Cold Atmospheric Pressure Plasma (CAP) as a New Tool for the Management of Vulva Cancer and Vulvar Premalignant Lesions in Gynaecological Oncology
Abstract
1. Introduction
- (a)
- Vulva cancer is technically easy to approach using CAP.
- (b)
- The effect of radioresistance in subtypes of this malignancy is becoming a clinical problem.
- (c)
- VIN lesions are commonly treated/managed with local drugs or by applying tracer, which may be suitable for large PAM (plasma-activated medium) treatment.
- (d)
- (e)
- Anatomical circumstances usually restrict re-excisions after primary surgery, which is often combined with advanced plastic flaps (e.g., in the case of “worrisome” surgical margins).
- (f)
2. Epidemiology and the Prevalence of Vulvar Cancer
3. Aetiopathology, Clinical Aspects and Current Treatment of Vulvar Cancer and Its Premalignant Lesions
3.1. Precursors and Classification of the Disease
3.2. Current Treatment of the Disease
4. Current Knowledge of In Vitro Cell Lines and Further Potential for Clinical Application of CAP Oncogynaecology
5. Plasma Physical and Chemical Characteristics and Plasma Sources in Medicine
Sources of Cold Atmospheric Plasma
- Direct plasma sources: These plasmas use the human body (such as the skin, internal tissues, etc.) as an electrode. Thus, the current produced by plasmas has to pass through the body. The most commonly utilised technology in this category is the dielectric barrier discharge (DBD) plasma source. The major disadvantage of this technique is the application distance (between the electrodes) which must remain within a close range, generally less than three mm2, thus limiting its use for small areas of the human body [15].
- Indirect plasma sources: These plasmas are generated between two electrodes. Active species that are created by the plasmas are subsequently transported to target application areas. Several devices are available, ranging from very narrow plasma needles or jets to larger plasma torches such as the kINPen® MED, Atmospheric Pressure MicroPlasma Jet (APMPJ), InvivoPen, and MicroPlaSter® α and β. Plasma jets can be classified according to parameters such as discharge geometry, electrode arrangement, excitation frequency or pattern.
- Hybrid plasma sources: These plasmas combine the benefits of the two aforementioned plasma source types (e.g., using the plasma production technique of direct plasma sources and the essentially current-free property of indirect plasma sources). This is achieved by introducing a grounded wire mesh electrode, which has significantly smaller electrical resistance than that of the tissue. Thus, in principle, all current can pass through the wire mesh. The MiniFlatPlaSter is an example of a hybrid plasma source.
6. Plasma Interaction with Human Tissue
7. Plasma Promoted Wound Healing and Its Possibilities in the Surgical Treatment of VSCC
8. CAP Specific Abilities Predisposing Its Application in Anticancer Therapy
8.1. CAP Effect on Cellular and Extracellular Level
8.2. CAP and Apoptosis
8.3. CAP and Induced Gene Expressions, Proteomic and Epigenetic Changes
8.4. CAP Induced DNA Breaks and Modifications
8.5. CAP and Induced Redox ROS and RNS Effect
9. CAP as a Novel Anticancer Treatment Modality, Including Vulvar Pathologies
9.1. Direct Anti-Tumour Effects of CAP
9.2. Indirect Anti-Tumour Effects of CAP
9.3. Dual Cancer Therapeutic Approach: Synergy of CAP and Nanotechnology
9.4. Immunotherapy and CAP
10. Advancements in VC Therapy Based on Better Profiling and Novel Technologies Combining CAP with Existing Treatments
11. A paradigm Shift from Reactive to Predictive, Preventive and Personalised Medicine (3PM)—Prominent Examples in the Context of Vulva Cancer and Premalignant Lesions
11.1. The Primary Level of Targeted Prevention
11.2. The Secondary Level of Targeted Prevention
11.3. The Tertiary Level of Targeted Prevention
12. Status Quo and Clinically Relevant Perspectives
13. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 3001. [Google Scholar] [CrossRef]
- Rehman, M.U.; Jawaid, P.; Uchiyama, H.; Kondo, T. Comparison of free radicals formation induced by cold atmospheric plasma, ultrasound, and ionizing radiation. Arch. Biochem. Biophys. 2016, 605, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Miyazaki, A.; Uchida, G.; Setsuhara, Y. Atmospheric-pressure plasma interaction with soft materials as fundamental processes in plasma medicine. J. Nanosci. Nanotechnol. 2015, 15, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, S.; Ono, R. Effect of discharge polarity on the propagation of atmospheric-pressure helium plasma jets and the densities of OH, NO, and O radicals. Biointerphases 2015, 10, 029514. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Uddin, N.; Sim, G.B.; Hong, Y.J.; Baik, K.Y.; Kim, C.H.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Responses of Solid Tumor Cells in DMEM to Reactive Oxygen Species Generated by Non-Thermal Plasma and Chemically Induced ROS Systems. Sci. Rep. 2015, 5, srep08587. [Google Scholar] [CrossRef] [PubMed]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.-D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from physical plasmas: Redox chemistry for biomedical therapy. Oxidative Med. Cell. Longev. 2019, 2019, 1–29. [Google Scholar] [CrossRef]
- Gorbanev, Y.; O’Connell, D.; Chechik, V. Non-thermal plasma in contact with water: The origin of species. Chemistry 2016, 22, 3496–3505. [Google Scholar] [CrossRef]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.-D. Plasma Medicine: A field of applied redox biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef]
- Ji, W.-O.; Lee, M.-H.; Kim, G.-H.; Kim, E.-H. Quantitation of the ROS production in plasma and radiation treatments of biotargets. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Xu, D.; Luo, X.; Xu, Y.; Cui, Q.; Yang, Y.; Liu, D.; Chen, H.; Kong, M.G. The effects of cold atmospheric plasma on cell adhesion, differentiation, migration, apoptosis and drug sensitivity of multiple myeloma. Biochem. Biophys. Res. Commun. 2016, 473, 1125–1132. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F. Variable susceptibility of ovarian cancer cells to non-thermal plasma-activated medium. Oncol. Rep. 2016, 35, 3169–3177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kwon, B.-S.; Choi, E.H.; Chang, B.; Choi, J.-H.; Kim, K.S.; Park, H.-K. Selective cytotoxic effect of non-thermal micro-DBD plasma. Phys. Biol. 2016, 13, 056001. [Google Scholar] [CrossRef]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Stepp, M.A.; Srinivasan, P.; Sandler, A.; Trink, B. Cold atmospheric plasma in cancer therapy. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Aryal, S.; Bisht, G. New paradigm for a targeted cancer therapeutic approach: A short review on potential synergy of gold nanoparticles and cold atmospheric plasma. Biomedicine 2017, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Shimizu, T.; Li, Y.-F.; Stolz, W.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L. Cold atmospheric plasma devices for medical issues. Exp. Rev. Med. Dev. 2013, 10, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.M.; Lee, B.K.; Yap, S.S.; Thong, K.L. Cold plasma inactivation of chronic wound bacteria. Arch. Biochem. Biophys. 2016, 605, 76–85. [Google Scholar] [CrossRef]
- Nguyen, L.; Lu, P.; Boehm, D.; Bourke, P.; Gilmore, B.F.; Hickok, N.J.; Freeman, T.A. Cold atmospheric plasma is a viable solution for treating orthopedic infection: A review. Biol. Chem. 2018, 400, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical applications of cold atmospheric plasma: State of the science. J. Wound Care 2018, 27 (Suppl. S9), S4–S10. [Google Scholar] [CrossRef]
- Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Czaika, V.A.; Richter, H.; Bob, A.; Von Hutten, J.; Painsi, C.; Hüge, R.; et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care 2015, 24, 196–203. [Google Scholar] [CrossRef]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of production and application in dentistry and oncology. Med. Gas Res. 2013, 3, 21. [Google Scholar] [CrossRef]
- Boeckmann, L.; Bernhardt, T.; Schäfer, M.; Semmler, M.L.; Kordt, M.; Waldner, A.; Wendt, F.; Sagwal, S.; Bekeschus, S.; Berner, J.; et al. Aktuelle indikationen der plasmatherapie in der dermatologie. Hautarzt 2020, 71, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma medicine: Applications of cold atmospheric pressure plasma in dermatology. Oxidative Med. Cell. Longev. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Gareri, C.; Bennardo, L.; De Masi, G. Use of a new cold plasma tool for psoriasis treatment: A case report. SAGE Open Med. Case Rep. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Ikehara, S.; Sakakita, H.; Ikehara, Y. Low temperature plasma equipment applied on surgical hemostasis and wound healings. J. Clin. Biochem. Nutr. 2017, 60, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Filis, K.; Galyfos, G.; Sigala, F.; Zografos, G. Utilization of low-temperature helium plasma (J-Plasma) for dissection and hemostasis during carotid endarterectomy. J. Vasc. Surg. Cases Innov. Tech. 2020, 6, 152–155. [Google Scholar] [CrossRef]
- Rosani, U.; Tarricone, E.; Venier, P.; Brun, P.; Deligianni, V.; Zuin, M.; Martines, E.; Leonardi, A. Atmospheric-pressure cold plasma induces transcriptional changes in ex vivo human corneas. PLoS ONE 2015, 10, e0133173. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.-P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef]
- Setsuhara, Y. Low-temperature atmospheric-pressure plasma sources for plasma medicine. Arch. Biochem. Biophys. 2016, 605, 3–10. [Google Scholar] [CrossRef]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A. Acidification is an essential process of cold atmospheric plasma and promotes the anti-cancer effect on malignant melanoma cells. Cancers 2019, 11, 671. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Yang, C.; Gao, J.; Zhao, Y.; Cheng, C.; Zhao, G.; Liu, S. The inhibition effect of cold atmospheric plasma-activated media in cutaneous squamous carcinoma cells. Futur. Oncol. 2019, 15, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.-P.; Heidecke, C.-D.; Von Bernstorff, W.; Von Woedtke, T.; Partecke, L.I. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gweon, B.; Kim, M.; Kim, D.B.; Kim, D.; Kim, H.; Jung, H.; Shin, J.H.; Choe, W. Differential responses of human liver cancer and normal cells to atmospheric pressure plasma. Appl. Phys. Lett. 2011, 99, 63701. [Google Scholar] [CrossRef]
- Torii, K.; Yamada, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Tanahashi, K.; Iwata, N.; Kanda, M.; Kobayashi, D.; Tanaka, C.; et al. Effectiveness of plasma treatment on gastric cancer cells. Gastric Cancer 2014, 18, 635–643. [Google Scholar] [CrossRef]
- Schneider, C.; Arndt, S.; Zimmermann, J.L.; Li, Y.; Karrer, S.; Bosserhoff, A.-K. Cold atmospheric plasma treatment inhibits growth in colorectal cancer cells. Biol. Chem. 2018, 400, 111–122. [Google Scholar] [CrossRef]
- Weiss, M.; Gümbel, D.; Gelbrich, N.; Brandenburg, L.-O.; Mandelkow, R.; Zimmermann, U.; Ziegler, P.; Burchardt, M.; Stope, M.B. Inhibition of cell growth of the prostate cancer cell model LNCaP by cold atmospheric plasma. In Vivo 2015, 29, 611. [Google Scholar]
- Gelbrich, N.; Stope, M.B.; Burchardt, M. Kaltes atmosphärisches Plasma für die urologische Tumortherapie. Urol. A 2018, 58, 673–679. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Bae, H.; Choi, E.H.; Kim, S.J. Epigenetic silencing of miR-19a-3p by cold atmospheric plasma contributes to proliferation inhibition of the MCF-7 breast cancer cell. Sci. Rep. 2016, 6, 30005. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.; Jeong, D.; Ham, J.; Park, S.; Choi, E.H.; Kim, S.J. Cold atmospheric plasma restores tamoxifen sensitivity in resistant MCF-7 breast cancer cell. Free Radic. Biol. Med. 2017, 110, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Lee, S.-J.; Castro, N.J.; Yan, D.; Keidar, M.; Zhang, L.G. Synergistic effect of cold atmospheric plasma and drug loaded core-shell nanoparticles on inhibiting breast cancer cell growth. Sci. Rep. 2016, 6, 21974. [Google Scholar] [CrossRef] [PubMed]
- Mirpour, S.; Ghomi, H.; Piroozmand, S.; Nikkhah, M.; Tavassoli, S.H.; Azad, S.Z. The selective characterization of nonthermal atmospheric pressure plasma jet on treatment of human breast cancer and normal cells. IEEE Trans. Plasma Sci. 2014, 42, 315–322. [Google Scholar] [CrossRef]
- Wang, M.; Holmes, B.; Cheng, X.; Zhu, W.; Keidar, M.; Zhang, L.G. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS ONE 2013, 8, e73741. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Chung, T.; Bae, S.H.; Leem, S.H. Induction of apoptosis in human breast cancer cells by a pulsed atmospheric pressure plasma jet. Appl. Phys. Lett. 2010, 97, 23702. [Google Scholar] [CrossRef]
- Ninomiya, K.; Ishijima, T.; Imamura, M.; Yamahara, T.; Enomoto, H.; Takahashi, K.; Tanaka, Y.; Uesugi, Y.; Shimizu, N. Evaluation of extra- and intracellular OH radical generation, cancer cell injury, and apoptosis induced by a non-thermal atmospheric-pressure plasma jet. J. Phys. D Appl. Phys. 2013, 46. [Google Scholar] [CrossRef]
- Kang, S.U.; Cho, J.-H.; Chang, J.W.; Shin, Y.S.; Kim, K.I.; Park, J.K.; Yang, S.S.; Lee, J.-S.; Moon, E.; Lee, K.; et al. Nonthermal plasma induces head and neck cancer cell death: The potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014, 5, e1056. [Google Scholar] [CrossRef] [PubMed]
- Gümbel, D.; Bekeschus, S.; Gelbrich, N.; Napp, M.; Ekkernkamp, A.; Kramer, A.; Stope, M.B. Cold atmospheric plasma in the treatment of osteosarcoma. Int. J. Mol. Sci. 2017, 18, 2004. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Tornín, J.; Brulin, B.; Khlyustova, A.; Ginebra, M.-P.; Layrolle, P.; Canal, C. Cold plasma-treated Ringer’s saline: A weapon to target osteosarcoma. Cancers 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, K.; Scally, L.; Manaloto, E.; Gunes, S.; Ng, S.W.; Maher, M.; Tiwari, B.; Byrne, H.J.; Bourke, P.; et al. Cold atmospheric plasma stimulates clathrin-dependent endocytosis to repair oxidised membrane and enhance uptake of nanomaterial in glioblastoma multiforme cells. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Wolff, C.M.; Kolb, J.F.; Weltmann, K.-D.; Von Woedtke, T.; Bekeschus, S. Combination treatment with cold physical plasma and pulsed electric fields augments ROS production and cytotoxicity in lymphoma. Cancers 2020, 12, 845. [Google Scholar] [CrossRef]
- Xu, D.; Ning, N.; Xu, Y.; Wang, B.; Cui, Q.; Liu, Z.; Wang, X.; Liu, D.; Chen, H.; Kong, M.G. Effect of cold atmospheric plasma treatment on the metabolites of human leukemia cells. Cancer Cell Int. 2019, 19, 135. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Y.; Cui, Q.; Liu, D.; Liu, Z.; Wang, X.; Yang, Y.; Feng, M.; Liang, R.; Chen, H.; et al. Cold atmospheric plasma as a potential tool for multiple myeloma treatment. Oncotarget 2018, 9, 18002–18017. [Google Scholar] [CrossRef]
- Chang, C.-H.; Yano, K.-I.; Sato, T. Nanosecond pulsed current under plasma-producing conditions induces morphological alterations and stress fiber formation in human fibrosarcoma HT-1080 cells. Arch. Biochem. Biophys. 2020, 681, 108252. [Google Scholar] [CrossRef] [PubMed]
- Golubitskaya, E.A.; Troitskaya, O.S.; Yelak, E.V.; Gugin, P.P.; Richter, V.A.; Schweigert, I.V.; Zakrevsky, D.E.; Koval, O.A. Cold physical plasma decreases the viability of lung adenocarcinoma cells. Acta Nat. 2019, 11, 16–19. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; Van Der Linde, J.; Metelmann, H.-R.; Heidecke, C.-D.; Partecke, L.-I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef]
- Mirpour, S.; Piroozmand, S.; Soleimani, N.; Faharani, N.J.; Ghomi, H.; Eskandari, H.F.; Sharifi, A.M.; Mirpour, S.; Eftekhari, M.; Nikkhah, M. Utilizing the micron sized non-thermal atmospheric pressure plasma inside the animal body for the tumor treatment application. Sci. Rep. 2016, 6, 29048. [Google Scholar] [CrossRef]
- Hirst, A.M.; Simms, M.S.; Mann, V.M.; Maitland, N.J.; Oconnell, D.; Frame, F.M. Low-temperature plasma treatment induces DNA damage leading to necrotic cell death in primary prostate epithelial cells. Br. J. Cancer 2015, 112, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, J.; Judée, F.; Vallette, M.; Decauchy, H.; Arbelaiz, A.; Aoudjehane, L.; Scatton, O.; Gonzalez-Sanchez, E.; Merabtene, F.; Augustin, J.; et al. Cold-atmospheric plasma induces tumor cell death in preclinical in vivo and in vitro models of human cholangiocarcinoma. Cancers 2020, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.J.; Suh, M.J.; Lee, H.Y.; Lee, H.J.; Choi, E.H.; Moon, I.S.; Song, K. Anti-tumor effects of cold atmospheric pressure plasma on vestibular schwannoma demonstrate its feasibility as an intra-operative adjuvant treatment. Free Radic. Biol. Med. 2018, 115, 43–56. [Google Scholar] [CrossRef]
- Chen, Z.; Simonyan, H.; Cheng, X.; Gjika, E.; Lin, L.; Canady, J.; Sherman, J.H.; Young, C.; Keidar, M. A novel micro cold atmospheric plasma device for glioblastoma both in vitro and in vivo. Cancers 2017, 9, 61. [Google Scholar] [CrossRef]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.-A.; Abdollahifar, M.-A.; Khoramgah, M.S.; Shokri, B. Comparison of Direct and Indirect cold atmospheric-pressure plasma methods in the B16F10 melanoma cancer cells treatment. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kang, M.H.; Uhm, H.S.; Lee, G.J.; Choi, E.H.; Han, I. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma activated nitric oxide water on cervical cancer cells. Sci. Rep. 2017, 7, srep45781. [Google Scholar] [CrossRef] [PubMed]
- Lingzhi, B.; Yu, K.N.; Bao, L.; Shen, J.; Cheng, C.; Han, W. Non-thermal plasma inhibits human cervical cancer HeLa cells invasiveness by suppressing the MAPK pathway and decreasing matrix metalloproteinase-9 expression. Sci. Rep. 2016, 6, 19720. [Google Scholar] [CrossRef]
- Feil, L.; Koch, A.; Utz, R.; Ackermann, M.; Barz, J.; Stope, M.B.; Krämer, B.; Wallwiener, D.; Brucker, S.Y.; Weiss, M. Cancer-selective treatment of cancerous and non-cancerous human cervical cell models by a non-thermally operated electrosurgical argon plasma device. Cancers 2020, 12, 1037. [Google Scholar] [CrossRef]
- Ryan, H.A.; Neuber, J.; Song, S.; Beebe, S.J.; Jiang, C. Effects of a non-thermal plasma needle device on HPV-16 positive cervical cancer cell viability in vitro. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016, 2016, 537–540. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.-S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef]
- Tan, X.; Zhao, S.; Lei, Q.; Lu, X.; He, G.; Ostrikov, K. (Ken) single-cell-precision microplasma-induced cancer cell apoptosis. PLoS ONE 2014, 9, e101299. [Google Scholar] [CrossRef]
- Kim, K.; Ahn, H.J.; Lee, J.-H.; Kim, J.-H.; Yang, S.S.; Lee, J.-S. Cellular membrane collapse by atmospheric-pressure plasma jet. Appl. Phys. Lett. 2014, 104, 13701. [Google Scholar] [CrossRef]
- Wenzel, T.; Berrio, D.A.C.; Reisenauer, C.; Layland, S.; Koch, A.; Wallwiener, D.; Brucker, S.Y.; Schenke-Layland, K.; Brauchle, E.-M.; Weiss, M. Trans-mucosal efficacy of non-thermal plasma treatment on cervical cancer tissue and human cervix uteri by a next generation electrosurgical argon plasma device. Cancers 2020, 12, 267. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Liu, W.; Nakamura, K.; Yoshida, K.; Ikeda, Y.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F.; et al. Plasma-activated medium promotes autophagic cell death along with alteration of the mTOR pathway. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Ikeda, J.-I.; Tsuruta, Y.; Nojima, S.; Sakakita, H.; Hori, M.; Ikehara, Y. Anti-cancer effects of nonequilibrium atmospheric pressure plasma on cancer-initiating cells in human endometrioid adenocarcinoma cells. Plasma Process. Polym. 2015, 12, 1370–1376. [Google Scholar] [CrossRef]
- Ikeda, J.-I. Effect of Nonequilibrium Atmospheric Pressure Plasma on Cancer-Initiating Cells. Plasma Med. 2014, 4, 49–56. [Google Scholar] [CrossRef]
- Koensgen, D.; Besic, I.; Gümbel, D.; Kaul, A.; Weiss, M.; Diesing, K.; Kramer, A.; Bekeschus, S.; Mustea, A.; Stope, M.B. Cold atmospheric plasma (CAP) and CAP-stimulated cell culture media suppress ovarian cancer cell growth–a putative treatment option in ovarian cancer therapy. Anticancer Res. 2017, 37. [Google Scholar] [CrossRef]
- Bisag, A.; Bucci, C.; Coluccelli, S.; Girolimetti, G.; Laurita, R.; De Iaco, P.; Perrone, A.M.; Gherardi, M.; Marchio, L.; Porcelli, A.M.; et al. Plasma-activated Ringer’s lactate solution displays a selective cytotoxic effect on ovarian cancer cells. Cancers 2020, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Hori, M.; Kikkawa, F. Selective cytotoxicity of indirect nonequilibrium atmospheric pressure plasma against ovarian clear-cell carcinoma. SpringerPlus 2014, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kajiyama, H.; Utsumi, F.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F. Possible therapeutic option of aqueous plasma for refractory ovarian cancer. Clin. Plasma Med. 2016, 4, 14–18. [Google Scholar] [CrossRef]
- Iseki, S.; Nakamura, K.; Hayashi, M.; Tanaka, H.; Kondo, H.; Kajiyama, H.; Kano, H.; Kikkawa, F.; Hori, M. Selective killing of ovarian cancer cells through induction of apoptosis by nonequilibrium atmospheric pressure plasma. Appl. Phys. Lett. 2012, 100, 113702. [Google Scholar] [CrossRef]
- Mullen, M.M.; Merfeld, E.C.; Palisoul, M.L.; Massad, L.S.; WoolFolk, C.; Powell, M.A.; Mutch, D.G.; Thaker, P.H.; Hagemann, A.R.; Kuroki, L.M. Wound complication rates after vulvar excisions for premalignant lesions. Obstet. Gynecol. 2019, 133, 658–665. [Google Scholar] [CrossRef]
- Leminen, A.; Forss, M.; Paavonen, J. Wound complications in patients with carcinoma of the vulva. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 93, 193–197. [Google Scholar] [CrossRef]
- Weinberg, D.; Gomez-Martinez, R.A. Vulvar cancer. Obstet. Gynecol. Clin. N. Am. 2019, 46, 125–135. [Google Scholar] [CrossRef]
- Crum, C.P.; Herrington, C.S.; McCluggage, W.G.; Regauer, S.; Wilkinson, E.J. Epithelial tumours of vulva. In WHO Classification of Tumours of Female Reproductive Organs; Kurman, R.J., Carcangiu, M.L., Herrington, C.S., Young, R.H., Eds.; IARC: Lyon, France, 2014; pp. 232–241. ISBN 978-92-832-2435-8. [Google Scholar]
- Boer, F.L.; Eikelder, M.L.T.; Kapiteijn, E.H.; Creutzberg, C.L.; Galaal, K.; Van Poelgeest, M.I. Vulvar malignant melanoma: Pathogenesis, clinical behaviour and management: Review of the literature. Cancer Treat. Rev. 2019, 73, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Moxley, K.M.; Fader, A.N.; Rose, P.G.; Case, A.S.; Mutch, D.G.; Berry, E.; Schink, J.C.; Kim, C.; Chi, D.S.; Moore, K.N. Malignant melanoma of the vulva: An extension of cutaneous melanoma? Gynecol. Oncol. 2011, 122, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Kilts, T.P.; Long, B.; Glasgow, A.E.; Bakkum-Gamez, J.N.; Habermann, E.B.; Cliby, W.A. Invasive vulvar extramammary Paget’s disease in the United States. Gynecol. Oncol. 2020, 157, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Van Der Linden, M.; Meeuwis, K.; Bulten, J.; Bosse, T.; Van Poelgeest, M.; De Hullu, J. Paget disease of the vulva. Crit. Rev. Oncol. 2016, 101, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, R.M.; McDonnell, K.A.; Chebbi, A.; Callanan, J.J.; Dowling, D.P. Evaluation of the effectiveness of kINPen Med plasma jet and bioactive agent therapy in a rat model of wound healing. Biointerphases 2018, 13, 051002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cai, D.; Xiao, S.; Ning, M.; Zhou, R.; Zhang, S.; Chen, X.; Ostrikov, K.; Dai, X. InvivoPen: A novel plasma source for in vivo cancer treatment. J. Cancer 2020, 11, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, Z.; Wen, D.; Wang, Z.; Li, H.; Zeng, Y.; Dotti, G.; Wirz, R.E.; Gu, Z. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 3687–3692. [Google Scholar] [CrossRef]
- Almeida, N.D.; Klein, A.L.; Hogan, E.; Terhaar, S.J.; Kedda, J.; Uppal, P.; Sack, K.; Keidar, M.; Sherman, J.H.; Kedda, N. Cold atmospheric plasma as an adjunct to immunotherapy for glioblastoma multiforme. World Neurosurg. 2019, 130, 369–376. [Google Scholar] [CrossRef]
- Li, W.; Yu, H.; Ding, D.; Chen, Z.; Wang, Y.; Wang, S.; Li, X.; Keidar, M.; Zhang, W. Cold atmospheric plasma and iron oxide-based magnetic nanoparticles for synergetic lung cancer therapy. Free Radic. Biol. Med. 2019, 130, 71–81. [Google Scholar] [CrossRef]
- American Cancer Society: Key Statistics for Vulvar Cancer. Available online: https://www.cancer.org/cancer/vulvar-cancer/about/key-statistics.html (accessed on 20 October 2020).
- Siegel, R.L.; Mph, K.D.M.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Meltzer-Gunnes, C.J.; Småstuen, M.C.; Kristensen, G.B.; Trope, C.G.; Lie, A.K.; Vistad, I. Vulvar carcinoma in Norway: A 50-year perspective on trends in incidence, treatment and survival. Gynecol. Oncol. 2017, 145, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Shah, J.P.; Malone, J.M. Vulvar cancer in women less than fifty in United States, 1980–2005. Gynecol. Oncol. 2008, 112, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, M.S.; Einden, L.V.D.; Massuger, L.; Kiemeney, L.; Van Der Aa, M.; De Hullu, J. Trends in incidence and survival of Dutch women with vulvar squamous cell carcinoma. Eur. J. Cancer 2013, 49, 3872–3880. [Google Scholar] [CrossRef]
- Pleunis, N.; Schuurman, M.; Van Rossum, M.; Bulten, J.; Massuger, L.; De Hullu, J.; Van Der Aa, M. Rare vulvar malignancies; incidence, treatment and survival in the Netherlands. Gynecol. Oncol. 2016, 142, 440–445. [Google Scholar] [CrossRef]
- Dasgupta, S.; Ewing-Graham, P.C.; Swagemakers, S.M.; Van Der Spek, P.; Van Doorn, H.C.; Hegt, V.N.; Koljenović, S.; Van Kemenade, F.J. Precursor lesions of vulvar squamous cell carcinoma–histology and biomarkers: A systematic review. Crit. Rev. Oncol. 2020, 147, 102866. [Google Scholar] [CrossRef]
- Dittmer, C.; Katalinic, A.; Mundhenke, C.; Thill, M.; Fischer, D. Epidemiology of vulvar and vaginal cancer in Germany. Arch. Gynecol. Obstet. 2011, 284, 169–174. [Google Scholar] [CrossRef]
- Bray, F.; Me, J.F.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Eva, L.J.; Sadler, L.; Fong, K.L.; Sahota, S.; Jones, R.W.; Bigby, S.M. Trends in HPV-dependent and HPV-independent vulvar cancers: The changing face of vulvar squamous cell carcinoma. Gynecol. Oncol. 2020, 157, 450–455. [Google Scholar] [CrossRef]
- Buttmann-Schweiger, N.; Klug, S.J.; Luyten, A.; Holleczek, B.; Heitz, F.; Du Bois, A.; Kraywinkel, K. Incidence Patterns and Temporal Trends of Invasive Nonmelanotic Vulvar Tumors in Germany 1999–2011. A Population-Based Cancer Registry Analysis. PLoS ONE 2015, 10, e0128073. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Zhang, Z. Prevalence of human papillomavirus and its prognostic value in vulvar cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0204162. [Google Scholar] [CrossRef]
- Holleczek, B.; Sehouli, J.; Barinoff, J. Vulvar cancer in Germany: Increase in incidence and change in tumour biological characteristics from 1974 to 2013. Acta Oncol. 2017, 57, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Forman, D.; De Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30, F12–F23. [Google Scholar] [CrossRef]
- Reinholdt, K.; Thomsen, L.T.; Dehlendorff, C.; Larsen, H.K.; Sørensen, S.S.; Haedersdal, M.; Kjær, S.K. Human papillomavirus-related anogenital premalignancies and cancer in renal transplant recipients: A Danish nationwide, registry-based cohort study. Int. J. Cancer 2019, 146, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Bucchi, L.; Baldacchini, F.; Giuliani, O.; Ravaioli, A.; Vattiato, R.; Preti, M.; Tumino, R.; Ferretti, S.; Biggeri, A.; et al. Incidence trends of vulvar squamous cell carcinoma in Italy from 1990 to 2015. Gynecol. Oncol. 2020, 157, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Suneja, G.; Viswanathan, A.N. Gynecologic malignancies. Hematol. Clin. N. Am. 2020, 34, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Forte, S.; Ardighieri, L.; Bonetti, E.; Fernando, B.; Sartori, E.; Odicino, F. Multivariate analysis of prognostic factors in primary squamous cell vulvar cancer: The role of perineural invasion in recurrence and survival. Eur. J. Surg. Oncol. 2019, 45, 2115–2119. [Google Scholar] [CrossRef] [PubMed]
- Beller, U.; Quinn, M.; Benedet, J.; Creasman, W.; Ngan, H.Y.S.; Maisonneuve, P.; Pecorelli, S.; Odicino, F.; Heintz, A. Carcinoma of the Vulva. Int. J. Gynecol. Obstet. 2006, 95 (Suppl. S1), S7–S27. [Google Scholar] [CrossRef]
- Mantovani, G.; Fragomeni, S.M.; Inzani, F.; Fagotti, A.; Della Corte, L.; Gentileschi, S.; Tagliaferri, L.; Zannoni, G.F.; Scambia, G.; Garganese, G. Molecular pathways in vulvar squamous cell carcinoma: Implications for target therapeutic strategies. J. Cancer Res. Clin. Oncol. 2020, 146, 1647–1658. [Google Scholar] [CrossRef]
- Allbritton, J.I. Vulvar Neoplasms, Benign and Malignant. Obstet. Gynecol. Clin. N. Am. 2017, 44, 339–352. [Google Scholar] [CrossRef]
- Joura, E.A.; Lösch, A.; Haider-Angeler, M.G.; Breitenecker, G.; Leodolter, S. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. J. Reprod. Med. 2000, 45, 613–615. [Google Scholar]
- Judson, P.L.; Habermann, E.B.; Baxter, N.N.; Durham, S.B.; Virnig, B.A. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstet. Gynecol. 2006, 107, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Ridley, C.M.; Frankman, O.; Jones, I.S.; Pincus, S.H.; Wilkinson, E.J.; Fox, P.H.; Friedrich, E.G.; Kaufman, R.H.; Lynch, P.J. New nomenclature for vulvar disease: International society for the study of vulvar disease. Hum. Pathol. 1989, 20, 495–496. [Google Scholar] [CrossRef]
- Bornstein, J.; Bogliatto, F.; Haefner, H.K.; Stockdale, C.K.; Preti, M.; Bohl, T.G.; Reutter, J. The 2015 International society for the study of vulvovaginal disease (ISSVD) terminology of vulvar squamous intraepithelial lesions. Obstet. Gynecol. 2016, 127, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Ghatage, P. Etiology, Clinical features, and diagnosis of vulvar lichen sclerosus: A scoping review. Obstet. Gynecol. Int. 2020, 2020, 7480754. [Google Scholar] [CrossRef] [PubMed]
- Harmon, M.L. Premalignant and malignant squamous lesions of the vulva. Diagn. Histopathol. 2017, 23, 19–27. [Google Scholar] [CrossRef]
- Bleeker, M.C.G.; Visser, P.J.; Overbeek, L.I.; Van Beurden, M.; Berkhof, J. Lichen Sclerosus: Incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1224–1230. [Google Scholar] [CrossRef]
- Halonen, P.M.; Jakobsson, M.I.; Heikinheimo, O.; Riska, A.E.; Gissler, M.; Pukkala, E.I. Lichen sclerosus and risk of cancer. Int. J. Cancer 2017, 140, 1998–2002. [Google Scholar] [CrossRef] [PubMed]
- Pouwer, A.-F.W.; Einden, L.C.V.D.; Van Der Linden, M.; Hehir-Kwa, J.Y.; Yu, J.; Hendriks, K.M.; Kamping, E.J.; Eijkelenboom, A.; Massuger, L.F.; Bulten, J.; et al. Clonal Relationship Between Lichen Sclerosus, Differentiated Vulvar Intra-epithelial Neoplasia and Non HPV-related Vulvar Squamous Cell Carcinoma. Cancer Genom. Proteom. 2020, 17, 151–160. [Google Scholar] [CrossRef]
- Del Pino, M.; Rodriguez-Carunchio, L.; Ordi, J. Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology 2012, 62, 161–175. [Google Scholar] [CrossRef]
- Gensthaler, L.; Joura, E.; Alemany, L.; Horvat, R.; De Sanjosé, S.; Pils, S. The impact of p16ink4a positivity in invasive vulvar cancer on disease-free and disease-specific survival, a retrospective study. Arch. Gynecol. Obstet. 2020, 301, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Proctor, L.; Hoang, L.; Moore, J.; Thompson, E.; Leung, S.; Natesan, D.; Chino, J.; Gilks, B.; McAlpine, J.N. Association of human papilloma virus status and response to radiotherapy in vulvar squamous cell carcinoma. Int. J. Gynecol. Cancer 2020, 30, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.N.; Park, K.J.; Soslow, R.A.; Murali, R. Squamous precursor lesions of the vulva: Current classification and diagnostic challenges. Pathology 2016, 48, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Figueiredo, A.; Paula, T.; Borrego, J. Vulvar intraepithelial neoplasia. J. Low. Genit. Tract Dis. 2012, 16, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Tuncer, Z.S.; Doğan, L.; Yüce, K.; Küçükali, T. Skinning vulvectomy for the treatment of vulvar intraepithelial neoplasia 2-3: A study of 21 cases. Eur. J. Gynaecol. Oncol. 1998, 19, 508–510. [Google Scholar]
- Van Seters, M.; Van Beurden, M.; De Craen, A.J.M. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol. Oncol. 2005, 97, 645–651. [Google Scholar] [CrossRef]
- Penna, C.; Fallani, M.G.; Fambrini, M.; Zipoli, E.; Marchionni, M. CO2 laser surgery for vulvar intraepithelial neoplasia. Excisional, destructive and combined techniques. J. Reprod. Med. 2002, 47, 915–918. [Google Scholar]
- De Witte, C.J.; Van De Sande, A.J.M.; Van Beekhuizen, H.J.; Koeneman, M.M.; Kruse, A.; Gerestein, C.G. Imiquimod in cervical, vaginal and vulvar intraepithelial neoplasia: A review. Gynecol. Oncol. 2015, 139, 377–384. [Google Scholar] [CrossRef]
- Van Der Zee, A.G.J.; Oonk, M.H.; De Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; Van Dorst, E.B.; et al. sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J. Clin. Oncol. 2008, 26, 884–889. [Google Scholar] [CrossRef]
- Oonkm, M.H.; van Hemel, B.M.; Hollema, H.; de Hullu, J.A.; Ansink, A.C.; Vergote, I.; Verheijen, R.H.; Maggioni, A.; Gaarenstroom, K.N.; Baldwin, P.J.; et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: Results from GROINSS-V, a multicentre observational study. Lancet Oncol. 2010, 11, 646–652. [Google Scholar] [CrossRef]
- Woelber, L.; Jaeger, A.; Prieske, K. New treatment standards for vulvar cancer 2020. Curr. Opin. Obstet. Gynecol. 2020, 32, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Chiantera, V.; Rossi, M.; De Iaco, P.; Koehler, C.; Marnitz, S.; Fagotti, A.; Fanfani, F.; Parazzini, F.; Schiavina, R.; Scambia, G.; et al. Morbidity after pelvic exenteration for gynecological malignancies: A retrospective multicentric study of 230 patients. Int. J. Gynecol. Cancer 2014, 24, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Huisman, B.; Burggraaf, J.; Vahrmeijer, A.L.; Schoones, J.; Rissmann, R.; Sier, C.F.M.; Van Poelgeest, M. Potential targets for tumor-specific imaging of vulvar squamous cell carcinoma: A systematic review of candidate biomarkers. Gynecol. Oncol. 2020, 156, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Bidzinski, M.; Brännström, M.; Landoni, F.; Mahner, S.; Mahantshetty, U.; Mirza, M.; Petersen, C.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients With Vulvar Cancer. Int. J. Gynecol. Cancer 2017, 27, 832–837. [Google Scholar] [CrossRef]
- De Hullu, J.A.; Van Der Zee, A. Surgery and radiotherapy in vulvar cancer. Crit. Rev. Oncol. Hematol. 2006, 60, 38–58. [Google Scholar] [CrossRef]
- Nooij, L.; Brand, F.; Gaarenstroom, K.; Creutzberg, C.L.; De Hullu, J.; Van Poelgeest, M. Risk factors and treatment for recurrent vulvar squamous cell carcinoma. Crit. Rev. Oncol. Hematol. 2016, 106, 1–13. [Google Scholar] [CrossRef]
- Pirot, F.; Chaltiel, D.; Ouldamer, L.; Touboul, C.; Raimond, E.; Carcopino, X.; Daraï, E.; Bendifallah, S. Patterns of first recurrence and outcomes in surgically treated women with vulvar cancer: Results from FRANCOGYN study group. J. Gynecol. Obstet. Hum. Reprod. 2020, 101775, 101775. [Google Scholar] [CrossRef]
- Salani, R.; Khanna, N.; Frimer, M.; Bristow, R.E.; Chen, L.-M. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol. Oncol. 2017, 146, 3–10. [Google Scholar] [CrossRef]
- Grootenhuis, N.C.T.; Van Der Zee, A.G.; Van Doorn, H.C.; Van Der Velden, J.; Vergote, I.; Zanagnolo, V.; Baldwin, P.J.; Gaarenstroom, K.N.; Van Dorst, E.B.; Trum, J.W.; et al. Sentinel nodes in vulvar cancer: Long-term follow-up of the groningen international study on sentinel nodes in vulvar cancer (GROINSS-V) I. Gynecol. Oncol. 2016, 140, 8–14. [Google Scholar] [CrossRef]
- Moore, D.H.; Ali, S.; Koh, W.-J.; Michael, H.; Barnes, M.N.; McCourt, C.K.; Homesley, H.D.; Walker, J.L. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: A gynecologic oncology group study. Gynecol. Oncol. 2012, 124, 529–533. [Google Scholar] [CrossRef]
- Gill, B.S.; Bernard, M.E.; Lin, J.F.; Balasubramani, G.K.; Rajagopalan, M.S.; Sukumvanich, P.; Krivak, T.C.; Olawaiye, A.B.; Kelley, J.L.; Beriwal, S. Impact of adjuvant chemotherapy with radiation for node-positive vulvar cancer: A national cancer data base (NCDB) analysis. Gynecol. Oncol. 2015, 137, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Logar, H.B.Z. Long term results of radiotherapy in vulvar cancer patients in Slovenia between 1997–2004. Radiol. Oncol. 2017, 51, 447–454. [Google Scholar] [CrossRef]
- Lupi, G.; Raspagliesi, F.; Zucali, R.; Fontanelli, R.; Paladini, D.; Kenda, R.; di Re, F. Combined preoperative chemoradiotherapy followed by radical surgery in locally advanced vulvar carcinoma. A pilot study. Cancer 1996, 77, 1472–1478. [Google Scholar] [CrossRef]
- Mullen, M.M.; Cripe, J.C.; Thaker, P.H. Palliative care in gynecologic oncology. Obstet. Gynecol. Clin. N. Am. 2019, 46, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Grootenhuis, N.T.; Pouwer, A.; De Bock, G.; Hollema, H.; Bulten, J.; Van Der Zee, A.; De Hullu, J.; Oonk, M.H.M. Margin status revisited in vulvar squamous cell carcinoma. Gynecol. Oncol. 2019, 154, 266–275. [Google Scholar] [CrossRef]
- Grootenhuis, N.C.T.; Pouwer, A.-F.W.; De Bock, G.H.; Hollema, H.; Bulten, J.; Van Der Zee, A.G.J.; De Hullu, J.A.; Oonk, M.H.M. Prognostic factors for local recurrence of squamous cell carcinoma of the vulva: A systematic review. Gynecol. Oncol. 2018, 148, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Woelber, L.; Griebel, L.-F.; Eulenburg, C.; Sehouli, J.; Jueckstock, J.; Hilpert, F.; De Gregorio, N.; Hasenburg, A.; Ignatov, A.; Hillemanns, P.; et al. Role of tumour-free margin distance for loco-regional control in vulvar cancer—A subset analysis of the Arbeitsgemeinschaft Gynäkologische Onkologie CaRE-1 multicenter study. Eur. J. Cancer 2016, 69, 180–188. [Google Scholar] [CrossRef]
- Nguyen-Xuan, H.-T.; Macias, R.M.; Bonsang-Kitzis, H.; Deloménie, M.; Ngô, C.; Koual, M.; Bats, A.-S.; Hivelin, M.; Lécuru, F.; Balaya, V. Use of fluorescence to guide surgical resection in vulvo-vaginal neoplasia: Two case reports. J. Gynecol. Obstet. Hum. Reprod. 2020, 101768, 101768. [Google Scholar] [CrossRef]
- Xia, J.; Zeng, W.; Liu, X.-M.; Wang, B.; Xu, D.; Liu, D.; Kong, M.G.; Dong, Y. Cold atmospheric plasma induces apoptosis of melanoma cells via Sestrin2-mediated nitric oxide synthase signaling. J. Biophotonics 2018, 12, e201800046. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Nedrelow, D.S.; Seebauer, C.; Schuster, M.; Von Woedtke, T.; Weltmann, K.-D.; Kindler, S.; Metelmann, P.H.; Finkelstein, S.E.; Von Hoff, D.D.; et al. Head and neck cancer treatment and physical plasma. Clin. Plasma Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; Von Woedtke, T.; Hasse, S.; Weltmann, K.-D.; et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Craniomaxillofac. Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef]
- Canady, J. Clinical Application of Cold Atmospheric Plasma (CAP) and Hybrid Plasma for the Treatment of Stage IV Gastrointestinal Cancers: Update. IWPCT-2017, Oral Lecture, Session 6. 2017. Available online: https://iwpct2017.sciencesconf.org/resource/page/id/13 (accessed on 20 October 2020).
- Partecke, L.I.; Evert, K.; Haugk, J.; Doering, F.; Normann, L.; Diedrich, S.; Weiss, F.U.; Evert, M.; Huebner, N.-O.; Guenther, C.; et al. Tissue tolerable plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer 2012, 12, 473. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A.A. Deep penetration into tissues of reactive oxygen species generated in floating-electrode dielectric barrier discharge (FE-DBD): An in vitro agarose gel model mimicking an open wound. Plasma Med. 2012, 2, 71–83. [Google Scholar] [CrossRef]
- Nakamura, K.; Peng, Y.; Utsumi, F.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F.; Kajiyama, H. novel intraperitoneal treatment with non-thermal plasma-activated medium inhibits metastatic potential of ovarian cancer cells. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Ji, H.W.; Kim, H.W.; Yun, S.H.; Choi, E.H.; Kim, S.J. Cold Atmospheric plasma restores paclitaxel sensitivity to paclitaxel-resistant breast cancer cells by reversing expression of resistance-related genes. Cancers 2019, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- Burm, K.T.A.L. Plasma: The fourth state of matter. Plasma Chem. Plasma Process. 2012, 32, 401–407. [Google Scholar] [CrossRef]
- Bogle, M.A.; Arndt, K.A.; Dover, J.S. Plasma skin regeneration technology. J. Drugs Dermatol. 2007, 6, 1110–1112. [Google Scholar] [PubMed]
- Dai, X.; Bazaka, K.; Richard, D.J.; Thompson, E.; Rik, W.; Ostrikov, K. The emerging role of gas plasma in oncotherapy. Trends Biotechnol. 2018, 36, 1183–1198. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.D.; Kindel, E.; Von Woedtke, T.; Hähnel, M.; Stieber, M.; Brandenburg, R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl. Chem. 2010, 82, 1223–1237. [Google Scholar] [CrossRef]
- Manner, H. Argon plasma coagulation therapy. Curr. Opin. Gastroenterol. 2008, 24, 612–616. [Google Scholar] [CrossRef]
- Brunaldi, V.O.; Farias, G.F.A.; de Rezende, D.T.; Cairo-Nunes, G.; Riccioppo, D.; de Moura, D.T.H.; Santo, M.A.; de Moura, E.G.H. Argon plasma coagulation alone versus argon plasma coagulation plus full-thickness endoscopic suturing to treat weight regain after Roux-en-Y gastric bypass: A prospective randomized trial (with videos). Gastrointest. Endosc. 2020, 92, 33997–34003. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Zimmermann, J.; Shimizu, T.; Li, Y.-F.; Morfill, G.; Thomas, H.; Steffes, B.; Heinlin, J.; Karrer, S.; Stolz, W. Non-thermal plasma—More than five years of clinical experience. Clin. Plasma Med. 2013, 1, 19–23. [Google Scholar] [CrossRef]
- Gay-Mimbrera, J.; García, M.C.; Isla-Tejera, B.; Rodero-Serrano, A.; García-Nieto, A.V.; Ruano, J. Clinical and Biological Principles of Cold Atmospheric Plasma Application in Skin Cancer. Adv. Ther. 2016, 33, 894–909. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Conway, B.; Meissner, K.; Scholz, F.; Rauch, B.; Moroder, A.; Ehlers, A.; Meixner, A.; Heidecke, C.-D.; Partecke, L.; et al. Cold atmospheric pressure plasma for treatment of chronic wounds: Drug or medical device? J. Wound Care 2017, 26, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Šimončicová, J.; Kryštofová, S.; Medvecká, V.; Ďurišová, K.; Kaliňáková, B. Technical applications of plasma treatments: Current state and perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 5117–5129. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Laroussi, M.; Puech, V. On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sour. Sci. Technol. 2012, 21. [Google Scholar] [CrossRef]
- Duan, Y.; Huang, C.; Yu, Q. Cold plasma brush generated at atmospheric pressure. Rev. Sci. Instrum. 2007, 78, 015104. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Brandenburg, R.; Weltmann, K.-D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Attri, P.; Park, J.H.; Ali, A.; Choi, E.H. How does plasma activated media treatment differ from direct cold plasma treatment? Anti-Cancer Agents Med. Chem. 2018, 18, 805–814. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold Atmospheric Plasma: Charged Species and Their Interactions With Cells and Tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Weiss, M.; Barz, J.; Ackermann, M.; Utz, R.; Ghoul, A.; Weltmann, K.-D.; Stope, M.B.; Wallwiener, D.; Schenke-Layland, K.; Oehr, C.; et al. Dose-dependent tissue-level characterization of a medical atmospheric pressure argon plasma jet. ACS Appl. Mater. Interfaces 2019, 11, 19841–19853. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef]
- Ngo, M.-H.T.; Liao, J.-D.; Shao, P.-L.; Weng, C.-C.; Chang, C.-Y. Increased Fibroblast Cell Proliferation and Migration Using Atmospheric N2 /Ar Micro-Plasma for the Stimulated Release of Fibroblast Growth Factor-7. Plasma Process. Polym. 2014, 11, 80–88. [Google Scholar] [CrossRef]
- Haertel, B.; Wende, K.; Von Woedtke, T.; Weltmann, K.-D.; Lindequist, U. Non-thermal atmospheric-pressure plasma can influence cell adhesion molecules on HaCaT-keratinocytes. Exp. Dermatol. 2011, 20, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Kubinova, S.; Zaviskova, K.; Uherkova, L.; Zablotskii, V.; Churpita, O.; Lunov, O.; Dejneka, A. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci. Rep. 2017, 7, srep45183. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; Von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.-L.; Liao, J.-D.; Wong, T.-W.; Wang, Y.-C.; Leu, S.; Yip, H.-K. Enhancement of wound healing by non-thermal N2/Ar micro-plasma exposure in mice with fractional-CO2-laser-induced wounds. PLoS ONE 2016, 11, e0156699. [Google Scholar] [CrossRef]
- Cheng, K.-Y.; Lin, Z.-H.; Cheng, Y.-P.; Chiu, H.-Y.; Yeh, N.-L.; Wu, T.-K.; Wu, J.-S. Wound healing in streptozotocin-induced diabetic rats using atmospheric-pressure argon plasma jet. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2018, 27, 114–125. [Google Scholar] [CrossRef]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.; Partecke, L.-I.; Kindel, E.; Döring, F.; Lademann, J.; Heidecke, C.-D.; Kramer, A.; Hübner, N.-O. The modified HET-CAM as a model for the assessment of the inflammatory response to tissue tolerable plasma. Toxicol. Vitro 2011, 25, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Wacker, E.; Shimizu, T.; Heinlin, J.; Li, Y.-F.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L.; Bosserhoff, A.-K.; et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS ONE 2013, 8, e79325. [Google Scholar] [CrossRef] [PubMed]
- Kisch, T.; Helmke, A.; Schleusser, S.; Song, J.; Liodaki, E.; Stang, F.H.; Mailaender, P.; Kraemer, R. Improvement of cutaneous microcirculation by cold atmospheric plasma (CAP): Results of a controlled, prospective cohort study. Microvasc. Res. 2016, 104, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.-K.; Karrer, S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef]
- Bacalbasa, N.; Balescu, I.; Vilcu, M.; Dima, S.; Brezean, I. Risk Factors for Postoperative Complications After Vulvar Surgery. In Vivo 2019, 34, 447–451. [Google Scholar] [CrossRef]
- Gan, L.; Zhang, S.; Poorun, D.; Liu, D.; Lu, X.; He, M.; Duan, X.; Chen, H. Medical applications of nonthermal atmospheric pressure plasma in dermatology. J. Dtsch. Dermatol. Ges. 2018, 16, 7–13. [Google Scholar] [CrossRef]
- Assadian, O.; Ousey, K.J.; Daeschlein, G.; Kramer, A.; Parker, C.; Tanner, J.; Leaper, D.J. Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds and wound size reduction: A systematic review and meta-analysis. Int. Wound J. 2019, 16, 103–111. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Schmidt, H.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Görlitz, A.; Simon, D.; Schön, M.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm®VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venerol. 2014, 29, 148–155. [Google Scholar] [CrossRef]
- Heinlin, J.; Zimmermann, J.L.; Zeman, F.; Bunk, W.; Isbary, G.; Landthaler, M.; Maisch, T.; Monetti, R.; Morfill, G.; Shimizu, T.; et al. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Repair Regen. 2013, 21, 800–807. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Vu, T.T.; Do, H.T.; Le, T.N.B.; Hoang, T.H.A.; Phi, T.T.T.; Luong, T.M.L.; Doan, V.T.; Nguyen, T.T.H.; Nguyen, T.L.; et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: A clinical long term observation. Clin. Plasma Med. 2013, 1, 30–35. [Google Scholar] [CrossRef]
- Nishijima, A.; Fujimoto, T.; Hirata, T.; Nishijima, J. Effects of Cold Atmospheric Pressure Plasma on Accelerating Acute Wound Healing: A Comparative Study among 4 Different Treatment Groups. Mod. Plast. Surg. 2019, 9, 18–31. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Xia, C.; Yang, X.; Cao, Z.; Zheng, L.; Ko, R.; Shen, C.; Yang, C.; Cheng, C. Cold atmospheric plasma promotes different types of superficial skin erosion wounds healing. Int. Wound J. 2019, 16, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Dias-Jr, A.R.; Soares-Jr, J.; De Faria, M.B.S.; Genta, M.L.N.D.; Carvalho, J.P.; Baracat, E.C. Secondary healing strategy for difficult wound closure in invasive vulvar cancer: A pilot case-control study. Clinics 2019, 74, e1218. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.M.; Frame, F.M.; Maitland, N.J.; O’Connell, D. Low temperature plasma: A novel focal therapy for localized prostate cancer? BioMed Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Fengyi, W.; Lenardo, M.J. Specification of DNA binding activity of NF-kappaB proteins. Cold Spring Harb. Perspect. Biol. 2009, a000067. [Google Scholar] [CrossRef]
- Keidar, M.; Yan, D.; Beilis, I.I.; Trink, B.; Sherman, J.H. Plasmas for Treating Cancer: Opportunities for Adaptive and Self-Adaptive Approaches. Trends Biotechnol. 2018, 36, 586–593. [Google Scholar] [CrossRef]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef]
- Arndt, S.; Landthaler, M.; Zimmermann, J.L.; Unger, P.; Wacker, E.; Shimizu, T.; Li, Y.-F.; Morfill, G.E.; Bosserhoff, A.-K.; Karrer, S. Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS ONE 2015, 10, e0120041. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [PubMed]
- Xingmin, S.; Jingfen, C.; Guimin, X.; Hongbin, R.; Sile, C.; Zhengshi, C.; Xili, W. Effect of cold plasma on cell viability and collagen synthesis in cultured murine fibroblasts. Plasma Sci. Technol. 2016, 18, 353–359. [Google Scholar] [CrossRef]
- Kang, S.U.; Kim, Y.S.; Kim, Y.E.; Park, J.-K.; Lee, Y.S.; Kang, H.Y.; Jang, J.W.; Ryeo, J.B.; Lee, Y.; Shin, Y.S.; et al. Opposite effects of non-thermal plasma on cell migration and collagen production in keloid and normal fibroblasts. PLoS ONE 2017, 12, e0187978. [Google Scholar] [CrossRef] [PubMed]
- Keyvani, A.; Atyabi, S.M.; Sardari, S.; Norouzian, D.; Madanchi, H. Effects of cold atmospheric plasma jet on collagen structure in different treatment times. Basic Res. J. Med. Clin. Sci. 2017, 6, 84–90. [Google Scholar]
- Privat-Maldonado, A.; Bengtson, C.; Razzokov, J.; Smits, E.; Bogaerts, A. Modifying the tumour microenvironment: Challenges and future perspectives for anticancer plasma treatments. Cancers 2019, 11, 1920. [Google Scholar] [CrossRef]
- Bernardes, N.; Fialho, A.M. Perturbing the dynamics and organization of cell membrane components: A new paradigm for cancer-targeted therapies. Int. J. Mol. Sci. 2018, 19, 3871. [Google Scholar] [CrossRef]
- Zalba, S.; Hagen, T.L.T. Cell membrane modulation as adjuvant in cancer therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, W.; Li, S.; Yang, H. The role of cholesterol metabolism in cancer. Am. J. Cancer Res. 2019, 9, 219–227. [Google Scholar]
- Rivel, T.; Ramseyer, C.; Yesylevskyy, S. The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Van Der Paal, J.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem. Sci. 2016, 7, 489–498. [Google Scholar] [CrossRef]
- Bauer, G. Tumor cell-protective catalase as a novel target for rational therapeutic approaches based on specific intercellular ROS signaling. Anticancer Res. 2012, 32, 2599–2624. [Google Scholar] [PubMed]
- Bauer, G. Targeting extracellular ROS signaling of tumor cells. Anticancer Res. 2014, 34, 1467–1482. [Google Scholar] [PubMed]
- Bauer, G. Targeting protective catalase of tumor cells with cold atmospheric plasma- activated medium (PAM). Anti-Cancer Agents Med. Chem. 2018, 18, 784–804. [Google Scholar] [CrossRef] [PubMed]
- Dezest, M.; Chavatte, L.; Bourdens, M.; Quinton, D.; Camus, M.; Garrigues, L.; Descargues, P.; Arbault, S.; Burlet-Schiltz, O.; Casteilla, L.; et al. Mechanistic insights into the impact of Cold Atmospheric Pressure Plasma on human epithelial cell lines. Sci. Rep. 2017, 7, 41163. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.J.; Walker, B.; Irvine, A.E. Proteasome inhibitors in cancer therapy. J. Cell Commun. Sign. 2011, 5, 101–110. [Google Scholar] [CrossRef]
- Zhao, S.; Xiong, Z.; Mao, X.; Meng, D.; Lei, Q.; Li, Y.; Deng, P.; Chen, M.; Tu, M.; Lu, X.; et al. Atmospheric pressure room temperature plasma jets facilitate oxidative and nitrative stress and lead to endoplasmic reticulum stress dependent apoptosis in HepG2 cells. PLoS ONE 2013, 8, e73665. [Google Scholar] [CrossRef]
- Weathington, N.M.; Mallampalli, R.K. Emerging therapies targeting the ubiquitin proteasome system in cancer. J. Clin. Investig. 2014, 124, 6–12. [Google Scholar] [CrossRef]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef]
- Jan, R.; Chaudhry, G.-E.-S. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef]
- Ashkenazi, A.; Fairbrother, A.A.W.J.; Leverson, J.D.; Souers, J.D.L.A.J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 1–9. [Google Scholar] [CrossRef]
- Baig, S.M.; Seevasant, I.; Mohamad, J.A.; Mukheem, A.; Huri, H.Z.; Kamarul, T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016, 7, e2058. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.; Mirfakhraie, R.; Jalili, A. Combination of cold atmospheric plasma and iron nanoparticles in breast cancer: Gene expression and apoptosis study. OncoTargets Ther. 2016, 9, 5911–5917. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold atmospheric plasma: A powerful tool for modern medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [PubMed]
- Turrini, E.; Laurita, R.; Stancampiano, A.; Catanzaro, E.; Calcabrini, C.; Maffei, F.; Gherardi, M.; Colombo, V.; Fimognari, C. Cold atmospheric plasma induces apoptosis and oxidative stress pathway regulation in t-lymphoblastoid leukemia cells. Oxid. Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.-S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, S.U.; Kim, K.I.; Kang, S.; Shin, Y.S.; Chang, J.W.; Yang, S.S.; Lee, K.; Lee, J.-S.; Moon, E.; et al. Nonthermal plasma induces apoptosis in ATC cells: Involvement of JNK and p38 MAPK-dependent ROS. Yonsei Med. J. 2014, 55, 1640–1647. [Google Scholar] [CrossRef]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.-S.; Moon, E.; Baek, S.J.; Lee, K.; et al. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: Involvement of DNA-damage-triggering sub-G1 arrest via the ATM/p53 pathway. Arch. Biochem. Biophys. 2014, 545, 133–140. [Google Scholar] [CrossRef]
- Ma, Y.; Ha, C.S.; Hwang, S.W.; Lee, H.J.; Kim, G.C.; Lee, K.-W.; Song, K. Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ROS Stress-response pathways. PLoS ONE 2014, 9, e91947. [Google Scholar] [CrossRef]
- Xiong, B.Z. Cold Atmospheric Pressure Plasmas (CAPs) for Skin Wound Healing, Plasma Medicine-Concepts and Clinical Applications. IntechOpen 2018. [Google Scholar] [CrossRef]
- Kalghatgi, S.; Friedman, G.; Fridman, A.; Clyne, A.M. Endothelial cell proliferation is enhanced by low dose non-thermal plasma through fibroblast growth factor-2 release. Ann. Biomed. Eng. 2010, 38, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Lendeckel, D.; Eymann, C.; Emicke, P.; Daeschlein, G.; Darm, K.; O’Neil, S.; Beule, A.; Von Woedtke, T.; Völker, U.; Weltmann, K.-D.; et al. Proteomic changes of tissue-tolerable plasma treated airway epithelial cells and their relation to wound healing. BioMed Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Welz, C.; Emmert, S.; Canis, M.; Becker, S.; Baumeister, P.; Shimizu, T.; Morfill, G.E.; Harréus, U.; Zimmermann, J.L. Cold atmospheric plasma: A promising complementary therapy for squamous head and neck cancer. PLoS ONE 2015, 10, e0141827. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Talbot, A.; Nourmohammadi, N.; Cheng, X.; Canady, J.; Sherman, J.; Keidar, M. Principles of using cold atmospheric plasma stimulated media for cancer treatment. Sci. Rep. 2015, 5, 18339. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Hashemi, M. Cold atmospheric plasma manipulation of proteins in food systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 2583–2597. [Google Scholar] [CrossRef]
- Puač, N.; Živković, S.; Selaković, N.; Milutinović, M.; Boljević, J.; Malovic, G.; Petrovic, Z.L. Long and short term effects of plasma treatment on meristematic plant cells. Appl. Phys. Lett. 2014, 104, 214106. [Google Scholar] [CrossRef]
- Schaner, M.E.; Ross, D.T.; Ciaravino, G.; Sorlie, T.; Troyanskaya, O.; Diehn, M.; Wang, Y.C.; Duran, G.E.; Sikic, T.L.; Caldeira, S.; et al. Gene expression patterns in ovarian carcinomas. Mol. Biol. Cell 2003, 14, 4376–4386. [Google Scholar] [CrossRef]
- Knutsen, E.; Oslo Breast Cancer Research Consortium (OSBREAC); Lellahi, S.M.; Aure, M.R.; Nord, S.; Fismen, S.; Larsen, K.B.; Gabriel, M.T.; Hedberg, A.; Bjørklund, S.S.; et al. The expression of the long NEAT1_2 isoform is associated with human epidermal growth factor receptor 2-positive breast cancers. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Su, H.; Wu, S.; Yen, L.; Chiao, L.K.; Wang, J.K.; Chiu, Y.L.; Ho, C.L.; Huang, S.M. Gene expression profiling identifies the role of Zac1 in cervical cancer metastasis. Sci. Rep. 2020, 10, 11837. [Google Scholar] [CrossRef]
- Park, S.-B.; Kim, B.; Bae, H.; Lee, H.; Lee, S.; Choi, E.H.; Kim, S.J. Differential epigenetic effects of atmospheric cold plasma on MCF-7 and MDA-MB-231 breast cancer cells. PLoS ONE 2015, 10, e0129931. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Mao, L.; Wang, L.; Zhang, H.; Hu, X. miR-218 functions as a tumor suppressor gene in cervical cancer. Mol. Med. Rep. 2020, 21, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Wacker, E.; Li, Y.-F.; Shimizu, T.; Thomas, H.M.; Morfill, G.E.; Karrer, S.; Zimmermann, J.L.; Bosserhoff, A.-K. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp. Dermatol. 2013, 22, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Kurita, H.; Haruta, N.; Uchihashi, Y.; Seto, T.; Takashima, K. Strand breaks and chemical modification of intracellular DNA induced by cold atmospheric pressure plasma irradiation. PLoS ONE 2020, 15, e0232724. [Google Scholar] [CrossRef] [PubMed]
- Volotskova, O.; Hawley, T.S.; Stepp, M.A.; Keidar, M. Targeting the cancer cell cycle by cold atmospheric plasma. Sci. Rep. 2012, 2, 636. [Google Scholar] [CrossRef]
- Chung, W.-H. Mechanisms of a novel anticancer therapeutic strategy involving atmospheric pressure plasma-mediated apoptosis and DNA strand break formation. Arch. Pharmacal Res. 2015, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of atmospheric pressure plasmas on isolated and cellular DNA—A review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef]
- Lackmann, J.W.; Schneider, S.; Narberhaus, F.; Benedikt, J.; Bandow, J.E. Characterization of damage to bacteria and bio-macromolecules caused by (V)UV radiation and particles generated by a microscale atmospheric pressure plasma jet. In Plasma for Biodecontamination, Medicine and Food Security; Machala, Z., Hensel, K., Akishev, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 17–29. [Google Scholar]
- Guo, L.; Zhao, Y.; Liu, D.X.; Liu, Z.C.; Chen, C.; Xu, R.; Tian, M.; Wang, X.; Chen, H.; Kong, M.G. Cold atmospheric-pressure plasma induces DNA–protein crosslinks through protein oxidation. Free Radic. Res. 2018, 52, 783–798. [Google Scholar] [CrossRef]
- Kim, G.J.; Kim, W.; Kim, K.T.; Lee, J.K. DNA damage and mitochondria dysfunction in cell apoptosis induced by nonthermal air plasma. Appl. Phys. Lett. 2010, 96, 021502. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Schneider, S.; Edengeiser, E.; Jarzina, F.; Brinckmann, S.; Steinborn, E.; Havenith, M.; Benedikt, J.; Bandow, J.E. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J. R. Soc. Interface 2013, 10, 20130591. [Google Scholar] [CrossRef]
- Yan, X.; Qiao, Y.; Ouyang, J.; Jia, M.; Li, J.; Yuan, F. Protective effect of atmospheric pressure plasma on oxidative stress-induced neuronal injuries: Anin vitrostudy. J. Phys. D Appl. Phys. 2017, 50, 095401. [Google Scholar] [CrossRef]
- Niki, E. Antioxidants: Basic principles, emerging concepts, and problems. Biomed. J. 2014, 37. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, E.A.; Mahmoud, A.M.; Khalifa, A.M.; Ali, S.S. Physiological and pathophysiological reactive oxygen species as probed by EPR spectroscopy: The underutilized research window on muscle ageing. J. Physiol. 2016, 594, 4591–4613. [Google Scholar] [CrossRef]
- Murakami, T. Numerical modelling of the effects of cold atmospheric plasma on mitochondrial redox homeostasis and energy metabolism. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Maiorino, M.; Ursini, F. Signaling functions of reactive oxygen species. Biochemistry 2010, 49, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Hanschmann, E.-M.; Godoy, J.R.; Berndt, C.; Hudemann, C.; Lillig, C.H. Thioredoxins, glutaredoxins, and peroxiredoxins—Molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxid. Redox Sign. 2013, 19, 1539–1605. [Google Scholar] [CrossRef]
- Kushnir, C.L.; Fleury, A.C.; Hill, M.C.; Silver, D.F.; Spirtos, N.M. The use of argon beam coagulation in treating vulvar intraepithelial neoplasia III: A retrospective review. Gynecol. Oncol. 2013, 131, 386–388. [Google Scholar] [CrossRef]
- Miller, V.; Lin, A.; Fridman, A. Why target immune cells for plasma treatment of cancer. Plasma Chem. Plasma Process. 2015, 36, 259–268. [Google Scholar] [CrossRef]
- Schneider, C.; Gebhardt, L.; Arndt, S.; Karrer, S.; Zimmermann, J.L.; Fischer, M.J.M.; Bosserhoff, A.-K. Cold atmospheric plasma causes a calcium influx in melanoma cells triggering CAP-induced senescence. Sci. Rep. 2018, 8, 10048. [Google Scholar] [CrossRef]
- Kim, H.W.; Jeong, D.; Ham, J.; Kim, H.; Ji, H.W.; Choi, E.H.; Kim, S.J. ZNRD1 and its antisense long noncoding RNA ZNRD1-AS1 Are oppositely regulated by cold atmospheric plasma in breast cancer cells. Oxidative Med. Cell. Longev. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Mokhtari, H.; Farahmand, L.; Yaserian, K.; Jalili, N.; Majidzadeh-A, K. The antiproliferative effects of cold atmospheric plasma-activated media on different cancer cell lines, the implication of ozone as a possible underlying mechanism. J. Cell. Physiol. 2018, 234, 6778–6782. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Yamada, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Kobayashi, D.; Tanaka, C.; Fujii, T.; et al. Intraperitoneal administration of plasma-activated medium: Proposal of a novel treatment option for peritoneal metastasis from gastric cancer. Ann. Surg. Oncol. 2017, 24, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.; Adhikari, B.; Ghimire, B.; Baboota, S.; Choi, E.H. Cold atmospheric plasma and silymarin nanoemulsion activate autophagy in human melanoma cells. Int. J. Mol. Sci. 2020, 21, 1939. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kaushik, N.; Yoo, K.C.; Uddin, N.; Kim, J.S.; Lee, S.J.; Choi, E.H. Low doses of PEG-coated gold nanoparticles sensitize solid tumors to cold plasma by blocking the PI3K/AKT-driven signaling axis to suppress cellular transformation by inhibiting growth and EMT. Biomaterials 2016, 87, 118–130. [Google Scholar] [CrossRef]
- Irani, S.; Shahmirani, Z.; Atyabi, S.M.; Mirpoor, S. Induction of growth arrest in colorectal cancer cells by cold plasma and gold nanoparticles. Arch. Med. Sci. 2015, 6, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Jawaid, P.; Rehman, M.U.; Zhao, Q.L.; Takeda, K.; Ishikawa, K.; Hori, M.; Shimizu, T.; Kondo, T. Helium-based cold atmospheric plasma-induced reactive oxygen species-mediated apoptotic pathway attenuated by platinum nanoparticles. J. Cell. Mol. Med. 2016, 20, 1737–1748. [Google Scholar] [CrossRef]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Barcia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold atmospheric plasma induces ATP-dependent endocytosis of nanoparticles and synergistic U373MG cancer cell death. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Wang, S.; Li, X.; Li, W.; Ding, D.; Gong, X.; Keidar, M.; Zhang, W.-F. Paclitaxel-loaded core–shell magnetic nanoparticles and cold atmospheric plasma inhibit non-small cell lung cancer growth. ACS Appl. Mater. Interfaces 2018, 10, 43462–43471. [Google Scholar] [CrossRef]
- Kletschkus, K.; Haralambiev, L.; Nitsch, A.; Pfister, F.; Klinkmann, G.; Kramer, A.; Bekeschus, S.; Mustea, A.; Stope, M.B. The application of a low-temperature physical plasma device operating under atmospheric pressure leads to the production of toxic NO2. Anticancer. Res. 2020, 40, 2591–2599. [Google Scholar] [CrossRef]
- Yan, D.; Xu, W.; Yao, X.; Lin, L.; Sherman, J.H.; Keidar, M. The cell activation phenomena in the cold atmospheric plasma cancer treatment. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Nourmohammadi, N.; Bian, K.; Murad, F.; Sherman, J.H.; Keidar, M. Stabilizing the cold plasma-stimulated medium by regulating medium’s composition. Sci. Rep. 2016, 6, 26016. [Google Scholar] [CrossRef] [PubMed]
- Van Boxem, W.; Van Der Paal, J.; Gorbanev, Y.; Vanuytsel, S.; Smits, E.; Dewilde, S.; Bogaerts, A. Anti-cancer capacity of plasma-treated PBS: Effect of chemical composition on cancer cell cytotoxicity. Sci. Rep. 2017, 7, 16478. [Google Scholar] [CrossRef] [PubMed]
- Azzariti, A.; Iacobazzi, R.M.; Di Fonte, R.; Porcelli, L.; Gristina, R.; Favia, P.; Fracassi, F.; Trizio, I.; Silvestris, N.; Guida, G.; et al. Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Flieswasser, T.; Boullosa, L.F.; De Waele, J.; Van Audernaerde, J.R.; Marcq, E.; Jacobs, J.; Lin, A.; Lion, E.; Dewitte, H.; et al. Cold Atmospheric Plasma-Treated PBS Eliminates Immunosuppressive Pancreatic Stellate Cells and Induces Immunogenic Cell Death of Pancreatic Cancer Cells. Cancers 2019, 11, 1597. [Google Scholar] [CrossRef]
- Kaushik, N.; Kaushik, N.; Adhikari, M.; Ghimire, B.; Linh, N.N.; Mishra, Y.; Lee, S.-J.; Choi, E.H. Preventing the solid cancer progression via release of anticancer-cytokines in co-culture with cold plasma-stimulated macrophages. Cancers 2019, 11, 842. [Google Scholar] [CrossRef]
- Wang, Z.; Førsund, M.S.; Trope, C.G.; Nesland, J.M.; Holm, R.; Slipicevic, A. Evaluation of CHK1 activation in vulvar squamous cell carcinoma and its potential as a therapeutic target in vitro. Cancer Med. 2018, 7, 3955–3964. [Google Scholar] [CrossRef]
- Nooij, L.S.; Ter Haar, N.T.; Ruano, D.; Rakislova, N.; Van Wezel, T.; Smit, V.T.; Trimbos, B.J.; Ordi, J.; Van Poelgeest, M.I.; Bosse, T. Genomic characterization of vulvar (Pre)cancers identifies distinct molecular subtypes with prognostic significance. Clin. Cancer Res. 2017, 23, 6781–6789. [Google Scholar] [CrossRef]
- Zięba, S.; Kowalik, A.; Zalewski, K.; Rusetska, N.; Goryca, K.; Piascik, A.; Misiek, M.; Bakuła-Zalewska, E.; Kopczyński, J.; Kowalski, K.; et al. Somatic mutation profiling of vulvar cancer: Exploring therapeutic targets. Gynecol. Oncol. 2018, 150, 552–561. [Google Scholar] [CrossRef]
- Brunetti, M.; Agostini, A.; Davidson, B.; Tropé, C.G.; Heim, S.; Panagopoulos, I.; Micci, F. Recurrent fusion transcripts in squamous cell carcinomas of the vulva. Oncotarget 2017, 8, 16843–16850. [Google Scholar] [CrossRef]
- Agostini, A.; Brunetti, M.; Davidson, B.; Trope, C.G.; Heim, S.; Panagopoulos, I.; Micci, F. Expressions of miR-30c and let-7a are inversely correlated with HMGA2 expression in squamous cell carcinoma of the vulva. Oncotarget 2016, 7, 85058–85062. [Google Scholar] [CrossRef] [PubMed]
- Deppe, G.; Mert, I.; Belotte, J.; Winer, I. Chemotherapy of vulvar cancer: A review. Wien. Klin. Wochenschr. 2013, 125, 119–128. [Google Scholar] [CrossRef]
- Mahner, S.; Prieske, K.; Grimm, D.; Trillsch, F.; Prieske, S.; Von Amsberg, G.; Petersen, C.; Mueller, V.; Jaenicke, F.; Woelber, L. Systemic treatment of vulvar cancer. Exp. Rev. Anticancer. Ther. 2015, 15, 629–637. [Google Scholar] [CrossRef]
- Reade, C.J.; Eiriksson, L.R.; Mackay, H. Systemic therapy in squamous cell carcinoma of the vulva: Current status and future directions. Gynecol. Oncol. 2014, 132, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Bang, Y.-J.; Piha-Paul, S.A.; Razak, A.R.A.; Bennouna, J.; Soria, J.-C.; Rugo, H.S.; Cohen, R.B.; O’Neil, B.H.; Mehnert, J.M.; et al. T-cell–inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J. Clin. Oncol. 2019, 37, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Pekkola-Heino, K.; Kulmala, J.; Grenman, S.; Carey, T.E.; Grenman, R. Radiation response of vulvar squamous cell carcinoma (UM-SCV-1A, UM-SCV-1B, UM-SCV-2, and A-431) cells in vitro. Cancer Res. 1989, 49, 2758419. [Google Scholar]
- Corrado, G.; Cutillo, G.; Fragomeni, S.M.; Bruno, V.; Tagliaferri, L.; Mancini, E.; Certelli, C.; Paris, I.; Vizza, E.; Scambia, G.; et al. Palliative electrochemotherapy in primary or recurrent vulvar cancer. Int. J. Gynecol. Cancer 2020, 30, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Biscop, E.; Lin, A.; Van Boxem, W.; Van Loenhout, J.; De Backer, J.; Deben, C.; Dewilde, S.; Smits, E.L.; Bogaerts, A. Influence of cell type and culture medium on determining cancer selectivity of cold atmospheric plasma treatment. Cancers 2019, 11, 1287. [Google Scholar] [CrossRef]
- Tornin, J.; Mateu-Sanz, M.; Rodríguez, A.; Labay, C.; Rodríguez, R.; Canal, C. pyruvate plays a main role in the antitumoral selectivity of cold atmospheric plasma in osteosarcoma. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Köritzer, J.; Boxhammer, V.; Schäfer, A.; Shimizu, T.; Klämpfl, T.G.; Li, Y.-F.; Welz, C.; Schwenk-Zieger, S.; Morfill, G.E.; Zimmermann, J.L.; et al. Restoration of sensitivity in chemo—Resistant glioma cells by cold atmospheric plasma. PLoS ONE 2013, 8, e64498. [Google Scholar] [CrossRef]
- Lin, L.; Wang, L.; Liu, Y.; Xu, C.; Tu, Y.; Zhou, J. Non-thermal plasma inhibits tumor growth and proliferation and enhances the sensitivity to radiation in vitro and in vivo. Oncol. Rep. 2018, 40, 3405–3415. [Google Scholar] [CrossRef]
- Lin, L.; Yan, D.; Gjika, E.; Sherman, J.H.; Keidar, M. Atmospheric plasma meets cell: Plasma tailoring by living cells. ACS Appl. Mater. Interfaces 2019, 11, 30621–30630. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Rajjoub, K.; Shashurin, A.; Yan, D.; Sherman, J.H.; Bian, K.; Murad, F.; Keidar, M. Enhancing cold atmospheric plasma treatment of cancer cells by static magnetic field. Bioelectromagnetics 2017, 38, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Noghreiyan, A.V.; Imanparast, A.; Ara, E.S.; Soudmand, S.; Noghreiyan, V.V.; Sazgarnia, A. In-vitro investigation of cold atmospheric plasma induced photodynamic effect by Indocyanine green and Protoporphyrin IX. Photodiagnosis Photodyn. Ther. 2020, 101822. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, R.; Rehman, M.U.; Zhao, Q.-L.; Jawaid, P.; Takeda, K.; Ishikawa, K.; Hori, M.; Tomihara, K.; Noguchi, K.; Kondo, T.; et al. Cold atmospheric helium plasma causes synergistic enhancement in cell death with hyperthermia and an additive enhancement with radiation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Van Leeuwen, J.H.S.; Schreuder, H.W.; Hermans, R.H.; De Hingh, I.H.; Van Der Velden, J.; Arts, H.J.; Massuger, L.F.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Chaiswing, L.; Weiss, H.L.; Jayswal, R.D.; Clair, D.K.S.; Kyprianou, N. Profiles of radioresistance mechanisms in prostate cancer. Crit. Rev. Oncog. 2018, 23, 39–67. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Pham, C.T.; Juhasz, M.; Sung, C.T.; Mesinkovska, N.A. The human papillomavirus vaccine as a treatment for human papillomavirus-related dysplastic and neoplastic conditions: A literature review. J. Am. Acad. Dermatol. 2020, 82, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Golubnitschaja, O.; Baban, B.; Boniolo, G.; Wang, W.; Bubnov, R.; Kapalla, M.; Krapfenbauer, K.; Mozaffari, M.S.; Costigliola, V. Medicine in the early twenty-first century: Paradigm and anticipation - EPMA position paper 2016. EPMA J. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Golubnitschaja, O.; Flammer, J. Individualised patient profile: Clinical utility of Flammer syndrome phenotype and general lessons for predictive, preventive and personalised medicine. EPMA J. 2018, 9, 15–20. [Google Scholar] [CrossRef]
- Janssens, J.P.; Schuster, K.; Voss, A. Preventive, predictive, and personalized medicine for effective and affordable cancer care. EPMA J. 2018, 9, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Goncharenko, V.; Bubnov, R.; Polivka, J.; Zubor, P.; Biringer, K.; Bielik, T.; Kuhn, W.; Golubnitschaja, O. Vaginal dryness: Individualised patient profiles, risks and mitigating measures. EPMA J. 2019, 10, 73–79. [Google Scholar] [CrossRef]
- Kunin, A.; Polivka, J.; Moiseeva, N.; Golubnitschaja, O. “Dry mouth” and “Flammer” syndromes—Neglected risks in adolescents and new concepts by predictive, preventive and personalised approach. EPMA J. 2018, 9, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Maturo, M.G.; Soligo, M.; Gibson, G.; Manni, L.; Nardini, C. The greater inflammatory pathway—High clinical potential by innovative predictive, preventive, and personalized medical approach. EPMA J. 2019, 11, 1–16. [Google Scholar] [CrossRef]
- Avishai, E.; Yeghiazaryan, K.; Golubnitschaja, O. Impaired wound healing: Facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine. EPMA J. 2017, 8, 23–33. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Stolzenburg-Veeser, L.; Avishai, E.; Costigliola, V. Wound healing: Proof-of-principle model for the modern hospital-patient stratification, prediction, prevention and personalisation of treatment. In The Modern Hospital: Patients Centered, Disease Based, Research Oriented, Technology Driven; Latifi, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-030-01393-6. [Google Scholar]
- Gerner, C.; Costigliola, V.; Golubnitschaja, O. Multiomic patterns in body fluids: Technological Challenge with a great potential to implement the advanced paradigm of 3P medicine. Mass. Spectrom. Rev. 2019. [Google Scholar] [CrossRef]
- Lu, M.; Zhan, X. The crucial role of multiomic approach in cancer research and clinically relevant outcomes. EPMA J. 2018, 9, 77–102. [Google Scholar] [CrossRef]
- Golubnitschaja, O.; Polivka, J.; Yeghiazaryan, K.; Berliner, L. Liquid biopsy and multiparametric analysis in management of liver malignancies: New concepts of the patient stratification and prognostic approach. EPMA J. 2018, 9, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective activities of plant natural substances in cancer and chemopreventive strategies in the context of 3P medicine. EPMA J. 2020, 11, 261–287. [Google Scholar] [CrossRef] [PubMed]
- Zubor, P.; Dankova, Z.; Kolkova, Z.; Holubekova, V.; Brany, D.; Mersakova, S.; Samec, M.; Liskova, A.; Koklesova, L.; Kubatka, P.; et al. Rho GTPases in gynecologic cancers: In-depth analysis toward the paradigm change from reactive to predictive, preventive, and personalized medical approach benefiting the patient and healthcare. Cancers 2020, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.-R.; Seebauer, C.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; Metelmann, P. Treating cancer with cold physical plasma: On the way to evidence-based medicine. Contrib. Plasma Phys. 2018, 58, 415–419. [Google Scholar] [CrossRef]
- Apyx Medical. Available online: https://clinicaltrials.gov/NCT02658851 (accessed on 20 October 2020).
- Orvieto, M.A.; Coelho, R.F.; Chauhan, S.; Palmer, K.J.; Rocco, B.; Patel, V.R. Incidence of lymphoceles after robot-assisted pelvic lymph node dissection. BJU Int. 2011, 108, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- CAPCIN. Available online: https://clinicaltrials.gov/NCT03218436 (accessed on 20 October 2020).
- Marampon, F.; Gravina, G.L.; Popov, V.M.; Scarsella, L.; Festuccia, C.; La Verghetta, M.E.; Parente, S.; Cerasani, M.; Bruera, G.; Ficorella, C.; et al. Close correlation between MEK/ERK and Aurora-B signaling pathways in sustaining tumorigenic potential and radioresistance of gynecological cancer cell lines. Int. J. Oncol. 2014, 44, 285–294. [Google Scholar] [CrossRef]
- Golubnitschaja, O. Flammer Syndrome—From Phenotype to Associated Pathologies, Prediction, Prevention and Personalisation; Golubnitschaja, O., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-030-13549-2. [Google Scholar]
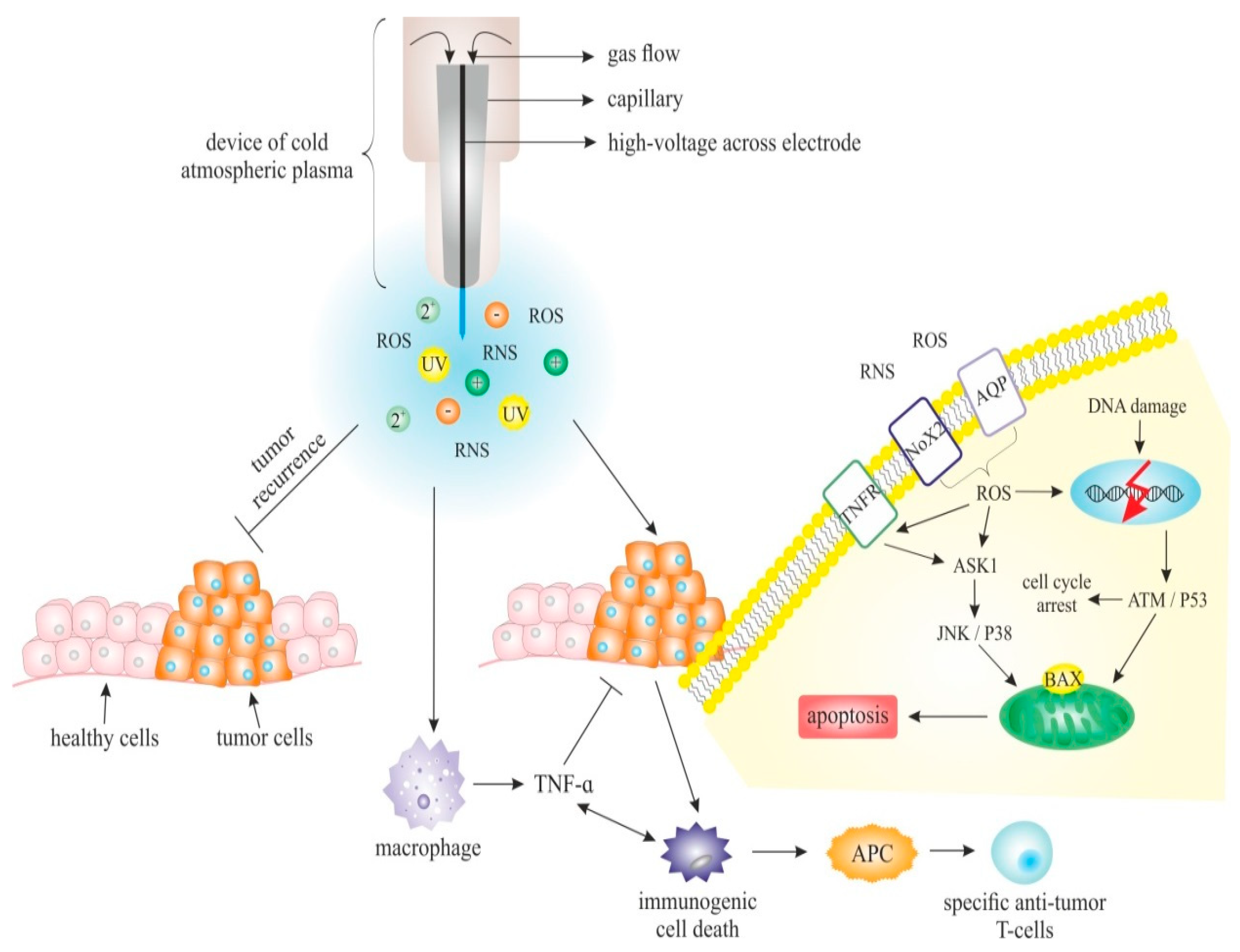
| Cell Line Origin | Cell Line/s | Main Effects of CAP on Cell Lines Observed in the Studies | Ref. |
|---|---|---|---|
| Cervix | HeLa SiHa HFB | ° Reduced viability of cells after plasma treatment in a dose-dependent manner ° Selective inhibition of proliferation in cancer cells compared to HFB ° Higher inhibition effect in the case of SiHa cells in comparison to Hela cells ° Significant increase of cells in subG0 phase cell and vice versa: reduction of populations in S phase and G2/M phase in a cell-type-specific manner ° Identification of caspase-3, -8 and -9 activation as an important mechanism underlying apoptosis in plasma-treated cells | [12] |
| Cervix | HeLa HFB detroit551 | ° Induction of HeLa cell apoptosis by facilitating an accumulation of intracellular reactive oxygen and nitrogen species (RONS) in a dose-dependent manner by both dielectric barrier discharge (DBD) plasma and nitric oxide-plasma activated water (NO-PAW) ° Higher selectivity of NO-PAW at given conditions | [62] |
| Cervix | HeLa | ° Inhibited proliferation and induced cell death in an exposure time-dependent manner ° Significant suppression of the migration and invasion ° Reduced activity and expression of the matrix metalloproteinase (MMP)-9 enzyme ° Decreased phosphorylation level of both ERK1/2 and JNK, but not p38 MAPK | [63] |
| Cervix | CaSki DoTc2-4510 SiHa C-33-A | ° Time- and energy-dependent effects of the treatment on cell proliferation ° Higher sensitivity of cervical cancer cells to plasma treatment in comparison to non-cancerous cervical tissue cells ° Decreased metabolic activity in cancer cells lines when compared to NCCT | [64] |
| Cervix | CaSki | ° Distance and flow rate-dependent effect of CAP on tumour cell viability ° Dose-dependent induction of tumour cell death by CAP treatment | [65] |
| Cervix | HeLa | ° Augmented number of early apoptotic cells, late apoptotic cells, but rarely necrotic cells by treatment with N2 and air plasma jets ° Induced apoptotic cell death in a dose-dependent manner ° Increased level of ROS and consequently, induction of apoptosis ° Induction of the mitochondria membrane depolarisation, causing increased mitochondrial transmembrane permeability and release of proapoptotic factors ° Blocking of ROS mediated plasma-induced apoptosis by D-mannitol, sodium pyruvate, carboxyl-PTIO or N-acetyl-cysteine ° Generation of different types and compositions of ROS by different plasma sources | [66] |
| Cervix | HeLa | ° After controlled application of plasma with the precision of tens of nanometres observed killing of plasma-treated cells, neighbouring cells were not affected significantly ° Induction of morphological changes as well as indicators of apoptosis in treated cells ° Crucial role of ROS in cancer cell death induction | [67] |
| Cervix | HeLa | ° Induction of cellular lipid membrane collapse by atmospheric-pressure plasma ° Alteration of electrical conductivity of the cells and induction of lipid oxidation by ROS | [68] |
| Cervix | SiHa + healthy human cervical tissue cells from cervical conus | ° Immediate and persisting decrease in CC cell growth and cell viability associated with significant plasma-dependent effects on lipid structures | [69] |
| Endometrium | AMEC HEC50 | ° Reduction of cell viability and induction of cell death by PAM ° Increased autophagic cell death ° Inactivation of the mTOR pathway by PAM ° G2/M-phase arrest in all PAM concentrations ° Induction of intracellular ROS accumulation | [70] |
| Endometrium | HEC-1 HEC-108 | ° Reduction of cells containing high levels of aldehyde dehydrogenase (ALDH) - a marker of cancer-initiating cells (CICs) ° Synergistic effect of combined treatment with cisplatin, especially at lower doses ° Combination of plasma and cisplatin treatment is effective both in ALDH high and low cells | [71] |
| Endometrium | HEC-1 GCIY | ° Reduction of cell viability ° Reduction of the number of cells with high aldehyde dehydrogenase (ALDH) production | [72] |
| Ovary | OVCAR-3 SKOV-3 TOV-21G TOV-112D | ° Variation of anti-proliferative efficacy of CAP dependent on treatment duration as well as on the OC cell line used ° Decreased motility, invasion, and metastasis potential ° Culture medium treated with plasma before addition mediates the CAP effect on the cells, however, this effect depends on the cell medium composition | [73] |
| Ovary | SKOV-3 OV-90 HOSE | ° Selective anticancer activity of plasma-activated Ringer’s Lactate solution (PA-RL) containing reactive oxygen and nitrogen species (RONS) | [74] |
| Ovary | TOV21G ES-2 SKOV3 NOS2 OHFC HPMC | ° Decreased viability of CCC cell line after plasma-activated medium treatment ° Induction of morphological changes in EOC cell lines treated with PAM ° Anti-tumour effects mediated by produced ROS ° Selective anti-proliferative effect on cancer cells without causing adverse reactions in normal cells | [75] |
| Ovary | NOS2 NOS3 NOS2TR NOS2CR NOS3TR NOS3CR | ° Decreased viability of ovarian cancer cells treated with PAM in plasma activation time-dependent manner ° Treatment with PAM decreased proliferation rate of paclitaxel and cisplatin-resistant cells derived from parental cell lines ° Addition of ROS scavenger into activated medium decreases anticancer activity, the addition of ROS scavenger inhibitor re-established anticancer activity, thus this point on the crucial role of ROS in an anti-tumour mechanism | [76] |
| Ovary | K2 K2R100 TOV-21G ES-2 | ° An anti-tumour effect of PAM on acquired chemo-resistant OC cells ° An anti-tumour effect of aqueous plasma against clear-cell carcinoma, which is natively chemo-refractory OC ° PAM has a selective cytotoxic effect on OC cells | [77] |
| Ovary | SKOV3 HRA | ° Effective killing of ovarian cancer cells lines by the plasma, while plasma-treated fibroblast cells were not damaged ° Plasma treatment induces apoptosis ° The exposure time of treatment affects the proliferation rate | [78] |
| Ovary | OVCAR-3 NOS2 TOV21G ES-2 | ° Negative impact of cell density on PAM-induced proliferation inhibition rate ° Selective, cell line dependent sensitivity to PAM ° Dependence of PAM effect on the proportion of ROS and the cell number ° Sensitivity to PAM affected by morphological characteristics of the cells ° TGF-β induced epithelial-mesenchymal morphological transition sensitised cancer cells to PAM | [11] |
| Ovary | ES2 SKOV3 WI-38 HPMCs | ° Inhibition of cell viability of ovarian cancer cells depends on the cell type, cell number, and plasma-activated medium (PAM) dilution ratio ° PAM mediated suppression of cell migration, invasion, and adhesion ° PAM-induced down-regulation of matrix metalloproteinase-9 (MMP-9) prevents cell plantation in co-culture with human peritoneal mesothelial cells ° Inhibition of anti-metastatic effect of PAM by the ROS scavenger | [157] |
| Breast | MCF-7 | ° CAP inhibitory effect on the cell proliferation is mediated by miR-19a-3p (miR-19a, oncomiR) ° CAP induces hypermethylation at the promoter CpG sites and subsequent downregulation of miR-19a ° CAP recovers production of ABCA1 and PTEN which are targets of miR-19a | [38] |
| Breast | MCF-7 MCF-7/TamR | ° CAP induces restoration of sensitivity to tamoxifen (Tam) in Tam-resistant cells ° Increase of ROS levels in CAP-treated cells ° Inhibition of the proliferation and promotion of the apoptosis in MCF-7/TamR ° Oppositely altered expression of 20 genes involved in Tam resistance in TamR cells and CAP-treated TamR cells ° MX1 and HOXC6 mediated the restoration of sensitivity against Tam | [39] |
| Breast | MSC MDA-MB-231 | ° Synergistic inhibition of breast cancer cell growth after treatment with the combination of CAP and drug (5FU) loaded core-shell nanoparticles ° Induction of down-regulation of metastasis-related genes (VEGF, MTDH, MMP9, and MMP2) ° Facilitation of the uptake of drug-loaded nanoparticles | [40] |
| Breast | MCF7 MCF10A MTT | ° Reduction of the viability of breast cancer cells ° Significantly lower CAP-induced damage on normal cells ° Enhanced reduction of cancer cells viability after addition of 5% oxygen to the helium plasma | [41] |
| Breast | metastatic BrCa cells MSC | ° CAP-induced selective ablation of metastatic BrCa cells in vitro without damaging healthy MSC ° Inhibition of the migration and invasion of BrCa cells after CAP treatment ° Different BrCa cell and MSC responses under varied CAP conditions | [42] |
| Breast | MCF-7 | ° Induction of apoptosis in cultured human breast cancer cells ° Significant portion of CAP-treated cells exhibits apoptotic fragmentation, with only limited necrosis | [43] |
| Breast | MDA-MB-231 MCF-7 HMEC | ° ROS in a liquid phase is generated via plasma irradiation of gas, producing the reactive species (electrons, ions, and radicals) and these species dissolve into the liquid phase and/or react with water ° Irradiation time, distance to the liquid surface and voltage affects OH radical generation in the extracellular culture medium | [44] |
| Breast | MDAMB231 MDAMB468 MCF7 MCF10A | ° Induction of apoptosis, inhibition of the proliferation and migration of triple-negative breast cancers (TNBC) after PAM treatment ° Significant increase of H2O2 concentration in the media after CAP treatment ° PAM selectively inhibits the activity of JNK and NF-κB in TNBC cells | [55] |
| Breast | 4T1 | ° Inhibition of cell migration after both plasma and doxorubicin treatment, assessed by wound healing assay | [56] |
| Breast | MCF-7 MCF-7/TxR | ° Restoration of sensitivity to paclitaxel in resistant cells ° Identification of altered expression of multiple drug resistance-related genes ° DAGLA and CEACAM1 were essential for the acquisition of resistance and the recovery of sensitivity | [158] |
| Anti-Cancer Potential of CAP | Cancer Types | Study Details | Reference | |
|---|---|---|---|---|
| Direct anti-tumour effects of CAP | Melanoma cells (Mel Im and Mel Juso) | → calcium influx → senescence | [264] | |
| ↑ acidification: → anti-cancer efficacy | [30] | |||
| Melanoma cell A375 and A875 | → apoptosis (Sestrin2-mediated nitric oxide synthase signalling) | [151] | ||
| Breast cancer cells MCF-7 | Opposite regulation of ZNRD1 and its lncRNA | [265] | ||
| Ovarian cancer cells | ↓ growth and mobility | [73] | ||
| Lung cancer cells A549 | Atmospheric pressure plasma irradiation: 8-oxoguanine formation DNA strand breaks | [247] | ||
| Indirect anti-tumour effects of CAP (PAM) | Breast cancer cells SKBR3 | O3 formation | [266] | |
| Triple negative breast cancer cells MDAMB231, MDAMB468 and Balb/c mice transplanted with MDAMB231 cells | → apoptosis ↓ proliferation, migration | [55] | ||
| Ovarian cancer cells ES2 and Balb/c mice injected with ES2 | ↓ migration, invasion, adhesion ↓ metastatic potential ↓ MMP9 ↓ MAPK activation ↓ phosphorylation of JNK1/2 and p38 MAPK | [157] | ||
| Gastric cancer cells SC-2-NU, AGS, GCIY-EGFP and peritoneal dissemination mouse model using GCIY-EGFP gastric cancer cells | ↓ migration, adhesion ↓ peritoneal metastatic modules | [267] | ||
| Synergy of CAP and nanotechnology | CAP + iron oxide-based magnetic NPs | Lung cancer cells A549 and Balb/c mice injected with A549 cells | ↓ proliferation, viability → apoptosis ↓ xenograft tumours | [91] |
| CAP + core-shell NPs | Breast cancer cells MDA-MB-231 | ↓ growth ↓ metastasis-related genes (VEGF. MTDH, MMP9, MMP2) → drug loaded NP uptake | [40] | |
| CAP + silymarin nanoemulsion | Melanoma cells | → autophagy PI3K/mTOR and EGFR activation Modulation of transcription factors (ZKSCAN3, TFEB, FOXO1, CRTC2, and CREBBP) and autophagy-related genes (BECN-1, AMBRA-1, MAP1LC3A, and SQSTM) | [268] | |
| CAP + PEG-coated gold NPs | Glioblastoma T98G and lung adenocarcinoma A549 and Balb/c female nude mice injected with glioma U87MG cells | PI3K/AKT blockage EMT reversion: ↑ E-cadherin ↓ N-cadherin, Slug, Zeb-1 | [269] | |
| CAP + gold NPs | Colon cancer cells HCT-116 | ↓ cell deaths | [270] | |
| CAP + platinum NPs | Human lymphoma U937 cells | Attenuated CAP-induced ROS-mediated apoptosis | [271] | |
| CAP + gold NPs | Glioblastoma multiforme U373MG cells | → clathrin-dependent endocytosis to repair oxidised membrane → uptake of nanomaterial | [48] | |
| CAP + gold NPs | Glioblastoma multiforme U373MG cells | Activation of NPs toxicity ↑ endocytosis ↑ trafficking to lysosomes | [272] | |
| CAP + paclitaxel-loaded core-shell magnetic NPs | Non-small cell lung cancer cells A549 | ↓ growth | [273] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubor, P.; Wang, Y.; Liskova, A.; Samec, M.; Koklesova, L.; Dankova, Z.; Dørum, A.; Kajo, K.; Dvorska, D.; Lucansky, V.; et al. Cold Atmospheric Pressure Plasma (CAP) as a New Tool for the Management of Vulva Cancer and Vulvar Premalignant Lesions in Gynaecological Oncology. Int. J. Mol. Sci. 2020, 21, 7988. https://doi.org/10.3390/ijms21217988
Zubor P, Wang Y, Liskova A, Samec M, Koklesova L, Dankova Z, Dørum A, Kajo K, Dvorska D, Lucansky V, et al. Cold Atmospheric Pressure Plasma (CAP) as a New Tool for the Management of Vulva Cancer and Vulvar Premalignant Lesions in Gynaecological Oncology. International Journal of Molecular Sciences. 2020; 21(21):7988. https://doi.org/10.3390/ijms21217988
Chicago/Turabian StyleZubor, Pavol, Yun Wang, Alena Liskova, Marek Samec, Lenka Koklesova, Zuzana Dankova, Anne Dørum, Karol Kajo, Dana Dvorska, Vincent Lucansky, and et al. 2020. "Cold Atmospheric Pressure Plasma (CAP) as a New Tool for the Management of Vulva Cancer and Vulvar Premalignant Lesions in Gynaecological Oncology" International Journal of Molecular Sciences 21, no. 21: 7988. https://doi.org/10.3390/ijms21217988
APA StyleZubor, P., Wang, Y., Liskova, A., Samec, M., Koklesova, L., Dankova, Z., Dørum, A., Kajo, K., Dvorska, D., Lucansky, V., Malicherova, B., Kasubova, I., Bujnak, J., Mlyncek, M., Dussan, C. A., Kubatka, P., Büsselberg, D., & Golubnitschaja, O. (2020). Cold Atmospheric Pressure Plasma (CAP) as a New Tool for the Management of Vulva Cancer and Vulvar Premalignant Lesions in Gynaecological Oncology. International Journal of Molecular Sciences, 21(21), 7988. https://doi.org/10.3390/ijms21217988







