iScience 2019, 11, 474–491. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.; Martin-Manso, G.; Pendrak, M.L.; Garfield, S.H.; Isenberg, J.S.; Roberts, D.D. Thrombospondin-1 inhibits VEGF Receptor-2 signaling by disrupting its association with CD47. J. Biol. Chem. 2010, 285, 38923–38932. [Google Scholar] [CrossRef] [Green Version]
- Nör, J.E.; Mitra, R.S.; Sutorik, M.M.; Mooney, D.J.; Castle, V.P.; Polverini, P.J. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J. Vasc. Res. 2000, 37, 209–218. [Google Scholar] [CrossRef]
- Bir, S.C.; Xiong, Y.; Kevil, C.G.; Luo, J. Emerging role of PKA/eNOS pathway in therapeutic angiogenesis for ischaemic tissue diseases. Cardiovasc. Res. 2012, 95, 7–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amano, K.; Matsubara, H.; Iba, O.; Okigaki, M.; Fujiyama, S.; Imada, T.; Kojima, H.; Nozawa, Y.; Kawashima, S.; Yokoyama, M.; et al. Enhancement of ischemia-induced angiogenesis by eNOS overexpression. Hypertension 2003, 41, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Koike, H.; Murakami, K.; Aoki, M.; Makino, H.; Hashiya, N.; Ogihara, T.; Kaneda, Y.; Kohno, M.; Morishita, R. Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation 2003, 108, 2250–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Demuinck, E.D.; Zhuang, Z.; Drinane, M.; Kauser, K.; Rubanyi, G.M.; Qian, H.S.; Murata, T.; Escalante, B.; Sessa, W. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc. Natl. Acad. Sci. USA 2005, 102, 10999–11004. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Guan, H.; He, J.; Zeng, L.; Yuan, Z.; Xu, M.; Zhang, W.; Wu, X.; Guan, J. VEGF increases the proliferative capacity and eNOS/NO levels of endothelial progenitor cells through the calcineurin/NFAT signalling pathway. Cell Biol. Int. 2012, 36, 21–27. [Google Scholar] [CrossRef]
- Kimura, H.; Esumi, H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim. Pol. 2003, 50, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Kroll, J.; Waltenberger, J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR). Biochem. Biophys. Res. Commun. 1998, 252, 743–746. [Google Scholar] [CrossRef]
- Zhao, D.; Xue, C.; Lin, S.; Shi, S.; Li, Q.; Liu, M.; Cai, X.; Lin, Y. Notch signaling pathway regulates angiogenesis via endothelial Cell in 3D co-culture model. J. Cell. Physiol. 2016, 232, 1548–1558. [Google Scholar] [CrossRef]
- Takeshita, K.; Satoh, M.; Ii, M.; Silver, M.; Limbourg, F.P.; Mukai, Y.; Rikitake, Y.; Radtke, F.; Gridley, T.; Losordo, U.W.; et al. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ. Res. 2007, 100, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Patenaude, A.; Fuller, M.; Chang, L.; Wong, F.; Paliouras, G.; Shaw, R.; Kyle, A.H.; Umlandt, P.; Baker, J.H.; Diaz, E.; et al. Endothelial-specific notch blockade inhibits vascular function and tumor growth through an eNOS-dependent mechanism. Cancer Res. 2014, 74, 2402–2411. [Google Scholar] [CrossRef] [Green Version]
- Sewduth, R.; Santoro, M.M. “Decoding” angiogenesis: New facets controlling endothelial cell behavior. Front. Physiol. 2016, 7, 306. [Google Scholar] [CrossRef] [Green Version]
- Vandekeere, S.; Dewerchin, M.; Carmeliet, P. Angiogenesis revisited: An overlooked role of endothelial cell metabolism in vessel sprouting. Microcirculation 2015, 22, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, A.F.; Covassin, L.; Lawson, N.D. Modulation of VEGF signalling output by the Notch pathway. BioEssays 2008, 30, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Smeda, M.; Kieronska, A.; Adamski, M.G.; Proniewski, B.; Sternak, M.; Mohaissen, T.; Przyborowski, K.; Derszniak, K.; Kaczor, D.; Stojak, M.; et al. Nitric oxide deficiency and endothelial–mesenchymal transition of pulmonary endothelium in the progression of 4T1 metastatic breast cancer in mice. Breast Cancer Res. 2018, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Ricard, N.; Scott, R.P.; Booth, C.J.; Velazquez, H.; Cilfone, N.A.; Baylon, J.L.; Gulcher, J.R.; Quaggin, S.E.; Chittenden, T.W.; Simons, M. Endothelial ERK1/2 signaling maintains integrity of the quiescent endothelium. J. Exp. Med. 2019, 216, 1874–1890. [Google Scholar] [CrossRef]
- Sabbineni, H.; Verma, A.; Artham, S.; Anderson, D.; Amaka, O.; Liu, F.; Narayanan, S.P.; Somanath, P.R. Pharmacological inhibition of β-catenin prevents EndMT in vitro and vascular remodeling in vivo resulting from endothelial Akt1 suppression. Biochem. Pharmacol. 2019, 164, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.L.; Xavier, R.; Wong, J.; Ezedi, I.; Mashimo, H.; Mizoguchi, A.; Mizoguchi, E.; Bhan, A.K.; Podolsky, D.K. Paradoxical roles of different nitric oxide synthase isoforms in colonic injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G137–G147. [Google Scholar] [CrossRef] [Green Version]
- Cirino, G.; Fiorucci, S.; Sessa, W. Endothelial nitric oxide synthase: The Cinderella of inflammation? Trends Pharmacol. Sci. 2003, 24, 91–95. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.-E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by extracellular vesicles: Where we are and where we need to go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.K.; Yang, Y.-P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019, 27, 940–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, D.; Jing, Q.; Zhao, W.; Yue, H.; Su, L.; Zhang, S.; Zhao, J. Finding ATF4/p75NTR/IL-8 signal pathway in endothelial–mesenchymal transition by safrole Oxide. PLoS ONE 2014, 9, e99378. [Google Scholar] [CrossRef] [Green Version]
- Mathiyalagan, P.; Liang, Y.; Kim, D.; Misener, S.; Thorne, T.; Kamide, C.E.; Klyachko, E.; Losordo, D.W.; Hajjar, R.J.; Sahoo, S. Angiogenic mechanisms of human CD34 + Stem cell exosomes in the repair of ischemic hindlimb. Circ. Res. 2017, 120, 1466–1476. [Google Scholar] [CrossRef] [Green Version]
- Mocharla, P.; Briand, S.; Giannotti, G.; Dörries, C.; Jakob, P.; Paneni, F.; Lüscher, T.; Landmesser, U. AngiomiR-126 expression and secretion from circulating CD34+ and CD14+ PBMCs: Role for proangiogenic effects and alterations in type 2 diabetics. Blood 2013, 121, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Ratajczak, J.; Kucia, M.; Mierzejewska, K.; Marlicz, W.; Pietrzkowski, Z.; Wojakowski, W.; Greco, N.J.; Tendera, M.; Ratajczak, M.Z. Paracrine proangiopoietic effects of human umbilical cord blood-derived purified CD133+cells—Implications for stem cell therapies in regenerative medicine. Stem Cells Dev. 2013, 22, 422–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantaluppi, V.; Biancone, L.; Figliolini, F.; Beltramo, S.; Medica, D.; Deregibus, M.C.; Galimi, F.; Romagnoli, R.; Salizzoni, M.; Tetta, C.; et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012, 21, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Turner, M.; Xiao, F.; Munkonda, M.N.; Akbari, S.; Burns, K.D. High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia 2017, 60, 1791–1800. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Shang, M.; Zhang, M.; Wang, Y.; Chen, Y.; Wu, Y.; Liu, M.; Song, J.; Liu, Y. Microvesicles derived from hypoxia/reoxygenation-treated human umbilical vein endothelial cells promote apoptosis and oxidative stress in H9c2 cardiomyocytes. BMC Cell Biol. 2016, 17, 25. [Google Scholar] [CrossRef] [Green Version]
- Lebedeva, A.; Vorobyeva, D.; Vagida, M.; Ivanova, O.; Felker, E.; Fitzgerald, W.; Danilova, N.; Gontarenko, V.; Shpektor, A.; Vasilieva, E.; et al. Ex vivo culture of human atherosclerotic plaques: A model to study immune cells in atherogenesis. Atherosclerosis 2017, 267, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Munemitsu, T.; Ishii, A.; Okada, E.; Chihara, H.; Yoshida, K.; Takahashi, J.C.; Takagi, Y.; Miyamoto, S. Ex vivo assessment of various histological differentiation in human carotid plaque with near-infrared spectroscopy using multiple wavelengths. Neurol. Med. Chir. 2019, 59, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aplin, A.C.; Nicosia, R.F. The plaque-aortic ring assay: A new method to study human atherosclerosis-induced angiogenesis. Angiogenesis 2019, 22, 421–431. [Google Scholar] [CrossRef]
- Prim, D.A.; Menon, V.; Hasanian, S.; Carter, L.; Shazly, T.; Potts, J.D.; Eberth, J.F. Perfusion tissue culture initiates differential remodeling of internal thoracic arteries, radial arteries, and saphenous veins. J. Vasc. Res. 2018, 55, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Mekontso-Dessap, A.; Kirsch, M.; Guignabert, C.; Zadigue, P.; Adnot, S.; Loisance, D.; Eddahibi, S. Vascular-wall remodeling of 3 human bypass vessels: Organ culture and smooth muscle cell properties. J. Thorac. Cardiovasc. Surg. 2006, 131, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Kutikhin, A.G.; Tupikin, A.E.; Matveeva, V.G.; Shishkova, D.K.; Antonova, L.V.; Kabilov, M.R.; Velikanova, E.A. Human peripheral blood-derived endothelial colony-forming cells are highly similar to mature vascular endothelial cells yet demonstrate a transitional transcriptomic signature. Cells 2020, 9, 876. [Google Scholar] [CrossRef] [Green Version]

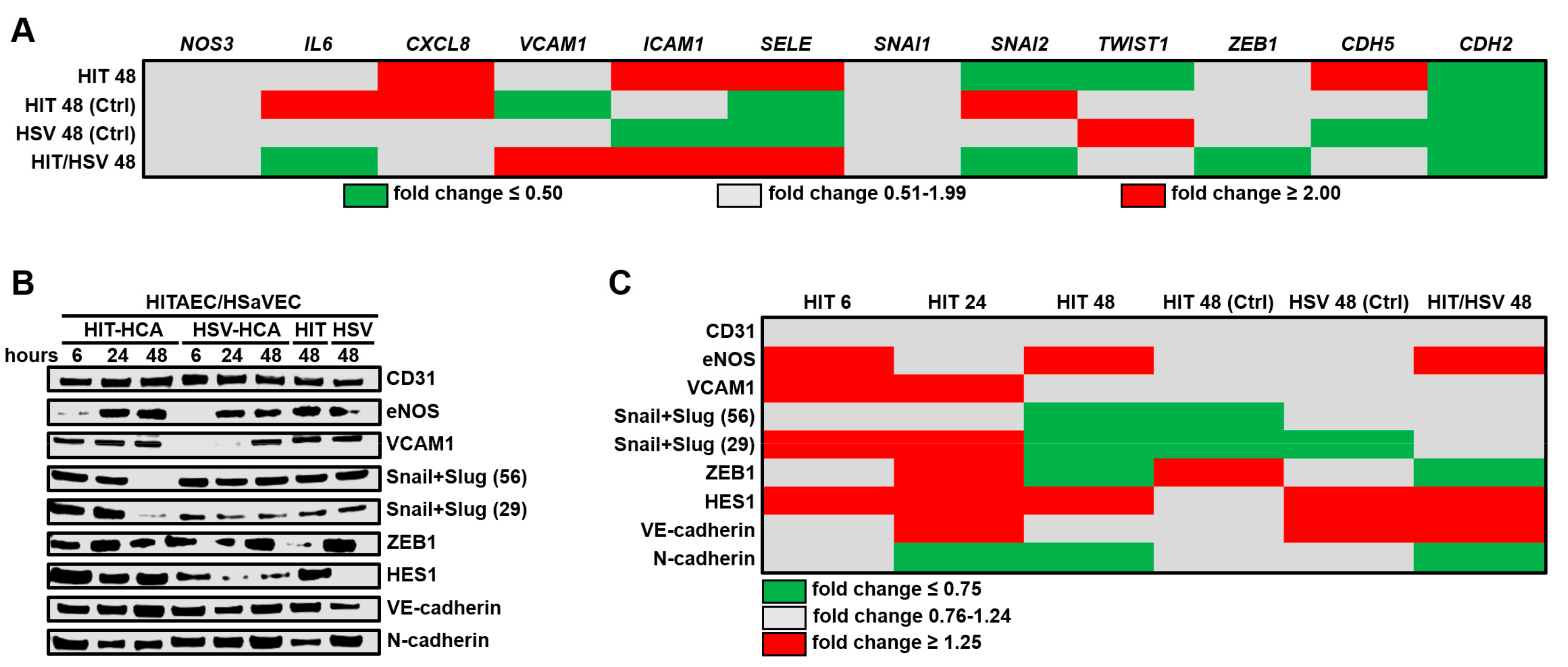

| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| NOS3 | 5′-GTGATGGCGAAGCGAGTGAAG-3′ | 5′-CCGAGCCCGAACACACAGAAC-3′ |
| IL6 | 5′-GGCACTGGCAGAAAACAACC-3′ | 5′-GCAAGTCTCCTCATTGAATCC-3′ |
| CXCL8 | 5′-CAGAGACAGCAGAGCACAC-3′ | 5′-AGTTCTTTAGCACTCCTTGGC-3′ |
| VCAM1 | 5′-CGTCTTGGTCAGCCCTTCCT-3′ | 5′-ACATTCATATACTCCCGCATCCTTC-3′ |
| ICAM1 | 5′-TTGGGCATAGAGACCCCGTT-3′ | 5′-GCACATTGCTCAGTTCATACACC-3′ |
| SELE | 5′-GCACAGCCTTGTCCAACC-3′ | 5′-ACCTCACCAAACCCTTCG-3′ |
| NAI1 | 5′-CAGACCCACTCAGATGTCAAGAA-3′ | 5′-GGGCAGGTATGGAGAGGAAGA-3′ |
| SNAI2 | 5′-ACTCCGAAGCCAAATGACAA-3′ | 5′-CTCTCTCTGTGGGTGTGTGT-3′ |
| TWIST1 | 5′-GTCCGCAGTCTTACGAGGAG-3′ | 5′-GCTTGAGGGTCTGAATCTTGCT-3′ |
| ZEB1 | 5′-GATGATGAATGCGAGTCAGATGC-3′ | 5′-ACAGCAGTGTCTTGTTGTTGT-3′ |
| CDH5 | 5′-AAGCGTGAGTCGCAAGAATG-3′ | 5′-TCTCCAGGTTTTCGCCAGTG-3′ |
| CDH2 | 5′-GCTTCTGGTGAAATCGCATTA-3′ | 5′-AGTCTCTCTTCTGCCTTTGTAG-3′ |
| PECAM1 | 5′-TGGCGCATGCCTGTAGTA-3′ | 5′-TCCGTTTCCTGGGTTCAA-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishkova, D.; Markova, V.; Sinitsky, M.; Tsepokina, A.; Frolov, A.; Zagorodnikov, N.; Bogdanov, L.; Kutikhin, A. Co-Culture of Primary Human Coronary Artery and Internal Thoracic Artery Endothelial Cells Results in Mutually Beneficial Paracrine Interactions. Int. J. Mol. Sci. 2020, 21, 8032. https://doi.org/10.3390/ijms21218032
Shishkova D, Markova V, Sinitsky M, Tsepokina A, Frolov A, Zagorodnikov N, Bogdanov L, Kutikhin A. Co-Culture of Primary Human Coronary Artery and Internal Thoracic Artery Endothelial Cells Results in Mutually Beneficial Paracrine Interactions. International Journal of Molecular Sciences. 2020; 21(21):8032. https://doi.org/10.3390/ijms21218032
Chicago/Turabian StyleShishkova, Daria, Victoria Markova, Maxim Sinitsky, Anna Tsepokina, Alexey Frolov, Nikita Zagorodnikov, Leo Bogdanov, and Anton Kutikhin. 2020. "Co-Culture of Primary Human Coronary Artery and Internal Thoracic Artery Endothelial Cells Results in Mutually Beneficial Paracrine Interactions" International Journal of Molecular Sciences 21, no. 21: 8032. https://doi.org/10.3390/ijms21218032
APA StyleShishkova, D., Markova, V., Sinitsky, M., Tsepokina, A., Frolov, A., Zagorodnikov, N., Bogdanov, L., & Kutikhin, A. (2020). Co-Culture of Primary Human Coronary Artery and Internal Thoracic Artery Endothelial Cells Results in Mutually Beneficial Paracrine Interactions. International Journal of Molecular Sciences, 21(21), 8032. https://doi.org/10.3390/ijms21218032