Hevein-Like Antimicrobial Peptides Wamps: Structure–Function Relationship in Antifungal Activity and Sensitization of Plant Pathogenic Fungi to Tebuconazole by WAMP-2-Derived Peptides
Abstract
:1. Introduction
2. Results
2.1. Recombinant Production and Physicochemical Properties of WAMP Homologues
2.2. Antifungal Activity of WAMP Homologues
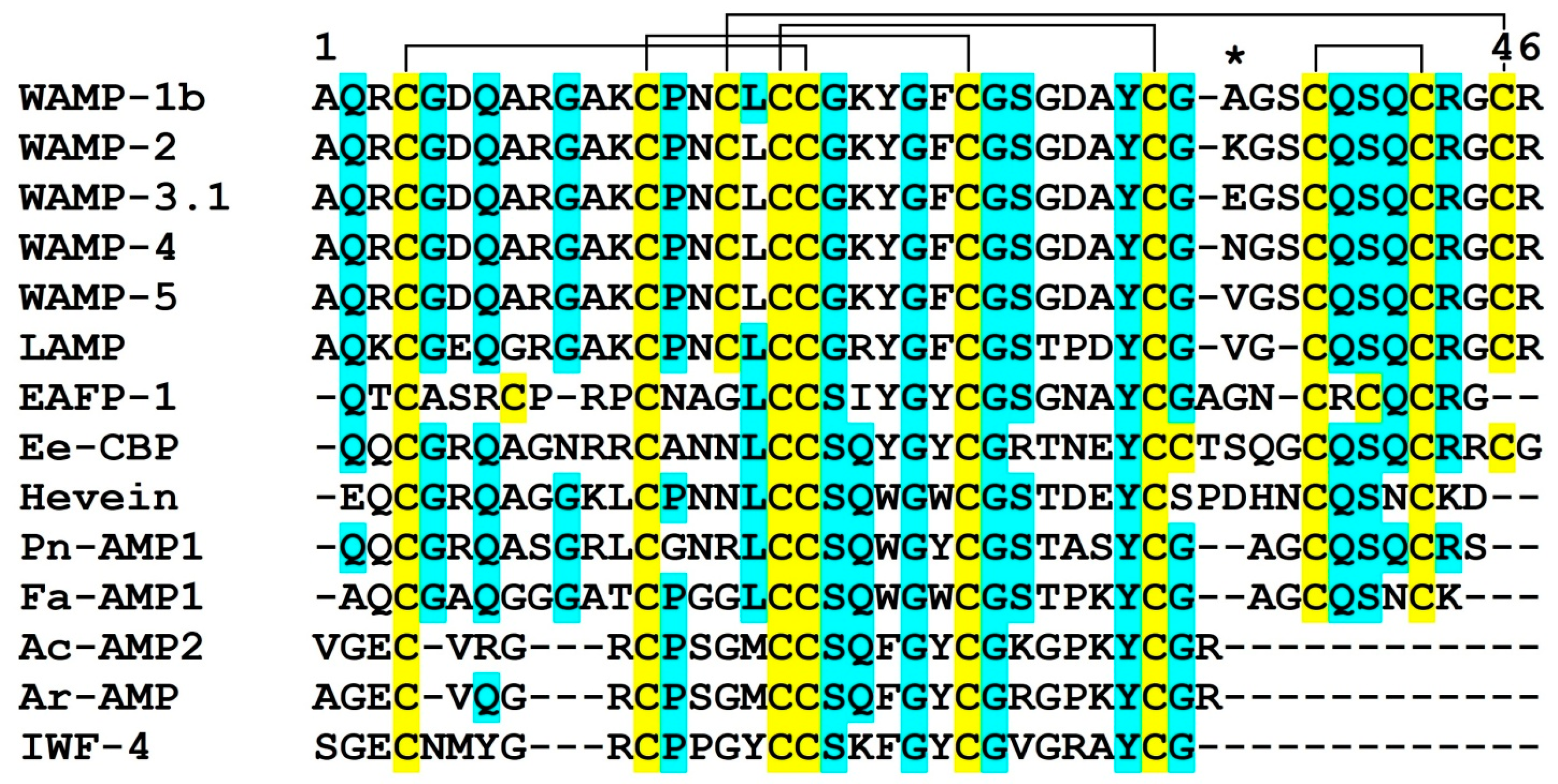
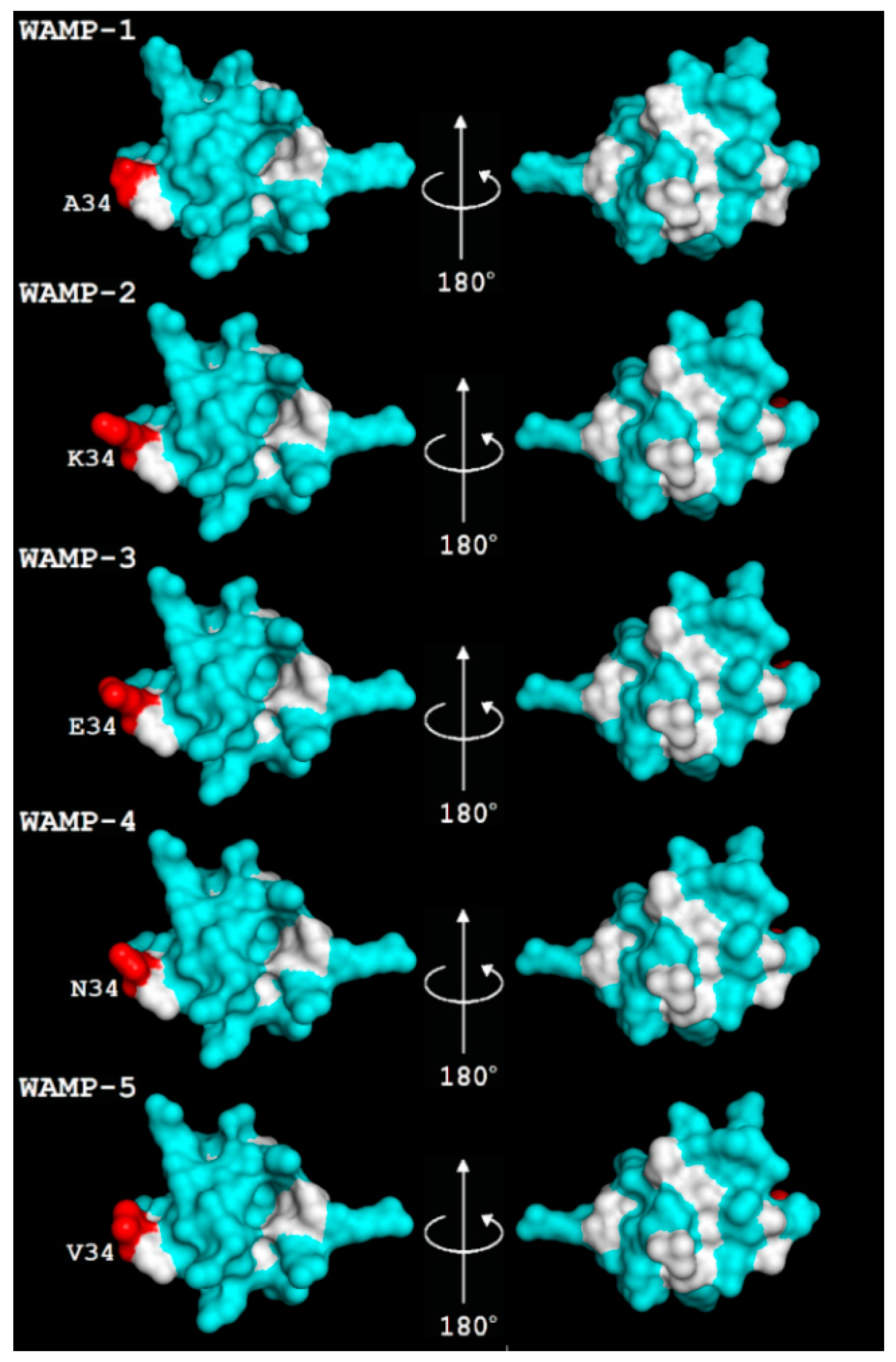
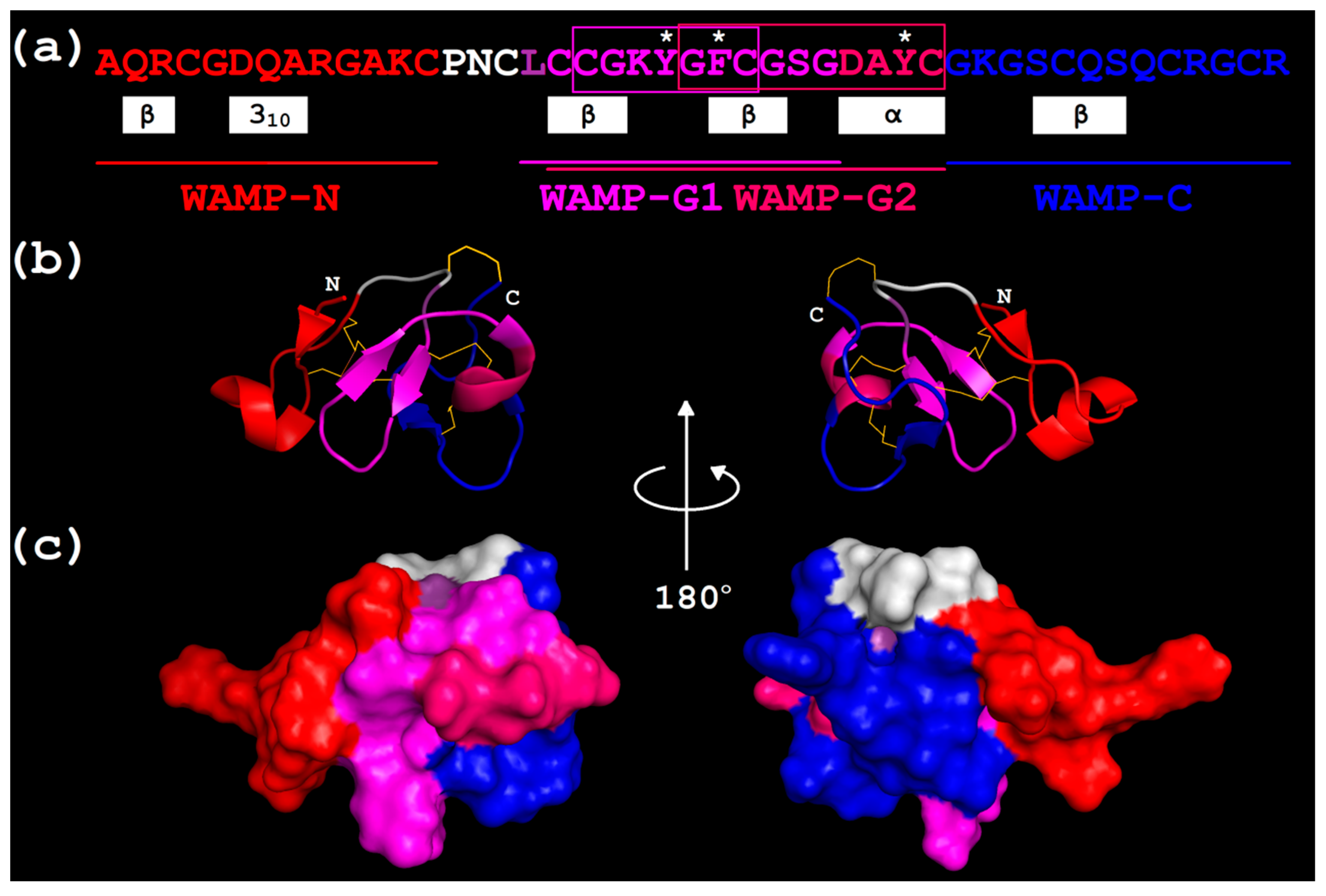
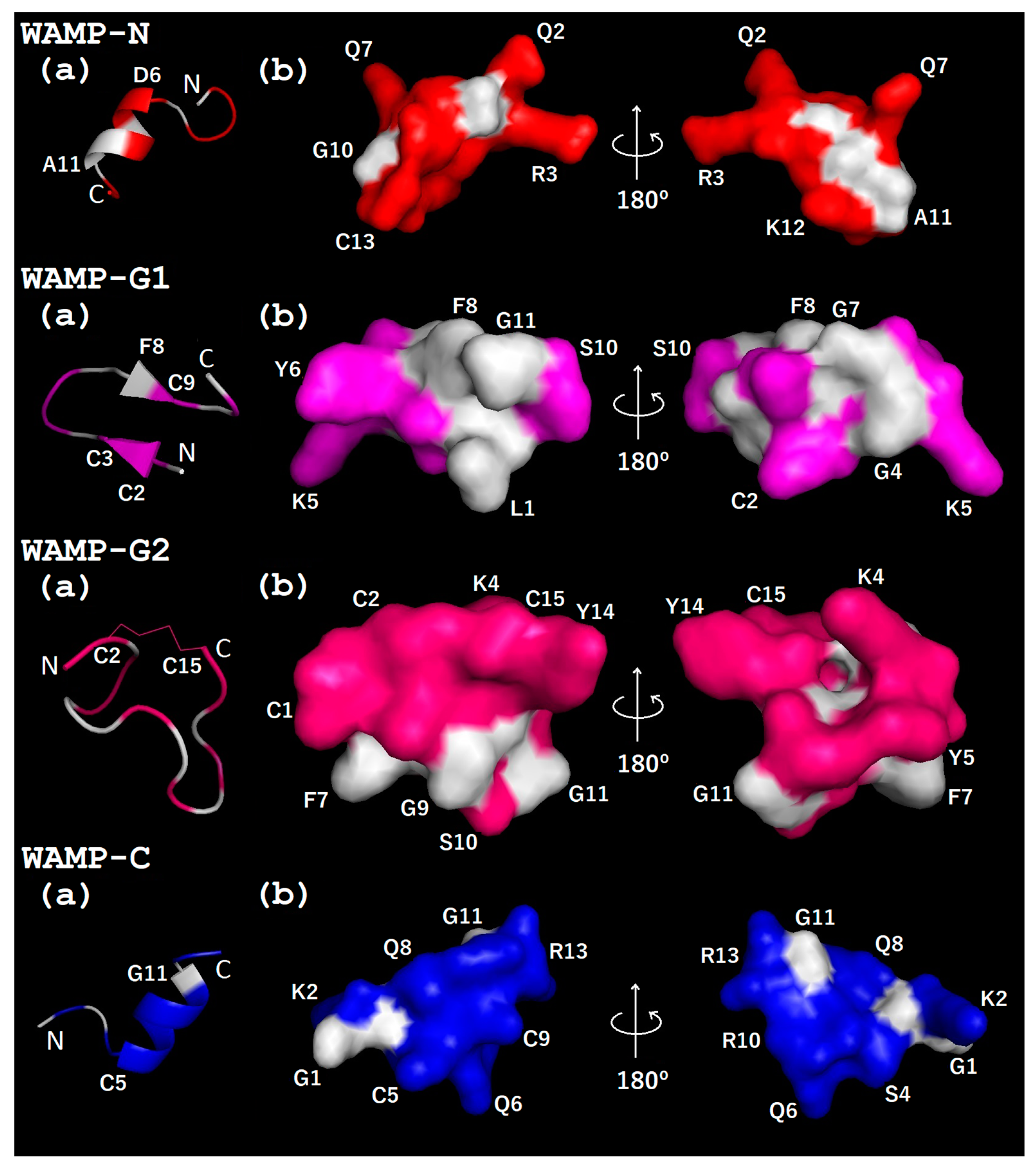
2.3. WAMP-2-Derived Peptides
2.3.1. Design
2.3.2. Physicochemical Characteristics
2.3.3. Three-Dimensional Structures of WAMP-2-Derived Peptides
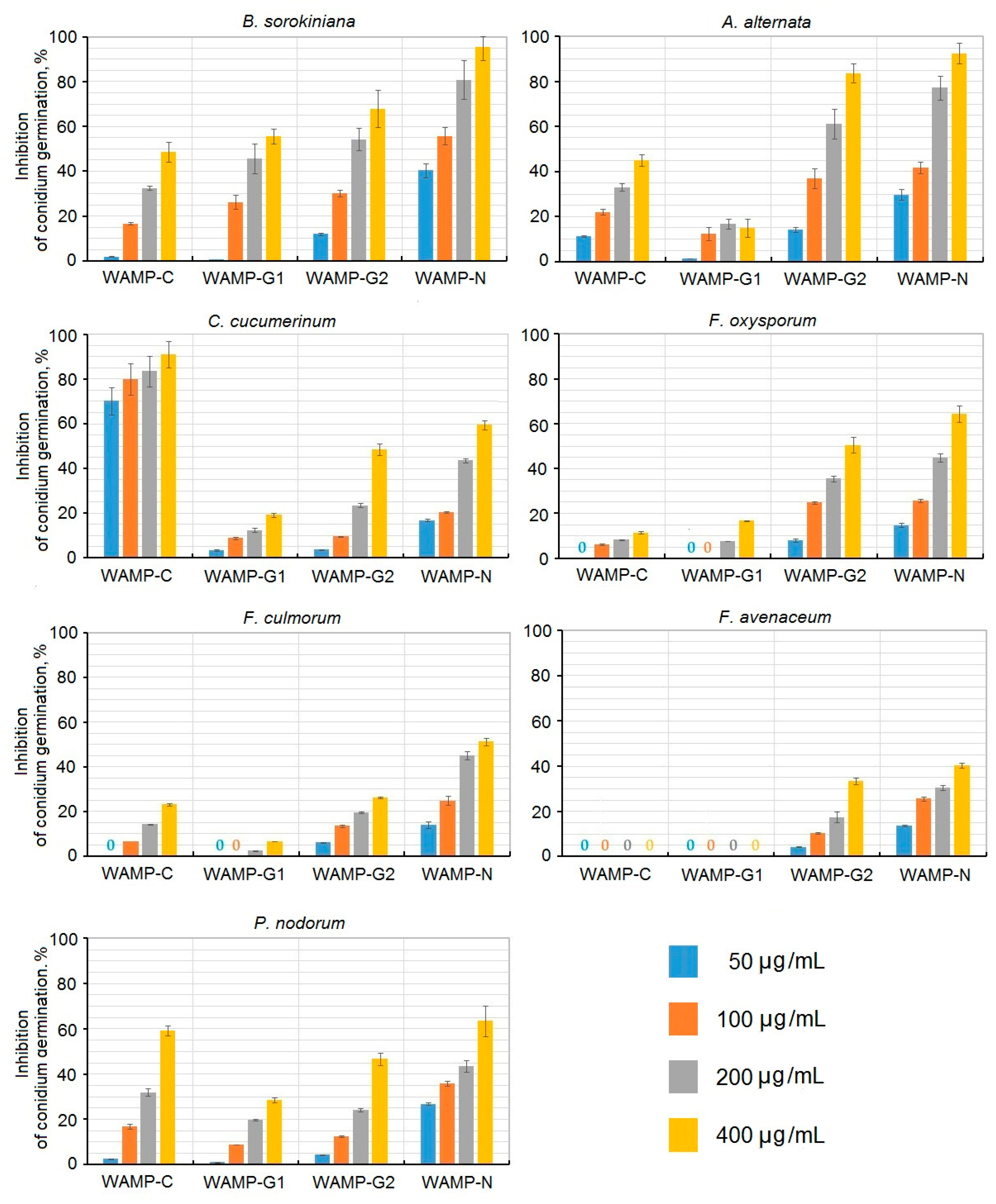
2.3.4. The Antifungal Activity of WAMP-2-Derived Peptides
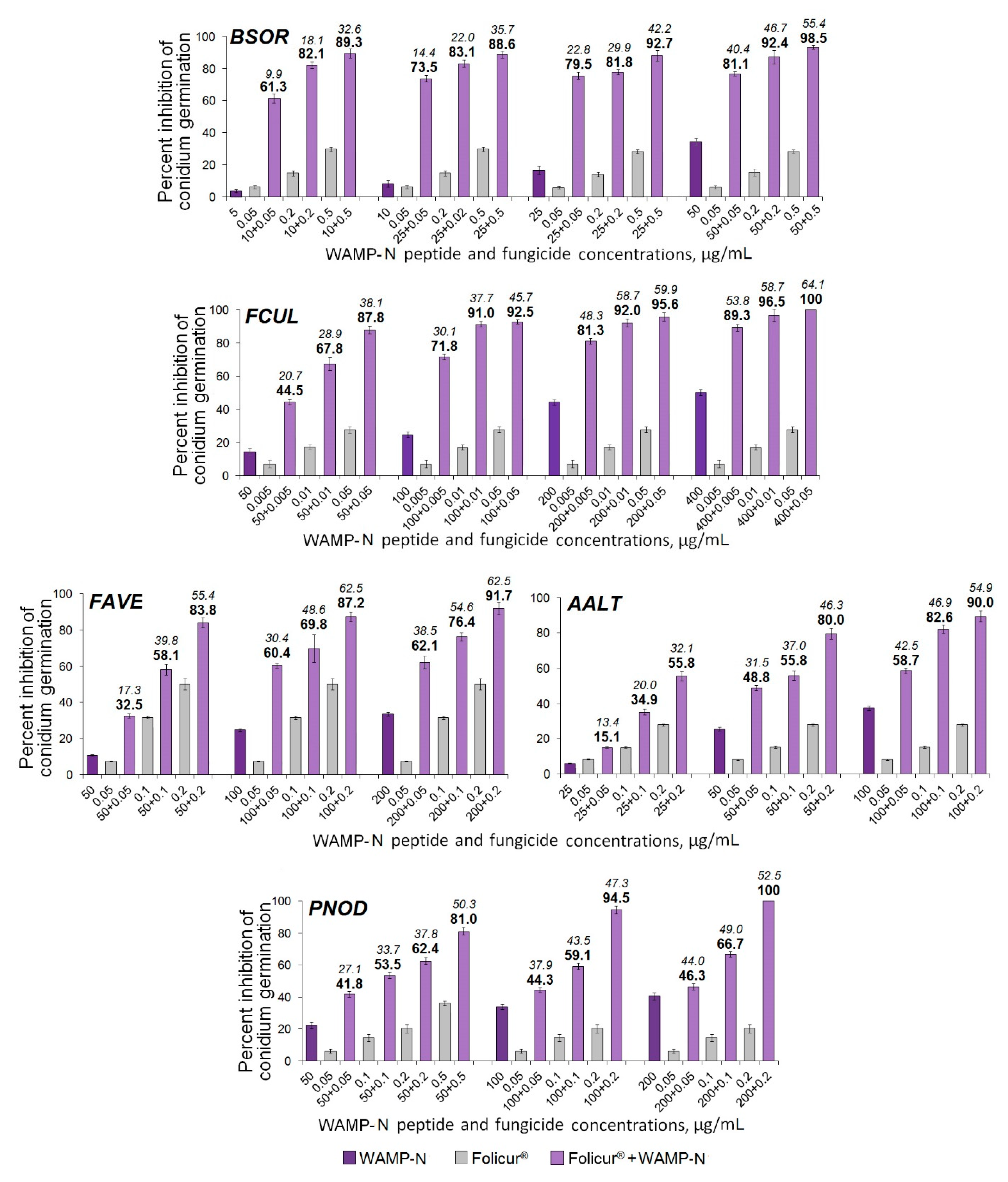
2.3.5. The Synergistic Interaction of WAMP-2-Derived Peptide Fragments with Tebuconazole
3. Discussion
4. Materials and Methods
4.1. Plant Pathogenic Fungi
4.2. Recombinant Production of WAMPs
4.3. Chemical Synthesis of WAMP-2-Derived Peptides
4.4. 3D Structure Modeling
4.5. MALDI-TOF MS
4.6. Antifungal Activity Assay
4.7. Revealing the Synergistic Interactions between WAMP-2-Derived Peptides and Tebuconazole
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silva, M.S.; Monteiro-Arraes, F.B.; de Araújo Campos, M.; Grossi-de-Sa, M.; Fernandez, D.; de Souza Cândido, E.; Cardoso, M.H.; Franco, O.L.; Grossi-de-Sa, M.F. Review: Potential biotechnological assets related to plant immunity modulation applicable in engineering disease-resistant crops. Plant Sci. 2018, 270, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Rosentrater, K.A.; Evers, A.D. Introduction to cereals and pseudocereals and their production. In Kent’s Technology of Cereals, 5th ed.; Woodhead Publishing: Duxford, UK, 2018; pp. 3–75. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Cammue, B.P.A.; De Bolle, M.F.C.; Thevissen, K.; De Samblanx, G.W.; Osborn, R.W. Antimicrobial peptides from plants. Crit. Rev. Plant Sci. 1997, 16, 297–323. [Google Scholar] [CrossRef]
- Manners, J.M. Hidden weapons of microbial destruction in plant genomes. Genome Biol. 2007, 8, 225:1–225:4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odintsova, T.I.; Egorov, T.A. Plant anti-microbial peptides. In Plant Signaling Peptides; Irving, R., Gehring, C.G., Eds.; Springer: Berlin, Germany, 2012; pp. 107–135. ISBN 978-3-642-27603-3. [Google Scholar]
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Goździcka-Józefiak, A. Plant antimicrobial peptides. Folia Microbiol. (Praha) 2014, 59, 181–196. [Google Scholar] [CrossRef] [Green Version]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial peptides from plants. Pharmaceuticals (Basel) 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Campos, M.L.; de Souza, C.M.; de Oliveira, K.B.S.; Dias, S.C.; Franco, O.L. The role of antimicrobial peptides in plant immunity. J. Exp. Bot. 2018, 69, 4997–5011. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Datta, K.; Karmakar, S.; Datta, S.K. Antimicrobial peptides ‒ small but mighty weapons for plants to fight phytopathogens. Protein Pept. Lett. 2019, 26, 720–742. [Google Scholar] [CrossRef]
- Slavokhotova, A.A.; Shelenkov, A.A.; Andreev, Y.A.; Odintsova, T.I. Hevein-like antimicrobial peptides of plants. Biochemistry (Moscow) 2017, 82, 1659–1674. [Google Scholar] [CrossRef]
- Beintema, J.J. Structural features of plant chitinases and chitin-binding proteins. FEBS Lett. 1994, 350, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, S.; Iida, Y.; Kawasaki, Y.; Minami, Y.; Watanabe, K.; Yagi, F. The chitin-binding capability of Cy-AMP1 from cycad is essential to antifungal activity. J. Pept. Sci. 2009, 15, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Chen, C.Y.; Ravinath, D.M.; Bungahot, A.; Cheng, C.P.; You, R.I. Functional characterization of chitin-binding lectin from Solanum integrifolium containing antifungal and insecticidal activities. BMC Plant Biol. 2018, 18, 3:1–3:11. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Vassilevski, A.A.; Slavokhotova, A.A.; Musolyamov, A.K.; Finkina, E.I.; Khadeeva, N.V.; Rogozhin, E.A.; Korostyleva, T.V.; Pukhalsky, V.A.; Grishin, E.V.; et al. A novel antifungal hevein-type peptide from Triticum kiharae seeds with a unique 10-cysteine motif. FEBS J. 2009, 276, 4266–4275. [Google Scholar] [CrossRef] [PubMed]
- Dubovskii, P.V.; Vassilevski, A.A.; Slavokhotova, A.A.; Odintsova, T.I.; Grishin, E.V.; Egorov, T.A.; Arseniev, A.S. Solution structure of a defense peptide from wheat with a 10-cysteine motif. Biochem. Biophys. Res. Commun. 2011, 411, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Korostyleva, T.V.; Slavokhotova, A.A.; Rogozhin, E.A.; Utkina, L.L.; Vassilevski, A.A.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. Genes encoding hevein-like defense peptides in wheat: Distribution, evolution, and role in stress response. Biochimie 2012, 94, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Istomina, E.A.; Slavokhotova, A.A.; Korostyleva, T.V.; Semina, Y.V.; Shcherbakova, L.A.; Pukhalskij, V.A.; Odintsova, T.I. Genes encoding hevein-like antimicrobial peptides WAMPs in the species of the genus Aegilops L. Russ. J. Genet. 2017, 53, 1320–1327. [Google Scholar] [CrossRef]
- Slezina, M.P.; Korostyleva, T.V.; Slavokhotova, A.A.; Istomina, E.A.; Shcherbakova, L.A.; Pukhalskij, V.A.; Odintsova, T.I. Genes encoding hevein-like antimicrobial peptides from Elytrigia repens (L.) Desv. ex Nevski. Russ. J. Genet. 2018, 54, 1152–1159. [Google Scholar] [CrossRef]
- Slavokhotova, A.A.; Naumann, T.A.; Price, N.P.; Rogozhin, E.A.; Andreev, Y.A.; Vassilevski, A.A.; Odintsova, T.I. Novel mode of action of plant defense peptides—Hevein-like antimicrobial peptides from wheat inhibit fungal metalloproteases. FEBS J. 2014, 281, 4754–4764. [Google Scholar] [CrossRef]
- Istomina, E.A.; Korostyleva, T.V.; Rozhnova, N.A.; Rogozhin, E.A.; Pukhalskiy, V.A.; Odintsova, T.I. Genes encoding hevein-like antimicrobial peptides WAMPs: Expression in response to phytohormones and environmental factors. Russ. J. Genet. 2016, 52, 1176–1185. [Google Scholar] [CrossRef]
- FRAC Code List ©* 2020. Available online: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2020-finalb16c2b2c512362eb9a1eff00004acf5d.pdf?sfvrsn=54f499a_2 (accessed on 19 October 2020).
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L.V. Efficacy of triazole-based fungicides for Fusarium head blight and deoxynivalenol control in wheat: A multivariate meta-analysis. Phytopathology 2008, 98, 999–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubos, T.; Pasquali, M.; Pogoda, F.; Hoffmann, L.; Beyer, M. Evidence for natural resistance towards trifloxystrobin in Fusarium graminearum. Eur. J. Plant Pathol. 2011, 130, 239–248. [Google Scholar] [CrossRef]
- Dubos, T.; Pasquali, M.; Pogoda, F.; Casanova, A.; Hoffmann, L.; Beyer, M. Differences between the succinate dehydrogenase sequences of isopyrazam sensitive Zymoseptoria tritici and insensitive Fusarium graminearum strains. Pestic Biochem Physiol. 2013, 105, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Z.; Zhang, B.; Zhang, Q. Degradation and adsorption of tebuconazole and tribenuron-methyl in wheat soil, alone and in combination. Chil. J. Agric. Res. 2017, 77, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.-Y.; Zhu, Y.-F.; Liu, Y.-Y.; Deng, Y.-Y.; Li, W.; Zhang, A.-X.; Chen, H.-G. Evaluation of tebuconazole for the management of Fusarium head blight in China. Australas. Plant Pathol. 2014, 43, 631–638. [Google Scholar] [CrossRef]
- Klix, M.B.; Verreet, J.-A.; Beyer, M. Comparison of the declining triazole sensitivity of Gibberella zeae and increased sensitivity achieved by advances in triazole fungicide development. Crop Prot. 2007, 26, 683–690. [Google Scholar] [CrossRef]
- Hellin, P.; Scauflaire, J.; Van Hese, V.; Munaut, F.; Legrève, A. Sensitivity of Fusarium culmorum to triazoles: Impact of trichothecene chemotypes, oxidative stress response and genetic diversity. Pest. Manag. Sci. 2017, 73, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.; Chan, K.L.; Kim, J.H. Chemosensitization as a means to augment commercial antifungal agents. Front. Microbiol. 2012, 3, 79:1–79:20. [Google Scholar] [CrossRef] [Green Version]
- Dzhavakhiya, V.; Shcherbakova, L.; Semina, Y.; Zhemchuzhina, N.; Campbell, B. Chemosensitization of plant pathogenic fungi to agricultural fungicides. Front. Microbiol. 2012, 3, 87:1–87:9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, T.B.; Chang, S.T. Synergistic effects of cinnamaldehyde in combination with eugenol against wood decay fungi. Bioresour. Technol. 2008, 99, 232–236. [Google Scholar] [CrossRef]
- Zuzarte, M.; Goncalves, M.J.; Cavaleiro, C.; Canhoto, J.; Vale-Silva, L.; Silva, M.J.; Pinto, E.; Salgueiro, L. Chemical composition and antifungal activity of the essential oils of Lavandula viridis L’Her. J. Med. Microbiol. 2011, 60, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Campbell, B.; Mahoney, N.; Chan, K.; Molyneux, R.; May, G. Chemosensitization prevents tolerance of Aspergillus fumigatus to antimycotic drugs. Biochem. Biophys. Res. Commun. 2008, 372, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Utkina, L.L.; Zhabon, E.O.; Slavokhotova, A.A.; Rogozhin, E.A.; Shiyan, A.N.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I.; Pukhal’skiy, V.A. Heterologous expression of a synthetic gene encoding a novel hevein-type antimicrobial peptide of Leymus arenarius in Escherichia coli cells. Russ. J. Genet. 2010, 46, 1449–1454. [Google Scholar] [CrossRef]
- Nielsen, K.K.; Nielsen, J.E.; Madrid, S.M.; Mikkelsen, J.D. Characterization of a new antifungal chitin-binding peptide from sugar beet leaves. Plant Physiol. 1997, 113, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yount, N.Y.; Yeaman, M.R. Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2004, 101, 7363–7368. [Google Scholar] [CrossRef] [Green Version]
- Sagaram, U.S.; Pandurangi, R.; Kaur, J.; Smith, T.J.; Shah, D.M. Structure-activity determinants in antifungal plant defensins MsDef1 and MtDef4 with different modes of action against Fusarium graminearum. PLoS ONE 2011, 6, e18550:1–e18550:13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhu, S.; Dennison, S.R.; Lea, B.; Snape, T.J.; Nicholl, I.D.; Radecka, I.; Harris, F. Anionic antimicrobial and anticancer peptides from plants. Crit. Rev. Plant Sci. 2013, 32, 303–320. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef] [Green Version]
- Ikai, A. Thermostability and aliphatic index of globular proteins. J. Biochem. 1980, 88, 1895–1898. [Google Scholar] [CrossRef]
- Moghadam, A.; Niazi, A.; Afsharifar, A.; Taghavi, S.M. Expression of a recombinant Anti-HIV and anti-tumor protein, MAP30, in Nicotiana tabacum hairy roots: A pH-stable and thermophilic antimicrobial protein. PLoS ONE 2016, 11, e0159653. [Google Scholar] [CrossRef]
- Guruprasad, K.; Reddy, B.V.B.; Pandit, M.W. Correlation between stability of a protein and its dipeptide composition: A novel approach for predicting in vivo stability of a protein from its primary sequence. Protein Eng. 1990, 4, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [Green Version]
- Boman, H.G. Antibacterial peptides: Basic facts and emerging concepts. J. Intern. Med. 2003, 254, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Lamiable, A.; Thévenet, P.; Rey, J.; Vavrusa, M.; Derreumaux, P.; Tufféry, P. PEP-FOLD3: Faster de novo structure prediction for linear peptides in solution and in complex. Nucleic Acids Res. 2016, 44, W449–W454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orhan, G.; Bayram, A.; Zer, Y.; Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J. Clin. Microbiol. 2005, 43, 140–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richer, D.L. Synergism: A patent view. Pesticide Sci. 1987, 19, 309–315. [Google Scholar] [CrossRef]
- Canton, E.; Peman, J.; Gobernado, M.; Viudes, A.; Espinel, I.A. Synergistic activities of fluconazole and voriconazole with terbinafine against four Candida species determined by checkerboard, time-kill, and E-test methods. Antimicrob. Agent Chemother. 2005, 49, 1593–1596. [Google Scholar] [CrossRef] [Green Version]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Matroodi, S.; Motallebi, M.; Zamani, M.; Moradyar, M. Designing a new chitinase with more chitin binding and antifungal activity. World J. Microbiol. Biotechnol. 2013, 29, 1517–1523. [Google Scholar] [CrossRef]
- Iseli, B.; Boller, T.; Neuhaus, J.M. The N-terminal cysteine-rich domain of tobacco class I chitinase is essential for chitin binding but not for catalytic or antifungal activity. Plant Physiol. 1993, 103, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Taira, T.; Ohnuma, T.; Yamagami, T.; Aso, Y.; Ishiguro, M.; Ishihara, M. Antifungal activity of rye (Secale cereale) seed chitinases: The different binding manner of class I and class II chitinases to the fungal cell walls. Biosci. Biotechnol. Biochem. 2002, 66, 970–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badrhadad, A.; Nazarian-Firouzabadi, F.; Ismaili, A. Fusion of a chitin-binding domain to an antibacterial peptide to enhance resistance to Fusarium solani in tobacco (Nicotiana tabacum). 3 Biotech 2018, 8, 391:1–391:10. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U.; et al. Fungal Morphogenesis, from the Polarized Growth of Hyphae to Complex Reproduction and Infection Structures. Microbiol. Mol. Biol. Rev. 2018, 82, e00068-17. [Google Scholar] [CrossRef] [Green Version]
- Van Parijs, J.; Broekart, W.F.; Goldstein, I.J.; Peumans, W.J. Hevein: An antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 1991, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Wessels, J.G.H. A Steady-State Model for apical wall growth in fungi. Acta Bot. Neerl. 1988, 37, 3–16. [Google Scholar] [CrossRef]
- Bartnicki-Garcia, S. Fundamental aspects of hyphal morphogenesis. In Microbial Differentiation: Symposium of the Society for General Microbiology; Ashworth, J.M., Smith, E., Eds.; Cambridge University Press: Cambridge, UK, 1973; pp. 245–267. ISBN 9780521201049. [Google Scholar]
- Cabib, E. The synthesis and degradation of chitin. Adv. Enzymol. Relat. Areas Mol. Biol. 1987, 59, 59–101. [Google Scholar] [CrossRef]
- Koo, J.C.; Lee, S.Y.; Chun, H.J.; Cheong, Y.H.; Choi, J.S.; Kawabata, S.; Miyagi, M.; Tsunasawa, S.; Ha, K.S.; Bae, D.W.; et al. Two hevein homologs isolated from the seed of Pharbitis nil L. exhibit potent anti-fungal activity. Biochim. Biophys. Acta 1998, 1382, 80–90. [Google Scholar] [CrossRef]
- Kam, A.; Loo, S.; Fan, J.S.; Sze, S.K.; Yang, D.; Tam, J.P. Roseltide rT7 is a disulfide-rich, anionic, and cell-penetrating peptide that inhibits proteasomal degradation. J. Biol. Chem. 2019, 294, 19604–19615. [Google Scholar] [CrossRef]
- Van den Bergh, K.P.B.; Rougé, P.; Proost, P.; Coosemans, J.; Krouglova, T.; Engelborghs, Y.; Peumans, W.J.; Van Damme, E.J.M. Synergistic antifungal activity of two chitin-binding proteins from spindle tree (Euonymus europaeus L.). Planta 2004, 219, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Ökmen, B.; Kemmerich, B.; Hilbig, D.; Wemhöner, R.; Aschenbroich, J.; Perrar, A.; Huesgen, P.F.; Schipper, K.; Doehlemann, G. Dual function of a secreted fungalysin metalloprotease in Ustilago maydis. New Phytol. 2018, 220, 249–261. [Google Scholar] [CrossRef] [Green Version]
- Duveiller, E.; García Altamirano, I. Pathogenicity of Bipolaris sorokiniana isolates from wheat roots, leaves and grains in Mexico. Plant Pathol. 2000, 49, 235–242. [Google Scholar] [CrossRef]
- Smiley, R.W.; Gourlie, J.A.; Easley, S.A.; Patterson, L.-M.; Whittaker, R.G. Crop damage estimates for crown rot of wheat and barley in the Pacific Northwest. Plant Dis. 2005, 89, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagkaeva, T.Y.; Gavrilova, O.P.; Levitin, M.M.; Novozhilov, K.V. Fusarium diseases of grain crops. Plant Protect. Quar. 2011, S5, 69–120. [Google Scholar]
- Cowger, C.; Brian, W.; Brown-Guedira, G.; Brown, J.K.M. Role of effector-sensitivity gene interactions and durability of quantitative resistance to Septoria nodorum blotch in Eastern U.S. wheat. Front. Plant Sci 2020, 11, 155-1–155-8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Lee, Y.; Ha, A.; Kim, J.I.; Park, A.R.; Yu, N.H.; Son, H.; Choi, G.J.; Park, H.W.; Lee, C.W.; et al. Chemosensitization of Fusarium graminearum to chemical fungicides using cyclic lipopeptides produced by Bacillus amyloliquefaciens strain JCK-12. Front. Plant Sci. 2017, 8, 2010-1–2010-16. [Google Scholar] [CrossRef] [Green Version]
- Vriens, K.; Cools, T.L.; Harvey, P.J.; Craig, D.J.; Braem, A.; Vleugels, J.; De Coninck, B.; Cammue, B.P.A.; Thevissen, K. The radish defensins RsAfp1 and RsAFP2 act synergistically with caspofungin against Candida albicans biofilms. Peptides 2016, 75, 71–79. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2005; pp. 571–607. ISBN 978-1-58829-343-5. [Google Scholar]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [Green Version]
- Zakharenkova, T.S.; Aver’yanov, A.A.; Pasechnik, T.D.; Lapikova, V.P.; Baker, C.J. Surface contact of cucumber or rice leaves with water can suppress their fungal diseases. Physiol. Mol. Plant Pathol. 2012, 79, 13–20. [Google Scholar] [CrossRef]
- Aver’yanov, A.A.; Lapikova, V.P.; Pasechnik, T.D.; Abramova, O.S.; Gaivoronskaya, L.M.; Kuznetsov, V.V.; Baker, C.J. Pre-illumination of rice blast conidia induces tolerance to subsequent oxidative stress. Fungal Biol. 2014, 118, 743–753. [Google Scholar] [CrossRef]



| Fungi | IC50, μg/mL | ||||
|---|---|---|---|---|---|
| WAMP-1b(A34) * | WAMP-2(K34) | WAMP-3.1(E34) | WAMP-4(N34) | WAMP-5(V34) * | |
| B. sorokiniana | 22.8 ± 3.2 | 30.6 ± 0,9 | 22.2 ± 0,5 | 24.3 ± 1.9 | 25.2 ± 2.3 |
| A. alternata | 82.8 ± 7.8 | 107.3 ± 7.9 | 82.8 ± 2.8 | 107.8 ± 13.4 | 63.8 ± 3.7 |
| C. cucumerinum | – | 37.1 ± 2.3 | – | 58.3 ± 3,2 | 53.5 ± 4.6 |
| F. oxysporum | 74.8 ± 4.6 | 41.0 ± 3.2 | 31.8 ± 1.9 | 52.3 ± 4.2 | 56.6 ± 5.1 |
| F. culmorum | n/d | 256.3 ± 18.1 | n/d | n/d | n/d |
| Peptide Property | Peptide Name | |||
|---|---|---|---|---|
| WAMP-N | WAMP-G1 | WAMP-G2 | WAMP-C | |
| Amino acid sequence | AQRCGDQARGAKC | LCCGKYGFCGSG | CCGKYGFCGSGDAYC | GKGSCQSQCRGCR |
| Length, aa residues | 13 (1–13) * | 12 (17–28) * | 15 (18–32) * | 13 (33–45) * |
| Molecular weight, Da | 1363.53 | 1194.40 | 1533.73 | 1369.55 |
| pI | 8.96 | 7.95 | 5.81 | 9.22 |
| Net charge at pH 7 | +2 | +1 | 0 | +3 |
| Aliphatic index | 23.08 | 32.5 | 6.67 | 0 |
| Instability index | −3.83 | −4.98 | −1.99 | 51.73 |
| GRAVY index | −1.062 | 0.542 | 0.147 | −1.169 |
| Boman index, kcal/mol | 3.48 | -0.53 | 0.28 | 3.58 |
| Fungi | IC50, μg/mL | |||
|---|---|---|---|---|
| WAMP-N | WAMP-G1 | WAMP-G2 | WAMP-C | |
| B. sorokiniana | 72.9 ± 12.4 | 273.1 ± 45.7 | 195.2 ± 36.8 | 429.5 ± 51.5 |
| A. alternata | 102.6 ± 19.6 | >700 | 145.6 ± 29.4 | 550.4 ± 41.3 |
| C. cucumerinum | 280.2 ± 29.7 | >700 | 410.2 ± 29.1 | 5.3 ± 0.1 |
| F. oxysporum | 238.0 ± 32.4 | >700 | 391.2 ± 34.2 | >700 |
| F. culmorum | 332.4 ± 26.1 | >700 | >700 | >700 |
| F. avenaceum | >700 | – | 602.8 ± 3.1 | – |
| P. nodorum | 220.2 ± 13.5 | >700 | 424.1 ± 12.1 | 329.6 ± 7.9 |
| Fungi | FFCIs | |||
|---|---|---|---|---|
| Sensitizing Peptides | ||||
| WAMP-N | WAMP-G1 | WAMP-G2 | WAMP-C | |
| B. sorokiniana | 0.1 | 0.02 | 0.13 | 0.005 |
| F. culmorum | 0.01 | n/d | 0.02 | 0.002 |
| F. avenaceum | 0.03 | n/d | 0.03 | 0.03 |
| A. alternata | 0.04 | n/d, n/s | 0.05 | 0.09 |
| P. nodorum | 0.06 | n/d | 0.04 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odintsova, T.; Shcherbakova, L.; Slezina, M.; Pasechnik, T.; Kartabaeva, B.; Istomina, E.; Dzhavakhiya, V. Hevein-Like Antimicrobial Peptides Wamps: Structure–Function Relationship in Antifungal Activity and Sensitization of Plant Pathogenic Fungi to Tebuconazole by WAMP-2-Derived Peptides. Int. J. Mol. Sci. 2020, 21, 7912. https://doi.org/10.3390/ijms21217912
Odintsova T, Shcherbakova L, Slezina M, Pasechnik T, Kartabaeva B, Istomina E, Dzhavakhiya V. Hevein-Like Antimicrobial Peptides Wamps: Structure–Function Relationship in Antifungal Activity and Sensitization of Plant Pathogenic Fungi to Tebuconazole by WAMP-2-Derived Peptides. International Journal of Molecular Sciences. 2020; 21(21):7912. https://doi.org/10.3390/ijms21217912
Chicago/Turabian StyleOdintsova, Tatyana, Larisa Shcherbakova, Marina Slezina, Tatyana Pasechnik, Bakhyt Kartabaeva, Ekaterina Istomina, and Vitaly Dzhavakhiya. 2020. "Hevein-Like Antimicrobial Peptides Wamps: Structure–Function Relationship in Antifungal Activity and Sensitization of Plant Pathogenic Fungi to Tebuconazole by WAMP-2-Derived Peptides" International Journal of Molecular Sciences 21, no. 21: 7912. https://doi.org/10.3390/ijms21217912
APA StyleOdintsova, T., Shcherbakova, L., Slezina, M., Pasechnik, T., Kartabaeva, B., Istomina, E., & Dzhavakhiya, V. (2020). Hevein-Like Antimicrobial Peptides Wamps: Structure–Function Relationship in Antifungal Activity and Sensitization of Plant Pathogenic Fungi to Tebuconazole by WAMP-2-Derived Peptides. International Journal of Molecular Sciences, 21(21), 7912. https://doi.org/10.3390/ijms21217912





