Survival of Lung Cancer Patients Dependent on the LOH Status for DMP1, ARF, and p53
Abstract
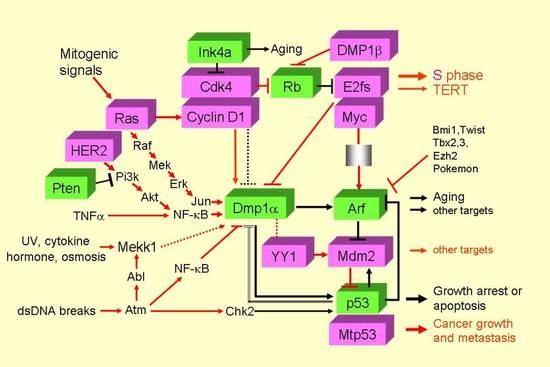
:1. Introduction
2. Results
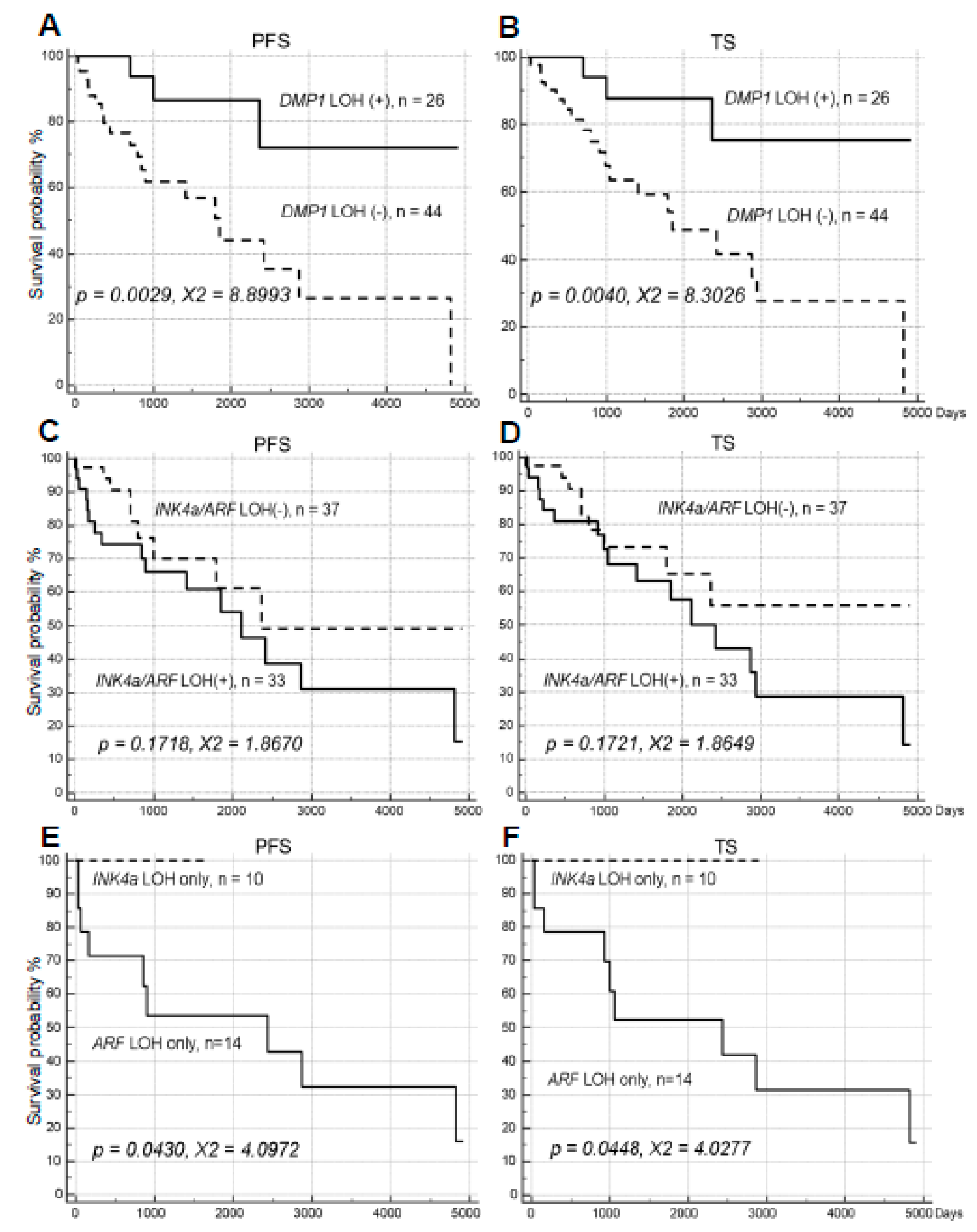
2.1. Impacts of LOH for hDMP1, ARF/INK4a, and p53 on NSCLC Survival
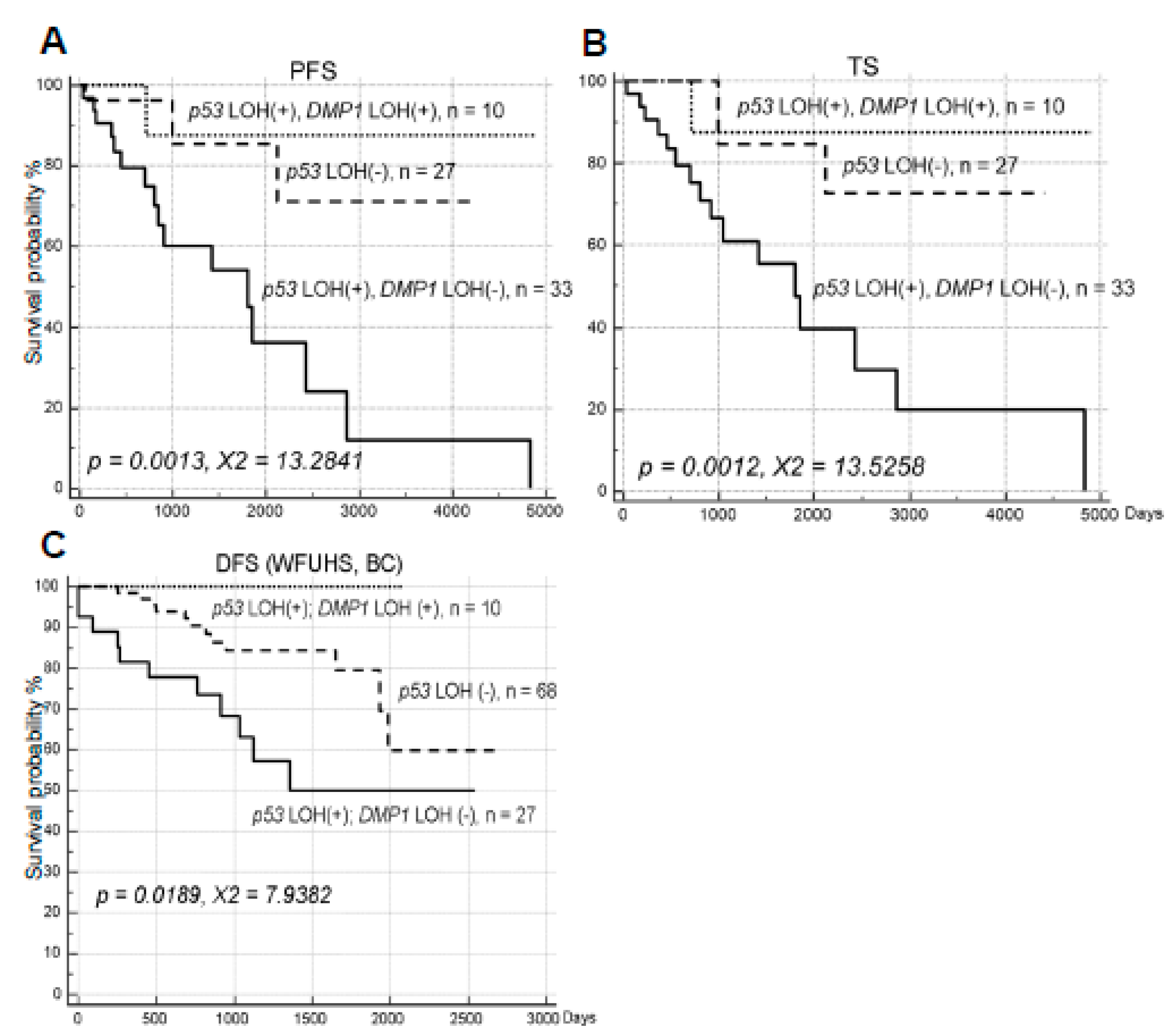
2.2. The Impact of hDMP1 LOH on NSCLC Survival with p53 LOH
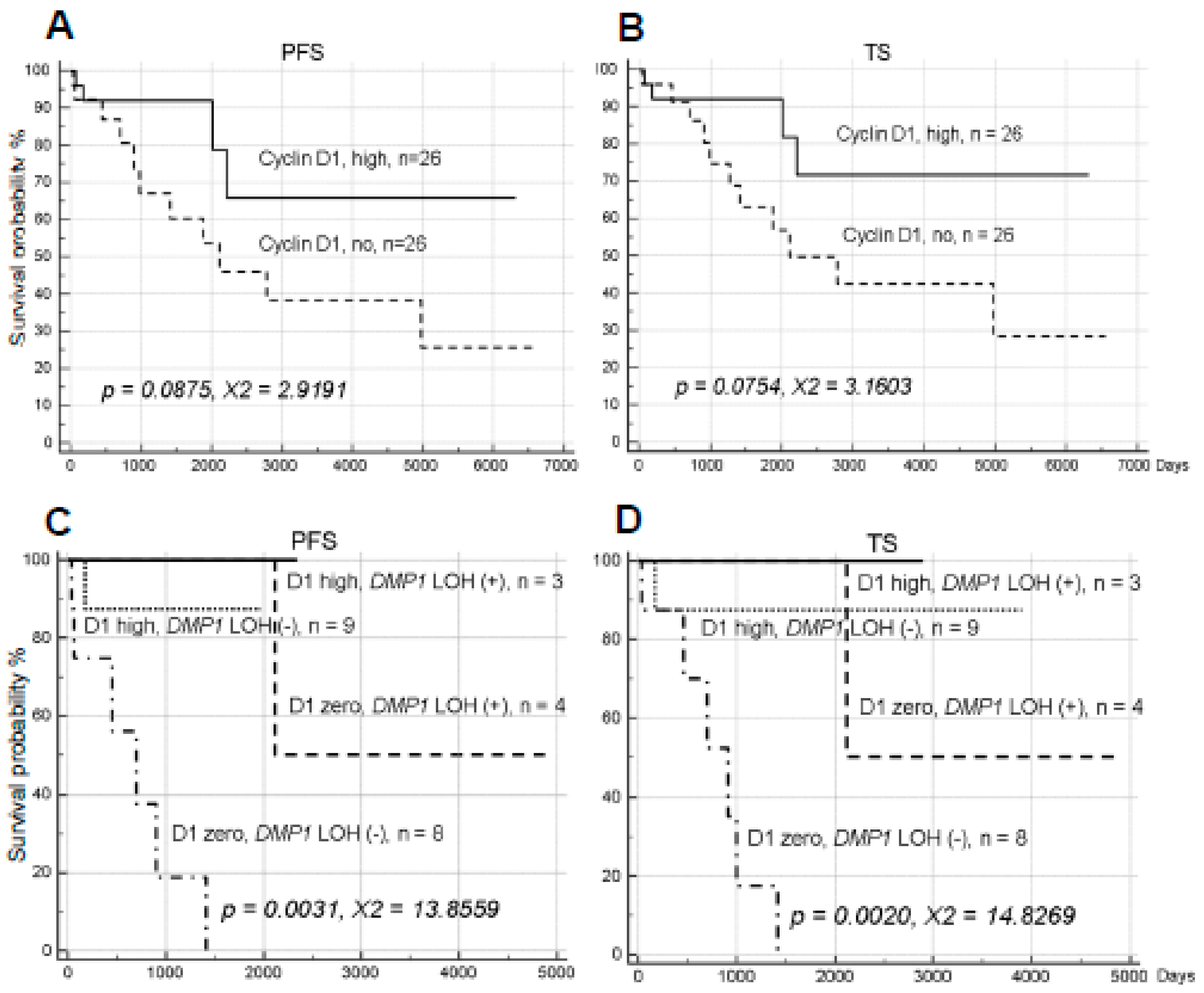
2.3. Cyclin D1 Overexpression and YY1 Amplification and NSCLC Survival
3. Discussion
4. Materials and Methods
4.1. Human Lung Cancer Samples
4.2. Loss of Heterozygosity (LOH) and Sequencing Analyses of Human Lung Cancer Specimen
4.3. Statistical Analyses for Mutual Exclusiveness of LOH
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Oser, M.G.; Niederst, M.J.; Sequist, L.V.; Engelman, J.A. Transformation from non-small-cell lung cancer to small-cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol. 2015, 16, e165–e172. [Google Scholar] [CrossRef] [Green Version]
- Satoh, Y.; Matsuo, Y.; Kuba, T.; Yamashita, K.; Sawano, M.; Tozaka, S.; Yamazaki, H.; Sonoda, D.; Mikubo, M.; Naito, M.; et al. EGFR mutation genotyping and ALK status determination in liquid-based cytology samples of non-small cell lung cancer. Virchows Arch. 2020, 476, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Di Maio, M.; Chiodini, P.; Rudd, R.M.; Okamoto, H.; Skarlos, D.V.; Fruh, M.; Qian, W.; Tamura, T.; Samantas, E.; et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: The COCIS meta-analysis of individual patient data. J. Clin. Oncol. 2012, 30, 1692. [Google Scholar] [CrossRef] [PubMed]
- Quelle, D.E.; Zindy, F.; Ashmun, R.A.; Sherr, C.J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell 1995, 83, 993–1000. [Google Scholar] [PubMed] [Green Version]
- Maggi, L.B., Jr.; Winkeler, C.L.; Miceli, A.P.; Apicelli, A.J.; Brady, S.N.; Kuchenreuther, M.J.; Weber, J.D. ARF tumor suppression in the nucleolus. Biochim. Biophys. Acta 2014, 1842, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Basu, S.; Murphy, E. Genetic Modifiers of the p53 Pathway. Cold Spring Harb. Perspect. Med. 2016, 6, a026302. [Google Scholar] [CrossRef]
- Carrasco-Garcia, E.; Moreno, M.; Moreno-Cugnon, L.; Mathew, A. Increased Arf/p53 activity in stem cells, aging and cancer. Aging Cell 2017, 16, 219–225. [Google Scholar] [CrossRef]
- Inoue, K.; Fry, E.A. Aberrant expression of p16INK4a in human cancer—A new biomarker? Cancer Rep. Rev. 2018, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Fry, E.A. Overexpression of ARF in human cancer—A new biomarker? Tumor Microenviron. 2018, 1, 37–44. [Google Scholar] [CrossRef]
- Palmero, I.; Pantoja, C.; Serrano, M. p19ARF links the tumour suppressor p53 to ras. Nature 1998, 395, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Zindy, F.; Eischen, C.M.; Randle, D.H.; Kamijo, T.; Cleveland, J.L.; Sherr, C.J.; Roussel, M.F. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998, 12, 2424–2433. [Google Scholar] [CrossRef] [Green Version]
- Bates, S.; Phillips, A.C.; Clarke, P.A.; Stott, F.; Peters, G.; Ludwig, R.L.; Vousden, K.H. p14ARF links tumor suppressors RB and p53. Nature 1998, 395, 124–125. [Google Scholar] [CrossRef]
- Taneja, P.; Maglic, D.; Kai, F.; Sugiyama, T.; Kendig, R.D.; Frazier, D.P.; Willingham, M.C.; Inoue, K. Critical role of Dmp1 in HER2/neu-p53 signaling and breast carcinogenesis. Cancer Res. 2010, 70, 9084–9094. [Google Scholar] [CrossRef] [Green Version]
- Sherr, C.J. Autophagy by ARF: A short story. Mol. Cell 2006, 22, 436–437. [Google Scholar] [CrossRef] [PubMed]
- Quelle, D.E.; Cheng, M.; Ashmun, R.A.; Sherr, C.J. Cancer-associated mutations at the INK4a locus cancel cell cycle arrest by p16INK4a but not by the alternative reading frame protein p19ARF. Proc. Natl. Acad. Sci. USA 1997, 94, 669–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krimpenfort, P.; Quon, K.C.; Mooi, W.J.; Loonstra, A.; Berns, A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 2001, 413, 83–86. [Google Scholar] [CrossRef]
- Krimpenfort, P.; Ijpenberg, A.; Song, J.Y.; van der Valk, M.; Nawijn, M.; Zevenhoven, J.; Berns, A. p15Ink4b is a critical tumour suppressor in the absence of p16Ink4a. Nature 2007, 448, 943–946. [Google Scholar] [CrossRef]
- Kamijo, T.; Zindy, F.; Roussel, M.F.; Quelle, D.E.; Downing, J.R.; Ashmun, R.A.; Grosveld, G.; Sherr, C.J. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 1997, 91, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Sharpless, N.E.; Bardeesy, N.; Lee, K.H.; Carrasco, D.; Castrillon, D.H.; Aguirre, A.J.; Wu, E.A.; Horner, J.W.; DePinho, R.A. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 2001, 413, 86–91. [Google Scholar] [CrossRef]
- Ruas, M.; Peters, G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta 1998, 1378, F115–F177. [Google Scholar] [CrossRef]
- Gil, J.; Peters, G. Regulation of the INK4b-ARF-INK4a tumour suppressor locus: All for one or one for all. Nat. Rev. Mol. Cell Biol. 2006, 7, 667–677. [Google Scholar] [CrossRef]
- Kuo, M.L.; den Besten, W.; Bertwistle, D.; Roussel, M.F.; Sherr, C.J. N-terminal polyubiquitination and degradation of the Arf tumor suppressor. Genes Dev. 2004, 18, 1862–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stott, F.J.; Bates, S.; James, M.C.; McConnell, B.B.; Starborg, M.; Brookes, S.; Palmero, I.; Ryan, K.; Hara, E.; Vousden, K.H.; et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998, 17, 5001–5014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, J.D.; Taylor, L.J.; Roussel, M.F.; Sherr, C.J.; Bar-Sagi, D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat. Cell Biol. 1999, 1, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Hamilton, T.; Silver, D.P.; Sharpless, N.E.; Bardeesy, N.; Rokas, M.; DePinho, R.A.; Livingston, D.M.; Grossman, S.R. p19ARF targets certain E2F species for degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 4455–4460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itahana, K.; Zhang, Y. Mitochondrial p32 is a critical mediator of ARF-induced apoptosis. Cancer Cell 2008, 13, 542–553. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Tavana, O.; Chu, B.; Erber, L.; Chen, Y.; Baer, R.; Gu, W. NRF2 is a major target of ARF in p53-independent tumor Suppression. Mol. Cell 2017, 68, 224–332. [Google Scholar] [CrossRef]
- Hirai, H.; Sherr, C.J. Interaction of D-type cyclins with a novel myb-like transcription factor, DMP1. Mol. Cell. Biol. 1996, 16, 6457–6467. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Sherr, C.J. Gene expression and cell cycle arrest mediated by transcription factor DMP1 is antagonized by D-type cyclins through a cyclin-dependent-kinase-independent mechanism. Mol. Cell. Biol. 1998, 18, 1590–1600. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Sherr, C.J.; Shapiro, L.H. Regulation of the CD13/aminopeptidase N gene by DMP1, a transcription factor antagonized by D-type cyclins. J. Biol. Chem. 1998, 273, 29188–29194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, K.; Roussel, M.F.; Sherr, C.J. Induction of ARF tumor suppressor gene expression and cell cycle arrest by transcription factor DMP1. Proc. Natl. Acad. Sci. USA 1999, 96, 3993–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodner, S.M.; Naeve, C.W.; Rakestraw, K.M.; Jones, B.G.; Valentine, V.A.; Valentine, M.B.; Luthardt, F.W.; Willman, C.L.; Raimondi, S.C.; Downing, J.R.; et al. Cloning and chromosomal localization of the gene encoding human cyclin D-binding Myb-like protein (hDMP1). Gene 1999, 229, 223–229. [Google Scholar] [CrossRef]
- Inoue, K.; Wen, R.; Rehg, J.E.; Adachi, M.; Cleveland, J.L.; Roussel, M.F.; Sherr, C.J. Disruption of the ARF transcriptional activator DMP1 facilitates cell immortalization, Ras transformation, and tumorigenesis. Genes Dev. 2000, 14, 1797–1809. [Google Scholar]
- Inoue, K.; Zindy, F.; Randle, D.H.; Rehg, J.E.; Sherr, C.J. Dmp1 is haplo-insufficient for tumor suppression and modifies the frequencies of Arf and p53 mutations in Myc-induced lymphomas. Genes Dev. 2001, 15, 2934–2939. [Google Scholar] [CrossRef] [Green Version]
- Sreeramaneni, R.; Chaudhry, A.; McMahon, M.; Sherr, C.J.; Inoue, K. Ras-Raf-Arf signaling critically depends on Dmp1 transcription factor. Mol. Cell. Biol. 2005, 25, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Mallakin, A.; Taneja, P.; Matise, L.A.; Willingham, M.C.; Inoue, K. Expression of Dmp1 in specific differentiated, nonproliferating cells and its repression by E2Fs. Oncogene 2006, 25, 7703–7713. [Google Scholar] [CrossRef] [Green Version]
- Taneja, P.; Mallakin, A.; Matise, L.A.; Frazier, D.P.; Choudhary, M.; Inoue, K. Repression of Dmp1 and Arf transcription by anthracyclins: Critical roles of the NF-kappaB subunit p65. Oncogene 2007, 26, 7457–7466. [Google Scholar] [CrossRef] [Green Version]
- Mallakin, A.; Sugiyama, T.; Taneja, P.; Matise, L.A.; Frazier, D.P.; Choudhary, M.; Hawkins, G.A.; D’Agostino, R.B., Jr.; Willingham, M.C.; Inoue, K. Mutually exclusive inactivation of DMP1 and ARF/p53 in lung cancer. Cancer Cell 2007, 12, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Mallakin, A.; Sugiyama, T.; Kai, F.; Taneja, P.; Kendig, R.D.; Frazier, D.P.; Maglic, D.; Matise, L.A.; Willingham, M.C.; Inoue, K. The Arf-inducing transcription factor Dmp1 encodes transcriptional activator of amphiregulin, thrombospondin-1, JunB and Egr1. Int. J. Cancer 2010, 126, 1403–1416. [Google Scholar] [CrossRef] [Green Version]
- Frazier, D.P.; Kendig, R.D.; Kai, F.; Maglic, D.; Sugiyama, T.; Morgan, R.L.; Fry, E.A.; Lagedrost, S.J.; Sui, G.; Inoue, K. Dmp1 physically interacts with p53 and positively regulates p53’s stabilization, nuclear localization, and function. Cancer Res. 2012, 72, 1740–1750. [Google Scholar] [CrossRef] [Green Version]
- Maglic, D.; Zhu, S.; Taneja, P.; Fry, E.A.; Kai, F.; Kendig, R.D.; Sugiyama, T.; Miller, L.D.; Willingham, M.C.; Inoue, K. Prognostic value of the hDMP1-ARF-Hdm2-p53 pathway in breast cancer. Oncogene 2013, 32, 4120–4129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.; Mott, R.T.; Fry, E.A.; Taneja, P.; Kulik, G.; Sui, G.; Inoue, K. Cooperation between cyclin D1 expression and Dmp1-loss in breast cancer. Am. J. Pathol. 2013, 183, 1339–1350. [Google Scholar] [CrossRef] [Green Version]
- Fry, E.A.; Taneja, P.; Maglic, D.; Zhu, S.; Sui, G.; Inoue, K. Dmp1α inhibits HER2/neu-induced mammary tumorigenesis. PLoS ONE 2013, 8, e77870. [Google Scholar] [CrossRef] [Green Version]
- Maglic, D.; Stovall, D.B.; Cline, J.M.; Fry, E.A.; Mallakin, A.; Taneja, P.; Caudell, D.L.; Willingham, M.C.; Sui, G.; Inoue, K. DMP1β, a splice isoform of the tumor suppressor DMP1 locus, induces proliferation and progression of breast cancer. J. Pathol. 2015, 236, 90–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschan, M.P.; Federzoni, E.A.; Haimovici, A.; Britschgi, C.; Moser, B.A.; Jin, J.; Reddy, V.A.; Sheeter, D.A.; Fischer, K.M.; Sun, P.; et al. Human DMTF1β antagonizes DMTF1α regulation of the p14(ARF) tumor suppressor and promotes cellular proliferation. Biochim. Biophys. Acta 2015, 1849, 1198–1208. [Google Scholar] [CrossRef] [Green Version]
- Kendig, R.D.; Kai, F.; Fry, E.A.; Inoue, K. Stabilization of the p53-DNA complex by the nuclear protein Dmp1α. Cancer Investig. 2017, 35, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Fry, E.A.; Frazier, D.P. Transcription factors that interact with p53 and Mdm2. Int. J. Cancer 2016, 138, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Fry, E.A. Aberrant splicing of the DMP1-INK4a/ARF-MDM2-p53 pathway in cancer. Int. J. Cancer 2016, 139, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fry, E.A.; Taneja, P.; Inoue, K. Oncogenic and tumor-suppressive mouse models for breast cancer employing HER2/neu. Int. J. Cancer 2017, 140, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Fry, E.A. Haplo-insufficient tumor suppressor genes. In Advances in Medicine and Biology; Nova Science Publishers, Inc.: Suite N Hauppauge, NY, USA, 2017; Volume 118, Chapter 6. [Google Scholar]
- Qie, S.; Diehl, J.A. Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. (Berl.) 2016, 94, 1313–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, S.C.; Lee, S.S.; Abraham, J. Mechanisms of therapeutic CDK4/6 inhibition in breast cancer. Semin. Oncol. 2017, 44, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.; Affar, B.; Shi, Y.; Brignone, C.; Wall, N.R.; Yin, P.; Donohoe, M.; Luke, M.P.; Calvo, D.; Grossman, S.R.; et al. Yin Yang 1 is a negative regulator of p53. Cell 2004, 117, 859–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Stovall, D.B.; Inoue, K.; Sui, G. The oncogenic role of Yin Yang 1. Crit. Rev. Oncog. 2011, 16, 163–197. [Google Scholar] [CrossRef]
- Kratzke, R.A.; Greatens, T.M.; Rubins, J.B.; Maddaus, M.A.; Niewoehner, D.E.; Niehans, G.A.; Geradts, J. Rb and p16INK4a expression in resected non-small cell lung tumors. Cancer Res. 1996, 56, 3415–3420. [Google Scholar]
- Zhou, J.X.; Niehans, G.A.; Shar, A.; Rubins, J.B.; Frizelle, S.P.; Kratzke, R.A. Mechanisms of G1 checkpoint loss in resected early stage non-small cell lung cancer. Lung Cancer 2001, 32, 27–38. [Google Scholar] [CrossRef]

| Patient ID | hDMP1 | INK4a/ARF | INK4a/ARF | I/A LOH ME | p53 | p53 LOH ME | p53 IHC | p53 IHC ME | ||
|---|---|---|---|---|---|---|---|---|---|---|
| #92465 | #198004 | #33647 | #27251 | #182 SE | #377 SE | |||||
| Group 1 | 5′ | 3′ | 5′ | 3′ | 5′ | 3′ | ||||
| 307 | 0.68 | 0.26 | 0.15 | 0.98 | NME | 2.01 | 2.04 | NME | ND | |
| 308 | 0.70 | 1.04 | 3.38 | 0.41 | ME | Deletion | 27.20 | ME | ND | |
| 309 | 1.21 | 2.51 | 1.42 | 1.11 | ME | 1.06 | 0.54 | ME | ND | |
| 310 | 0.06 | 4.31 | 0.68 | 0.93 | ME | 1.06 | 0.92 | ME | ND | |
| 311 | 1.05 | 1.08 | 1.31 | 1.26 | None | 1.50 | 0.84 | None | ND | |
| 313 | 0.94 | 0.47 | 1.46 | 1.32 | ME | 1.23 | 0.96 | ME | ND | |
| 314 | 0.46 | 6.09 | 2.41 | 0.63 | NME | 0.43 | 0.30 | NME | ND | |
| 315 | 1.09 | 1.87 | 1.12 | 0.98 | None | 9.97 | 0.28 | ME | ND | |
| 317 | 0.61 | 2.26 | 1.56 | 0.91 | ME | Deletion | 1.40 | NME | ND | |
| 318 | 1.23 | 0.90 | 5.48 | 2.21 | ME | 0.42 | 0.38 | ME | ND | |
| 319 | 1.86 | 2.29 | 1.30 | 0.82 | ME | 2.16 | 3.57 | NME | ND | |
| 321 | 0.77 | 0.29 | 0.69 | 1.03 | ME | 1.26 | 1.74 | None | ND | |
| 322 | 1.12 | 2.01 | 0.97 | 0.93 | ME | 1.16 | 0.07 | NME | ND | |
| 323 | 0.86 | 0.52 | 0.75 | 1.06 | None | 1.08 | 1.56 | None | ND | |
| 324 | 0.64 | 2.96 | 0.77 | 0.16 | NME | 2.31 | 0.31 | NME | ND | |
| 325 | 1.10 | 1.51 | 2.13 | 0.45 | ME | 0.34 | 0.29 | ME | ND | |
| 326 | 1.00 | 0.59 | 0.28 | 0.60 | ME | 2.37 | 0.62 | ME | ND | |
| 327 | 1.32 | >10.0 | 1.04 | 1.03 | ME | 1.80 | No deletion | ME | ND | |
| 328 | 2.33 | 4.97 | 1.53 | 1.34 | ME | No deletion | No deletion | ME | 2 | NME |
| 329 | 0.74 | 0.80 | 1.02 | 2.69 | ME | 0.25 | 0.9 | ME | 2 | ME |
| 330 | 0.87 | 0.72 | 0.96 | 0.64 | None | 0.88 | 13.97 | ME | 1 | None |
| 331 | 1.11 | 1.08 | 0.10 | 4.66 | ME | 1.34 | 0.48 | ME | 0 | None |
| 332 | 0.82 | 0.52 | 0.68 | 0.74 | None | 0.97 | 0.40 | ME | 2 | ME |
| 333 | 1.15 | 0.48 | 0.89 | 1.06 | ME | 1.89 | 1.09 | ME | 1 | ME |
| 277 | 0.41 | 0.41 | 1.19 | 1.02 | ME | 0.61 | No deletion | ME | 1 | ME |
| 295 | 0.74 | 0.13 | 1.24 | 1.01 | ME | 0.99 | 1.02 | ME | 1 | ME |
| 299 | 0.32 | 2.11 | 1.18 | 0.79 | ME | 0.87 | 0.51 | ME | 1 | ME |
| 338 | 1.73 | 1.27 | 1.54 | 0.04 | ME | 13.1 | 4.06 | ME | 0 | None |
| 349 | 2.95 | 20.00 | 0.93 | 1.21 | ME | 1.73 | 1.62 | ME | 1 | ME |
| 351 | 1.69 | 0.41 | No deletion | No deletion | ME | 0.83 | 1.08 | ME | 1 | ME |
| 379 | 0.98 | 0.18 | 0.76 | 0.64 | ME | 0.92 | 0.94 | ME | 2 | NME |
| 380 | 0.33 | 0.33 | 0.67 | 0.80 | ME | 0.55 | 1.82 | ME | ND | |
| 271 | 0.66 | 0.59 | 0.49 | 0.91 | ME | 0.65 | 3.39 | ME | 2 | ME |
| 272 | 0.87 | 0.92 | 0.78 | 1.93 | None | 0.92 | 0.82 | None | 0 | None |
| 273 | 0.57 | 0.41 | 0.32 | 0.83 | NME | 0.23 | Deletion | NME | 2 | NME |
| 274 | 1.02 | 0.87 | 0.70 | 0.95 | None | 0.69 | 0.17 | ME | 1 | None |
| 275 | 0.55 | 0.90 | 0.18 | 1.04 | ME | 0.35 | Deletion | ME | 2 | ME |
| 278 | 0.95 | No deletion | 0.76 | 3.11 | ME | 0.56 | 14.00 | ME | 2 | ME |
| 282 | 1.12 | 1.02 | 1.03 | 0.95 | None | 0.93 | 4.01 | ME | 2 | ME |
| 284 | 1.02 | 1.02 | 0.35 | 0.92 | ME | 2.27 | 10.40 | ME | 0 | None |
| 290 | 1.03 | 0.92 | 2.48 | 1.03 | ME | 0.96 | 0.92 | None | 2 | ME |
| 293 | 0.94 | 0.99 | 0.60 | 0.73 | None | 1.86 | 0.47 | ME | 0 | None |
| Group 2 | ||||||||||
| 269 | 0.74 | 0.74 | 0.76 | 0.15 | ME | 0.71 | 0.33 | ME | 2 | ME |
| 270 | 1.08 | 1.16 | 1.10 | 0.99 | None | 0.73 | 1.06 | None | 2 | ME |
| 276 | 0.85 | 1.07 | 4.00 | 1.41 | ME | 0.15 | 3.08 | ME | 2 | ME |
| 300 | 1.49 | 0.96 | 1.30 | 0.66 | None | 2.23 | 0.57 | ME | 2 | ME |
| 334 | 0.80 | 0.61 | 0.35 | 0.94 | ME | 1.05 | 4.73 | ME | 2 | ME |
| 337 | 1.06 | 0.81 | 1.09 | 0.88 | None | 1.66 | 2.07 | ME | 2 | ME |
| 339 | 0.66 | 1.75 | 1.27 | 0.07 | ME | 0.93 | 0.95 | None | 2 | ME |
| 340 | 3.90 | 0.91 | 1.30 | 1.25 | ME | 1.71 | No deletion | ME | 1 | ME |
| 353 | 4.28 | 0.37 | 1.20 | 0.59 | ME | 3.62 | No deletion | NME | 1 | ME |
| 356 | 1.68 | 1.03 | 1.65 | 0.59 | None | No deletion | No deletion | None | 0 | None |
| 357 | 1.17 | 1.26 | 1.88 | 0.18 | ME | 2.59 | 2.55 | ME | 1 | None |
| 358 | 1.07 | 1.23 | 2.91 | 1.45 | ME | 2.01 | 0.65 | ME | 1 | None |
| 360 | 0.95 | 1.02 | 0.25 | 0.41 | ME | 0.45 | 3.11 | ME | 2 | ME |
| 362 | 1.64 | No deletion | 1.33 | 1.03 | None | 0.88 | 0.22 | ME | 2 | ME |
| 363 | 0.84 | 0.88 | 0.27 | 1.22 | ME | 0.35 | 0.18 | ME | 1 | None |
| 364 | 1.04 | 1.07 | 0.11 | 0.06 | ME | 0.75 | 0.16 | ME | 0 | None |
| 365 | No deletion | 0.93 | 1.29 | 0.95 | None | 0.75 | 0.93 | None | 1 | None |
| 366 | 0.87 | 1.26 | 0.44 | 0.99 | ME | 1.04 | 0.98 | None | 2 | ME |
| 367 | 0.92 | 1.04 | 0.78 | 2.15 | ME | 0.70 | 1.20 | None | 0 | None |
| 368 | 0.97 | 0.48 | 0.35 | 0.27 | NME | 1.52 | 0.91 | ME | 1 | ME |
| 369 | 1.49 | 1.06 | 1.40 | 1.27 | None | 3.42 | No deletion | ME | 0 | None |
| 370 | 0.55 | 1.11 | 1.76 | 0.26 | ME | 0.47 | No deletion | ME | 2 | ME |
| 371 | 1.49 | 0.56 | 8.11 | 2.46 | ME | 0.71 | 0.25 | ME | 0 | None |
| 372 | 2.50 | 1.72 | 0.51 | 0.57 | ME | 0.27 | No deletion | NME | 2 | NME |
| 381 | 1.30 | 0.99 | 1.02 | 1.19 | None | 0.55 | 0.49 | ME | 0 | None |
| 384 | 1.63 | 0.80 | 2.53 | 0.48 | ME | 1.54 | 1.38 | None | 2 | ME |
| 387 | 1.23 | 0.26 | 0.39 | 1.99 | NME | 2.04 | 1.80 | NME | 0 | ME |
| 389 | 1.14 | 1.23 | 1.47 | 0.17 | ME | 1.16 | 0.42 | ME | 1 | None |
| Percentage | 26/70 = 37.1% | 33/70 = 47.1% | 47/53 = 88.7% | 43/70 = 61.4% | 48/58 = 82.8% | 29/33 = 87.9% | ||||
| p = 0.0023 | p = 0.0128 | |||||||||
| X2 = 9.330 | X2 = 6.195 | |||||||||
| 95% CI | 80.1–97.2% | 95% CI | 75.6–93.9% | 76.7–99.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fry, E.A.; Niehans, G.E.; Kratzke, R.A.; Kai, F.; Inoue, K. Survival of Lung Cancer Patients Dependent on the LOH Status for DMP1, ARF, and p53. Int. J. Mol. Sci. 2020, 21, 7971. https://doi.org/10.3390/ijms21217971
Fry EA, Niehans GE, Kratzke RA, Kai F, Inoue K. Survival of Lung Cancer Patients Dependent on the LOH Status for DMP1, ARF, and p53. International Journal of Molecular Sciences. 2020; 21(21):7971. https://doi.org/10.3390/ijms21217971
Chicago/Turabian StyleFry, Elizabeth A., Gloria E. Niehans, Robert A. Kratzke, Fumitake Kai, and Kazushi Inoue. 2020. "Survival of Lung Cancer Patients Dependent on the LOH Status for DMP1, ARF, and p53" International Journal of Molecular Sciences 21, no. 21: 7971. https://doi.org/10.3390/ijms21217971