In Cellulo Evaluation of the Therapeutic Potential of NHC Platinum Compounds in Metastatic Cutaneous Melanoma
Abstract
:1. Introduction
2. Results
2.1. Effects of Pt Compounds on Various Cell Lines Viability
2.1.1. Effects Measured on Melanoma Cell Lines
2.1.2. Impact on BRAF-Inhibitor-Resistant Cutaneous Melanoma Cell Lines
2.2. Investigation of Cell Pathways Involved in the Cytotoxicity of NHC-Pt Compounds
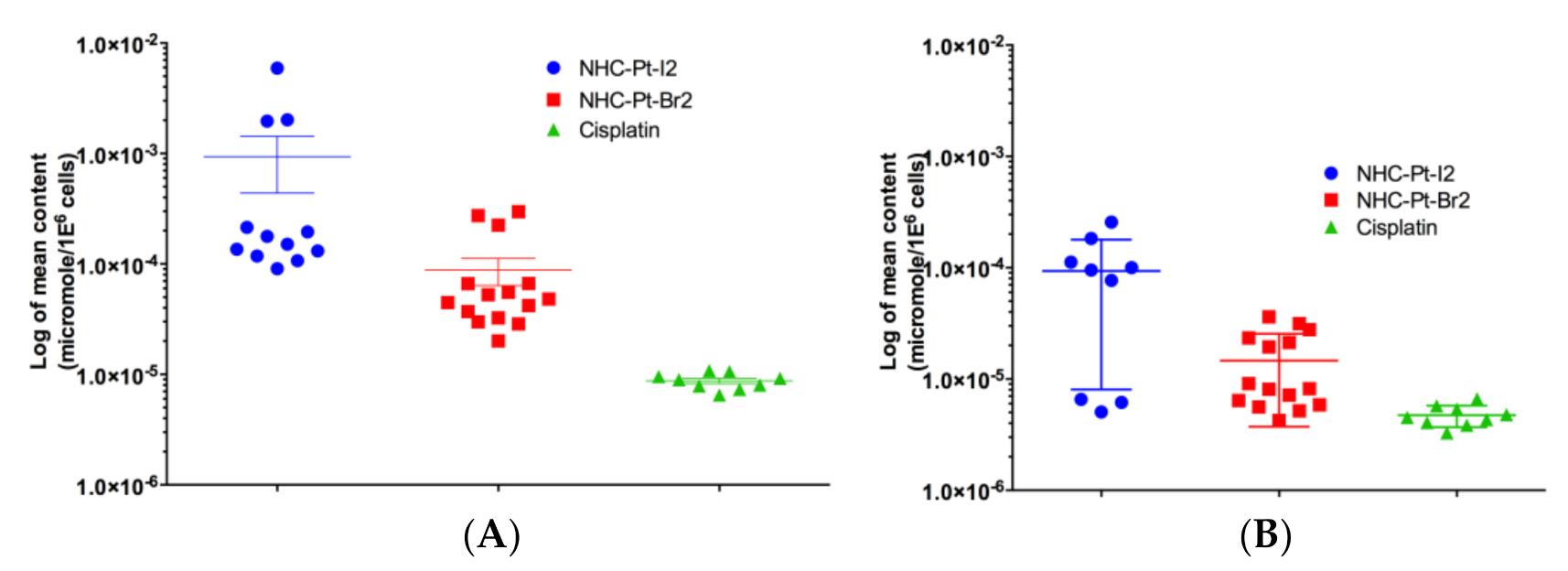
2.2.1. Evaluation of Pt Compound Uptake and Efflux Levels
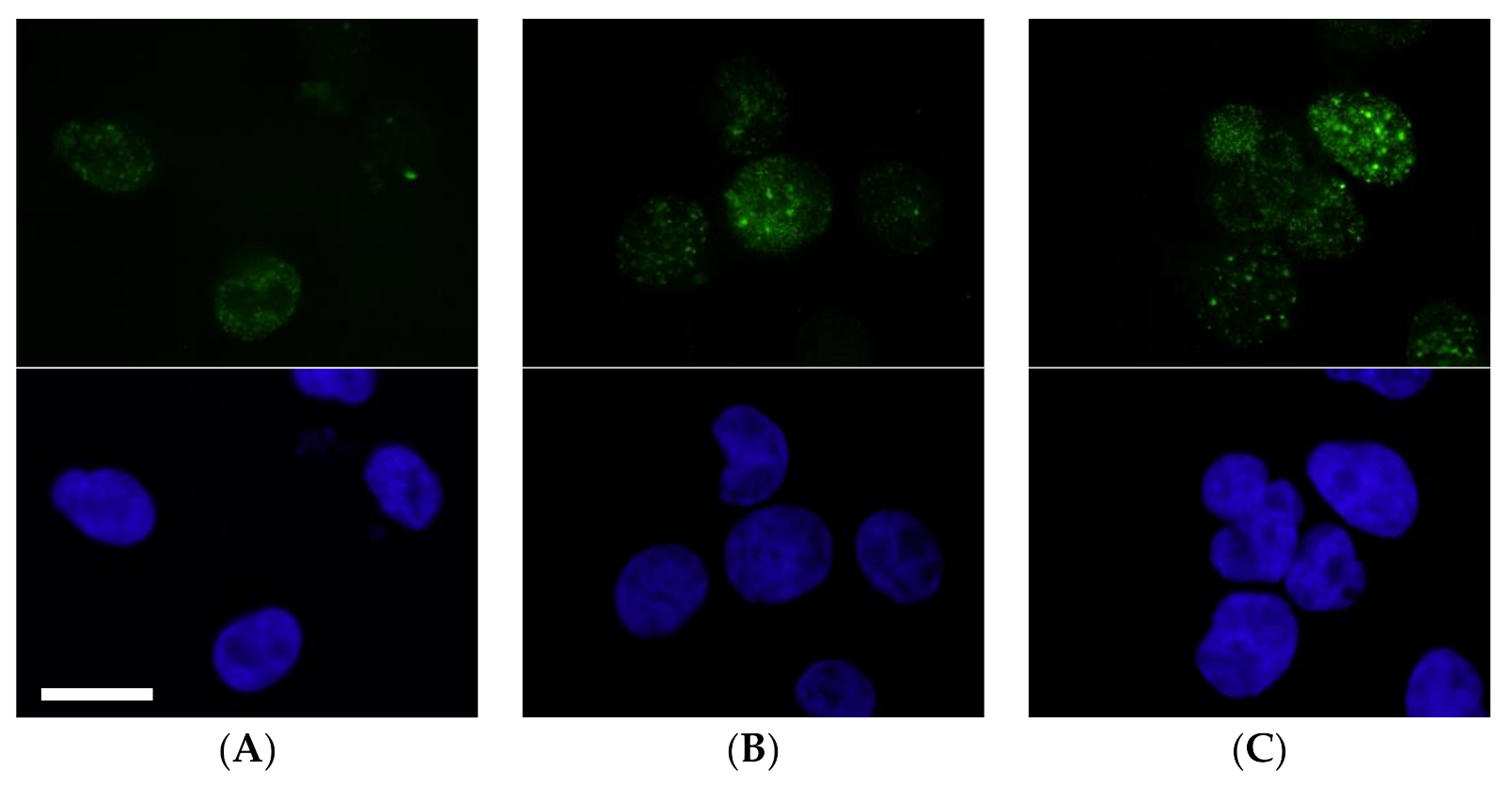
2.2.2. Evaluation of DNA Double-Strand Breaks
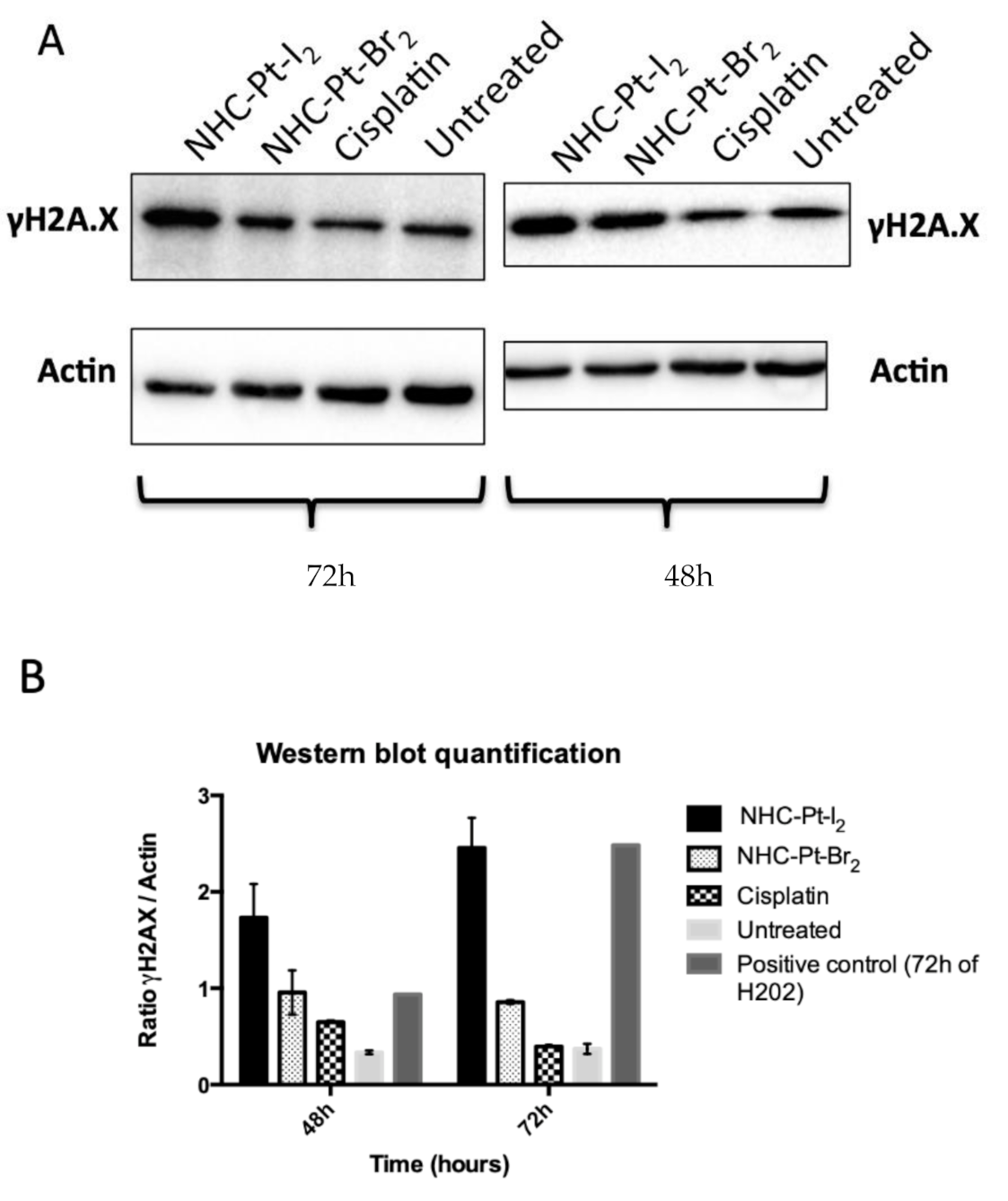
2.2.3. Assessing Cumulative DNA Damage
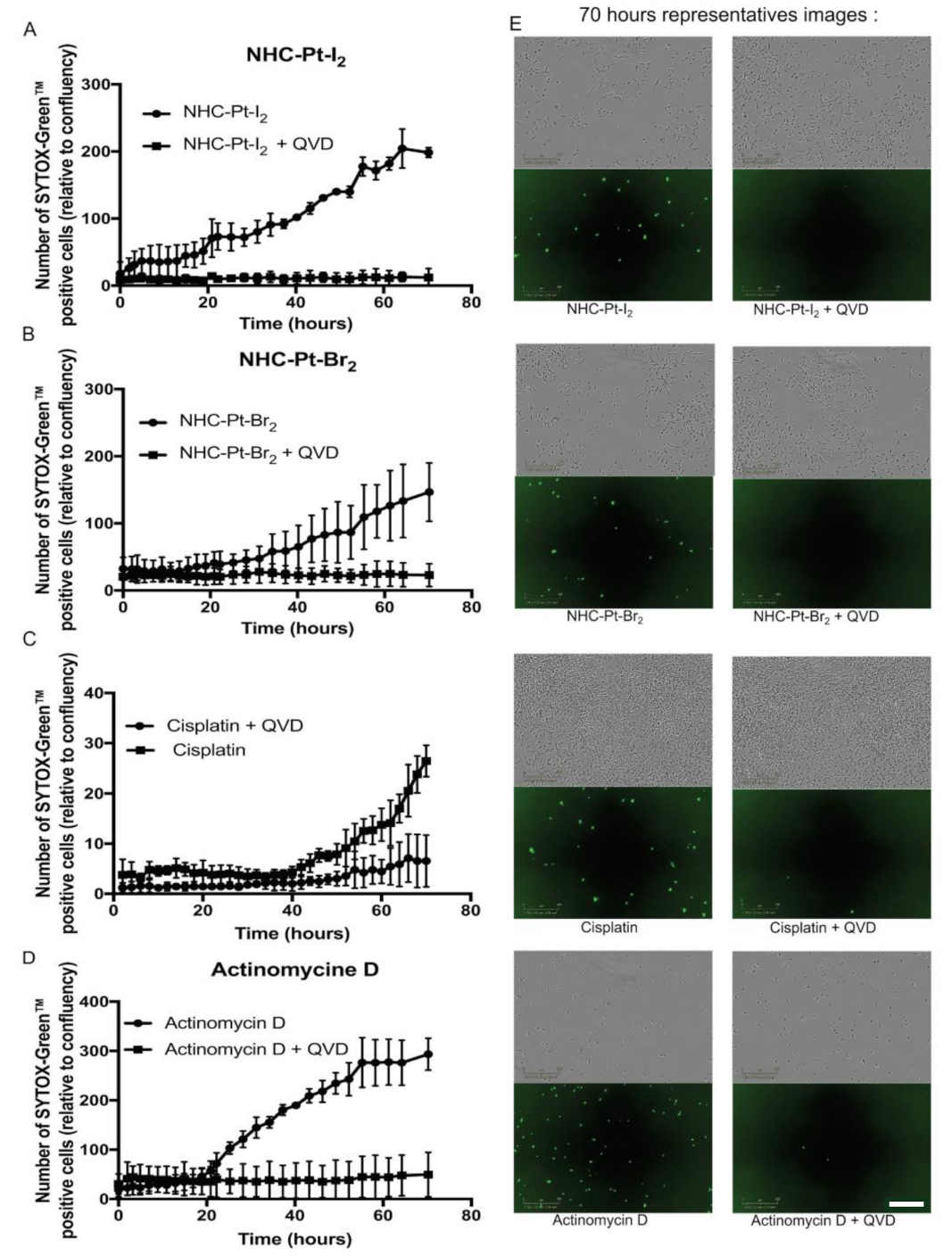
2.2.4. Cell Death Pathways
3. Discussion
4. Materials and Methods
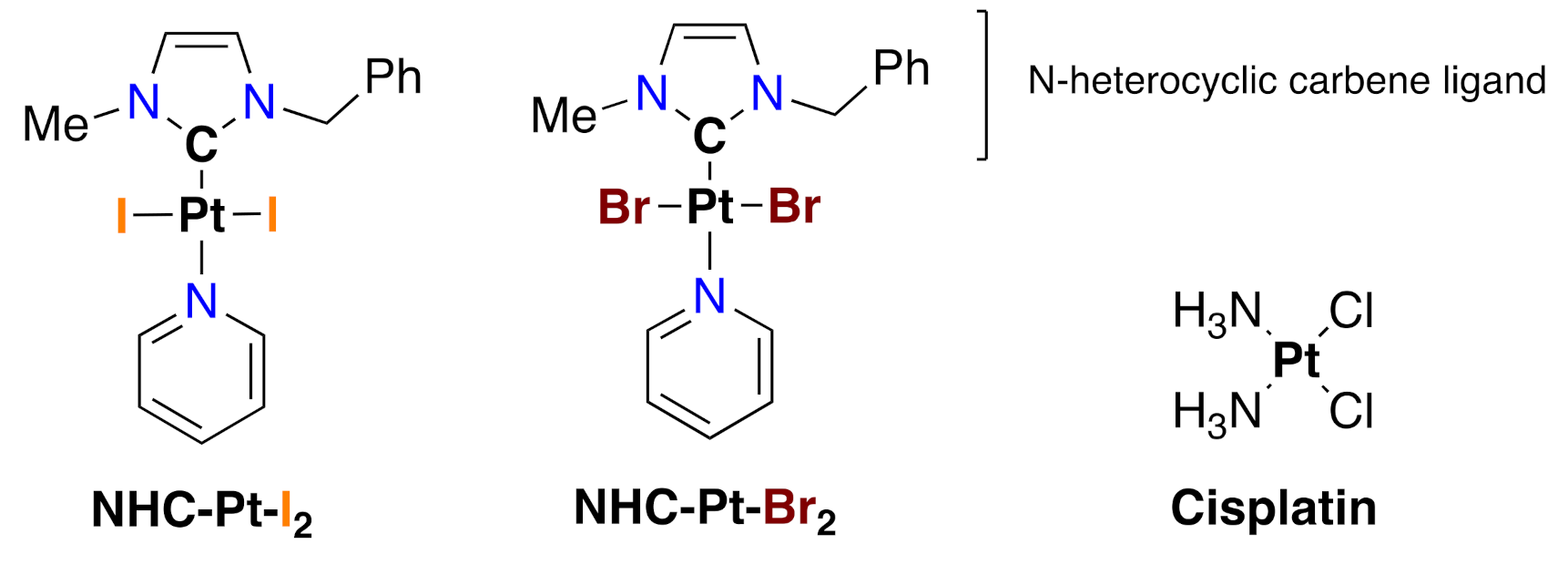
4.1. Compounds
4.1.1. Synthesis and Characterization of NHC-Pt Compounds
4.1.2. Solution Preparation
4.2. Cell Lines, Cell Culture, Treatments
4.2.1. Cell Lines and Cell Culture
4.2.2. MTT Assays
4.2.3. In Cellulo Pharmacokinetics
4.2.4. Evaluation of DNA Damage
4.2.5. Investigation of Cell Death Processes
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boniol, M.; Autier, P.; Boyle, P.; Gandini, S. Cutaneous melanoma attributable to sunbed use: Systematic review and meta-analysis. BMJ 2012, 345, e4757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; International Agency for Research on Cancer/World Health Organization: Lyon, France, 2014; ISBN 978-92-832-0443-5. [Google Scholar]
- Dummer, R.; Flaherty, K.T. Resistance patterns with tyrosine kinase inhibitors in melanoma: New insights. Curr. Opin. Oncol. 2012, 24, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, S.; Hadjinicolaou, A.V.; Chiarion Sileni, V.; Rossi, C.R.; Mocellin, S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst. Rev. 2018, 2, CD011123. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Poulalhon, N.; Thomas, L. Vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 365, 1448–1449. [Google Scholar]
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef]
- Garbe, C.; Basset-Seguin, N.; Poulin, Y.; Larsson, T.; Østerdal, M.L.; Venkata, R.; Lear, J.T. Efficacy and safety of follow-up field treatment of actinic keratosis with ingenol mebutate 0·015% gel: A randomized, controlled 12-month study. Br. J. Dermatol. 2016, 174, 505–513. [Google Scholar] [CrossRef]
- Hamid, O.; Puzanov, I.; Dummer, R.; Schachter, J.; Daud, A.; Schadendorf, D.; Blank, C.; Cranmer, L.D.; Robert, C.; Pavlick, A.C.; et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur. J. Cancer Oxf. Engl. 1990 2017, 86, 37–45. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Li, T.; Yue, W. Drug response to PD-1/PD-L1 blockade: Based on biomarkers. OncoTargets Ther. 2018, 11, 4673–4683. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Mohan, R.; Singh, J.K.; Samantaray, M.K.; Shaikh, M.M.; Panda, D.; Ghosh, P. Anticancer and antimicrobial metallopharmaceutical agents based on palladium, gold, and silver N-heterocyclic carbene complexes. J. Am. Chem. Soc. 2007, 129, 15042–15053. [Google Scholar] [CrossRef]
- N-Heterocyclic Carbene Platinum Complexes: A Big Step Forward for Effective Antitumor Compounds-Bellemin-Laponnaz-2020-European Journal of Inorganic Chemistry-Wiley Online Library. Available online: https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/ejic.201900960 (accessed on 31 July 2020).
- Skander, M.; Retailleau, P.; Bourrié, B.; Schio, L.; Mailliet, P.; Marinetti, A. N-heterocyclic carbene-amine Pt(II) complexes, a new chemical space for the development of platinum-based anticancer drugs. J. Med. Chem. 2010, 53, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Chardon, E.; Dahm, G.; Guichard, G.; Bellemin-Laponnaz, S. Derivatization of Preformed Platinum N-Heterocyclic Carbene Complexes with Amino Acid and Peptide Ligands and Cytotoxic Activities toward Human Cancer Cells. Organometallics 2012, 31, 7618–7621. [Google Scholar] [CrossRef]
- Liu, W.; Gust, R. Update on metal N-heterocyclic carbene complexes as potential anti-tumor metallodrugs. Coord. Chem. Rev. 2016, 329, 191–213. [Google Scholar] [CrossRef]
- Rehm, T.; Rothemund, M.; Muenzner, J.K.; Noor, A.; Kempe, R.; Schobert, R. Novel cis-[(NHC)1(NHC)2(L)Cl] platinum(II) complexes–synthesis, structures, and anticancer activities. Dalton Trans. 2016, 45, 15390–15398. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.D.; Bose, D.; Mitra, P.; Saha, K.D.; Bertolasi, V.; Dinda, J. Au(I)-and Pt(II)-N-heterocyclic carbene complexes with picoline functionalized benzimidazolin-2-ylidene ligands; synthesis, structures, electrochemistry and cytotoxicity studies. New J. Chem. 2012, 36, 759–767. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Harlepp, S.; Chardon, E.; Bouché, M.; Dahm, G.; Maaloum, M.; Bellemin-Laponnaz, S. N-Heterocyclic Carbene-Platinum Complexes Featuring an Anthracenyl Moiety: Anti-Cancer Activity and DNA Interaction. Int. J. Mol. Sci. 2019, 20, 4198. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, V.M.; Fuertes, M.A.; Alonso, C.; Perez, J.M. Is cisplatin-induced cell death always produced by apoptosis? Mol. Pharmacol. 2001, 59, 657–663. [Google Scholar] [CrossRef]
- Herceg, Z.; Wang, Z.Q. Functions of poly (ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat. Res. 2001, 477, 97–110. [Google Scholar] [CrossRef]
- Serrone, L.; Hersey, P. The chemoresistance of human malignant melanoma: An update. Melanoma Res. 1999, 9, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Panda, S.; Samal, S.K.; Shriwas, O.; Rath, R.; Pellecchia, M.; Emdad, L.; Das, S.K.; Fisher, P.B.; Dash, R. Bcl-2 Antiapoptotic Family Proteins and Chemoresistance in Cancer. Adv. Cancer Res. 2018, 137, 37–75. [Google Scholar]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.; Tykodi, S.S.; Thompson, J.A. Treatment of metastatic melanoma: An overview. Oncol. Williston Park N 2009, 23, 488–496. [Google Scholar]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef] [Green Version]
- Scolyer, R.A.; Long, G.V.; Thompson, J.F. Evolving concepts in melanoma classification and their relevance to multidisciplinary melanoma patient care. Mol. Oncol. 2011, 5, 124–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garbe, C.; Peris, K.; Hauschild, A.; Saiag, P.; Middleton, M.; Bastholt, L.; Grob, J.-J.; Malvehy, J.; Newton-Bishop, J.; Stratigos, A.J.; et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline-Update 2016. Eur. J. Cancer Oxf. Engl. 1990 2016, 63, 201–217. [Google Scholar] [CrossRef]
- Dahm, G.; Bouché, M.; Bailly, C.; Karmazin, L.; Bellemin-Laponnaz, S. Synthesis and structural characterization of benzyl-functionalized N-heterocyclic carbene platinum complexes: Dramatic substituent effect on anti-cancer activity. J. Organomet. Chem. 2019, 899, 120908. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, J.; Vultur, A.; Lee, J.T.; Somasundaram, R.; Fukunaga-Kalabis, M.; Cipolla, A.K.; Wubbenhorst, B.; Xu, X.; Gimotty, P.A.; Kee, D.; et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by co-targeting MEK and IGF-1R/PI3K. Cancer Cell 2010, 18, 683–695. [Google Scholar] [CrossRef] [Green Version]
- Karageorgis, A.; Claron, M.; Jugé, R.; Aspord, C.; Thoreau, F.; Leloup, C.; Kucharczak, J.; Plumas, J.; Henry, M.; Hurbin, A.; et al. Systemic Delivery of Tumor-Targeted Bax-Derived Membrane-Active Peptides for the Treatment of Melanoma Tumors in a Humanized SCID Mouse Model. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 534–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, G.; Dalle, S.; Monet, M.; Ligier, M.; Boespflug, A.; Pommier, R.M.; de la Fouchardière, A.; Perier-Muzet, M.; Depaepe, L.; Barnault, R.; et al. ZEB1-mediated melanoma cell plasticity enhances resistance to MAPK inhibitors. EMBO Mol. Med. 2016, 8, 1143–1161. [Google Scholar] [CrossRef] [PubMed]
- Ichim, G.; Lopez, J.; Ahmed, S.U.; Muthalagu, N.; Giampazolias, E.; Delgado, M.E.; Haller, M.; Riley, J.S.; Mason, S.M.; Athineos, D.; et al. Limited mitochondrial permeabilization causes DNA damage and genomic instability in the absence of cell death. Mol. Cell 2015, 57, 860–872. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.A.; Samuels, Y. Analysis of the genome to personalize therapy for melanoma. Oncogene 2010, 29, 5545–5555. [Google Scholar] [CrossRef] [Green Version]

| Compound | A375 1 | SK-MEL-28 1 | MeWo 2 | HMCB 2 |
|---|---|---|---|---|
| Dacarbazine | 9.2 ± 0.2 | 26.0 ± 16.0 | 394.0 ± 16.0 | >500.0 3 |
| Cisplatin | 1.6 ± 0.6 | 1.0 ± 0.2 | 13.2 ± 2.4 | 18.7 ± 4.7 |
| NHC-Pt-I2 | 2.5 ± 0.6 | 3.1 ± 1.0 | 4.8 ± 1.4 | 6.2 ± 3.3 |
| NHC-Pt-Br2 | 12.0 ± 1.0 | 25.7 ± 4.1 | >33.0 3 | >33.0 3 |
| Compound | A375 1 | SK-MEL-28 1 | MeWo 2 | HMCB 2 |
|---|---|---|---|---|
| Dacarbazine | >33.0 3 | >500.0 3 | >500.0 3 | >500.0 3 |
| Cisplatin | 24.6 ± 5.6 | >33.0 3 | >33.0 3 | >33.0 3 |
| NHC-Pt-I2 | 2.3 ± 0.3 | 5.2 ± 1.7 | 8.1 ± 0.5 | 5.4 ± 2.2 |
| NHC-Pt-Br2 | 11.6 ± 0.8 | >33.0 3 | >33.0 3 | >33.0 3 |
| A375-R 1 | SK-MEL-5-R 1 | 501MEL-R 1 | ||||
|---|---|---|---|---|---|---|
| Compound | 1 h | 72 h | 1 h | 72 h | 1 h | 72 h |
| Dacarbazine | >500.0 2 | 288.8 ± 182.6 | >500.0 2 | 182.8 ± 138.6 | >500.0 2 | 298.0 ± 68.0 |
| Cisplatin | 19.6 ± 9.5 | 1.0 ± 0.3 | 14.8 ± 7.4 | 1.5 ± 0.7 | >33.0 2 | 6.2 ± 2.6 |
| NHC-Pt-I2 | 1.7 ± 0.2 | 1.2 ± 0.4 | 1.3 ± 0.3 | 1.3 ± 0.3 | 2.5 ± 0.6 | 2.4 ± 0.5 |
| NHC-Pt-Br2 | 18.0 ± 5.0 | 16.3 ± 6.9 | 13.6 ± 2.7 | 15.3 ± 4.2 | 25.3 ± 3.2 | 23.1 ± 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charignon, E.; Bouché, M.; Clave-Darcissac, C.; Dahm, G.; Ichim, G.; Clotagatide, A.; Mertani, H.C.; Telouk, P.; Caramel, J.; Diaz, J.-J.; et al. In Cellulo Evaluation of the Therapeutic Potential of NHC Platinum Compounds in Metastatic Cutaneous Melanoma. Int. J. Mol. Sci. 2020, 21, 7826. https://doi.org/10.3390/ijms21217826
Charignon E, Bouché M, Clave-Darcissac C, Dahm G, Ichim G, Clotagatide A, Mertani HC, Telouk P, Caramel J, Diaz J-J, et al. In Cellulo Evaluation of the Therapeutic Potential of NHC Platinum Compounds in Metastatic Cutaneous Melanoma. International Journal of Molecular Sciences. 2020; 21(21):7826. https://doi.org/10.3390/ijms21217826
Chicago/Turabian StyleCharignon, Elsa, Mathilde Bouché, Caroline Clave-Darcissac, Georges Dahm, Gabriel Ichim, Anthony Clotagatide, Hichem C. Mertani, Philippe Telouk, Julie Caramel, Jean-Jacques Diaz, and et al. 2020. "In Cellulo Evaluation of the Therapeutic Potential of NHC Platinum Compounds in Metastatic Cutaneous Melanoma" International Journal of Molecular Sciences 21, no. 21: 7826. https://doi.org/10.3390/ijms21217826
APA StyleCharignon, E., Bouché, M., Clave-Darcissac, C., Dahm, G., Ichim, G., Clotagatide, A., Mertani, H. C., Telouk, P., Caramel, J., Diaz, J.-J., Bellemin-Laponnaz, S., Bouvet, P., & Billotey, C. (2020). In Cellulo Evaluation of the Therapeutic Potential of NHC Platinum Compounds in Metastatic Cutaneous Melanoma. International Journal of Molecular Sciences, 21(21), 7826. https://doi.org/10.3390/ijms21217826










