Leptin Downregulates Angulin-1 in Active Crohn’s Disease via STAT3
Abstract
:1. Introduction
2. Results
2.1. Patients Features
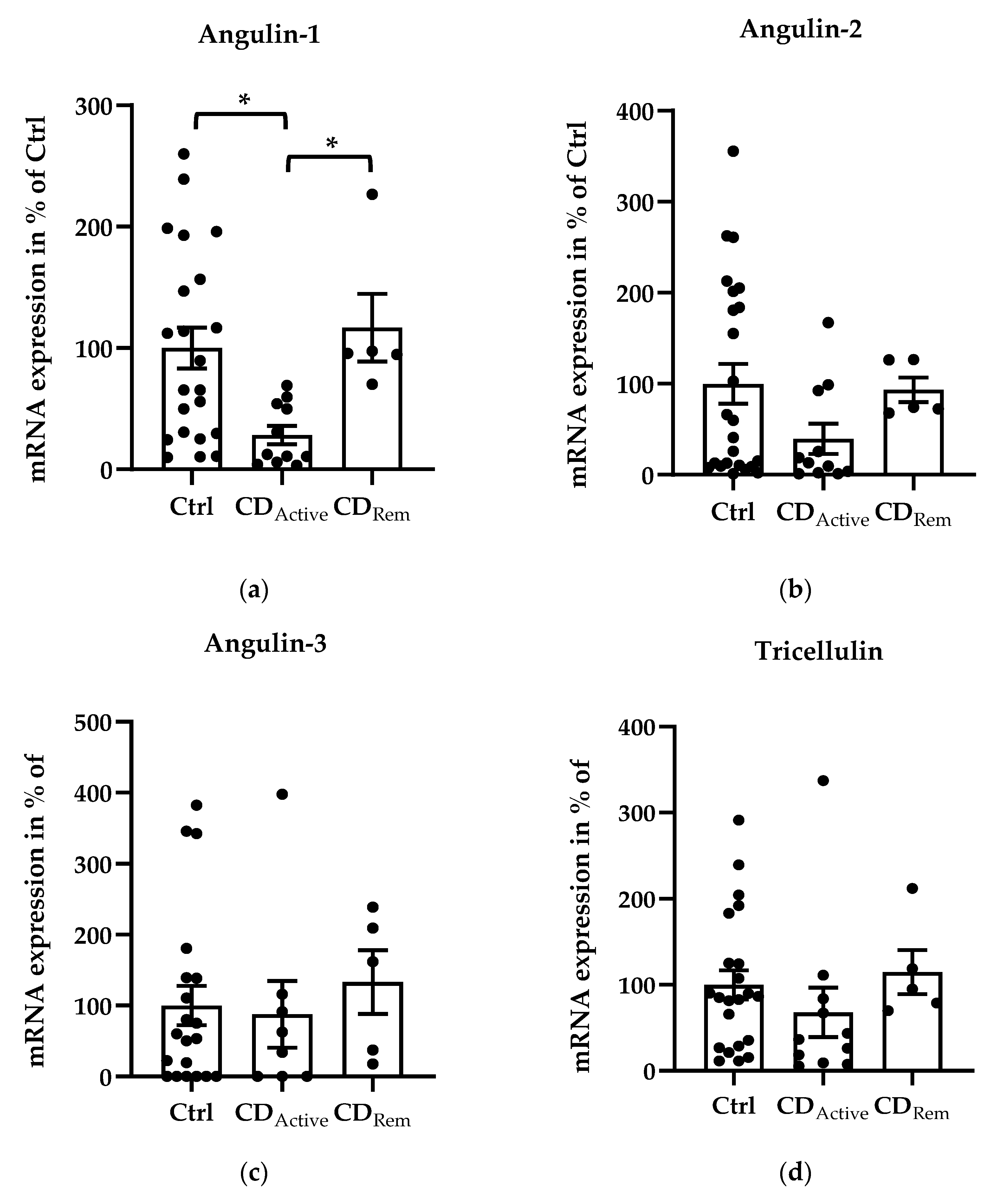
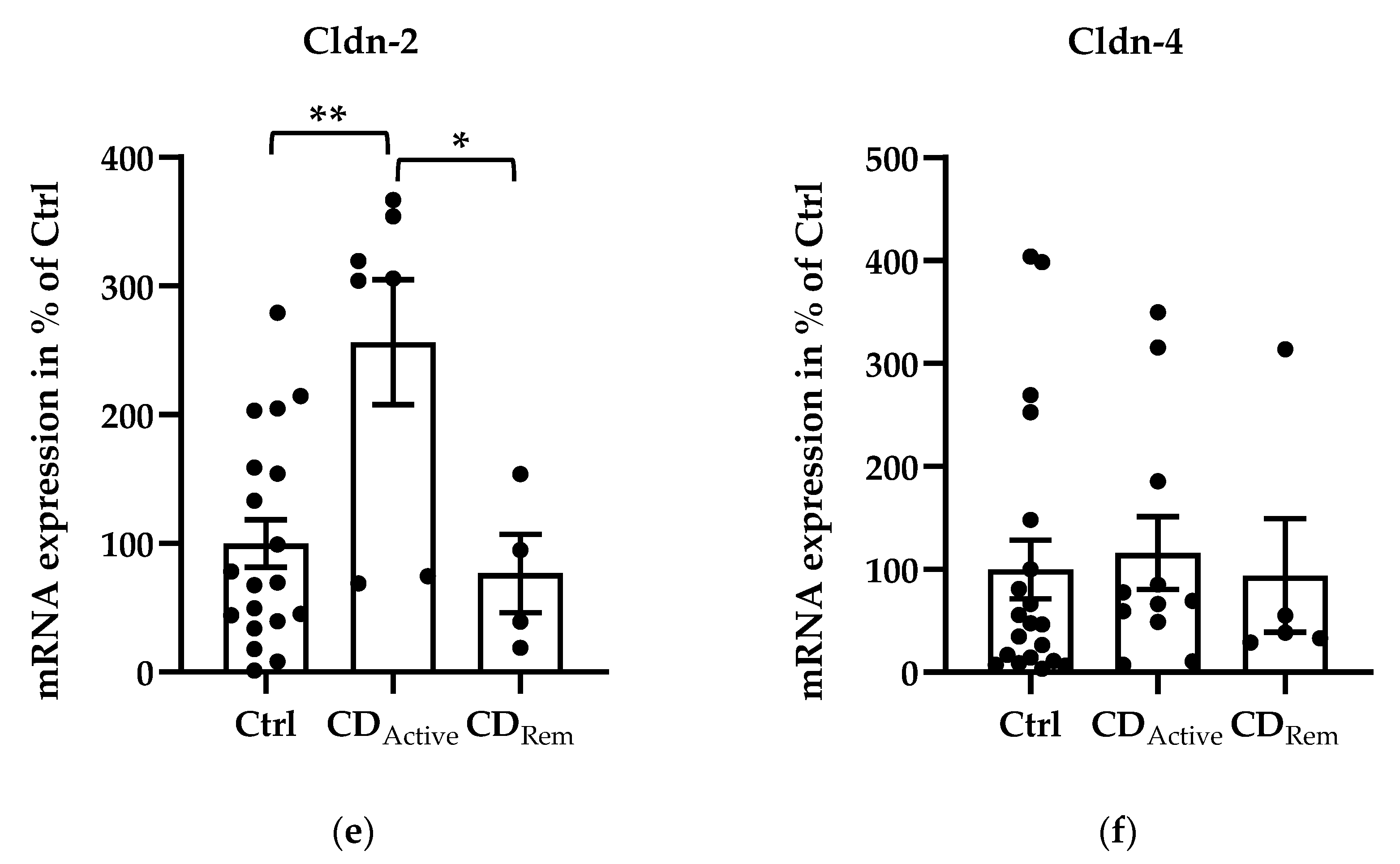
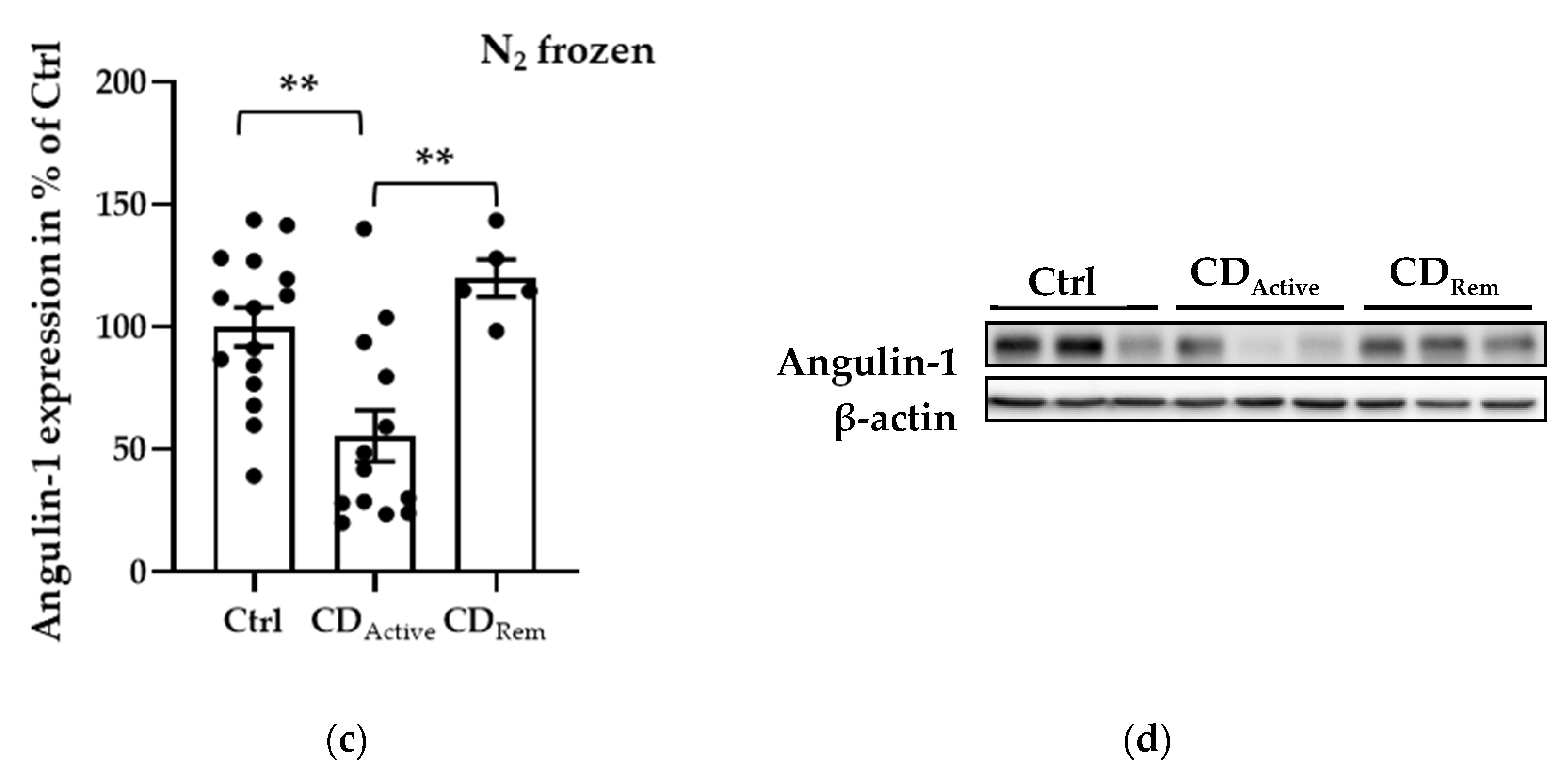
2.2. Expression of Angulins in Intestinal Biopsies
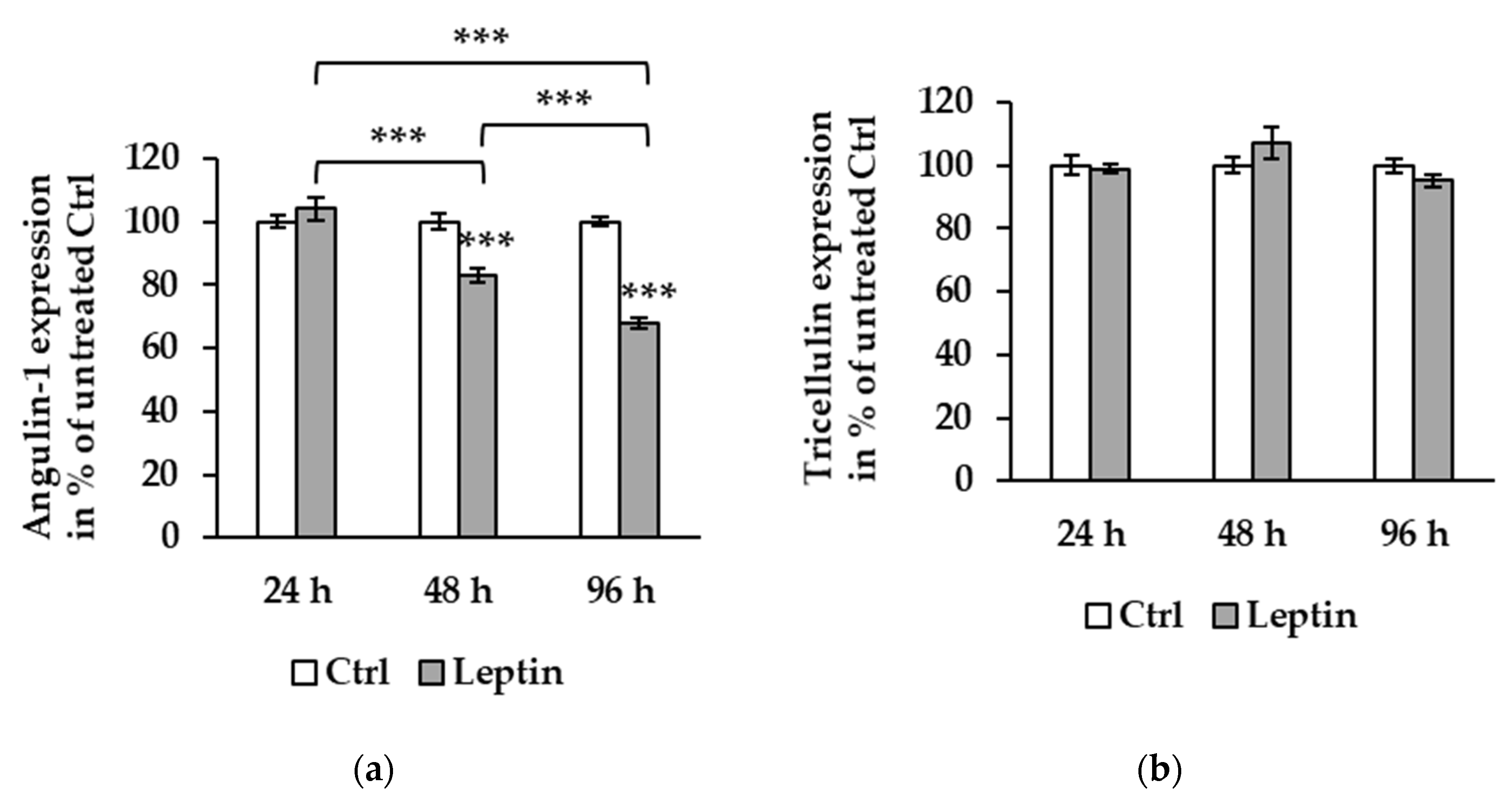
2.3. Cytokine Effects of Angulins in Human Intestinal Epithelial Cell Lines
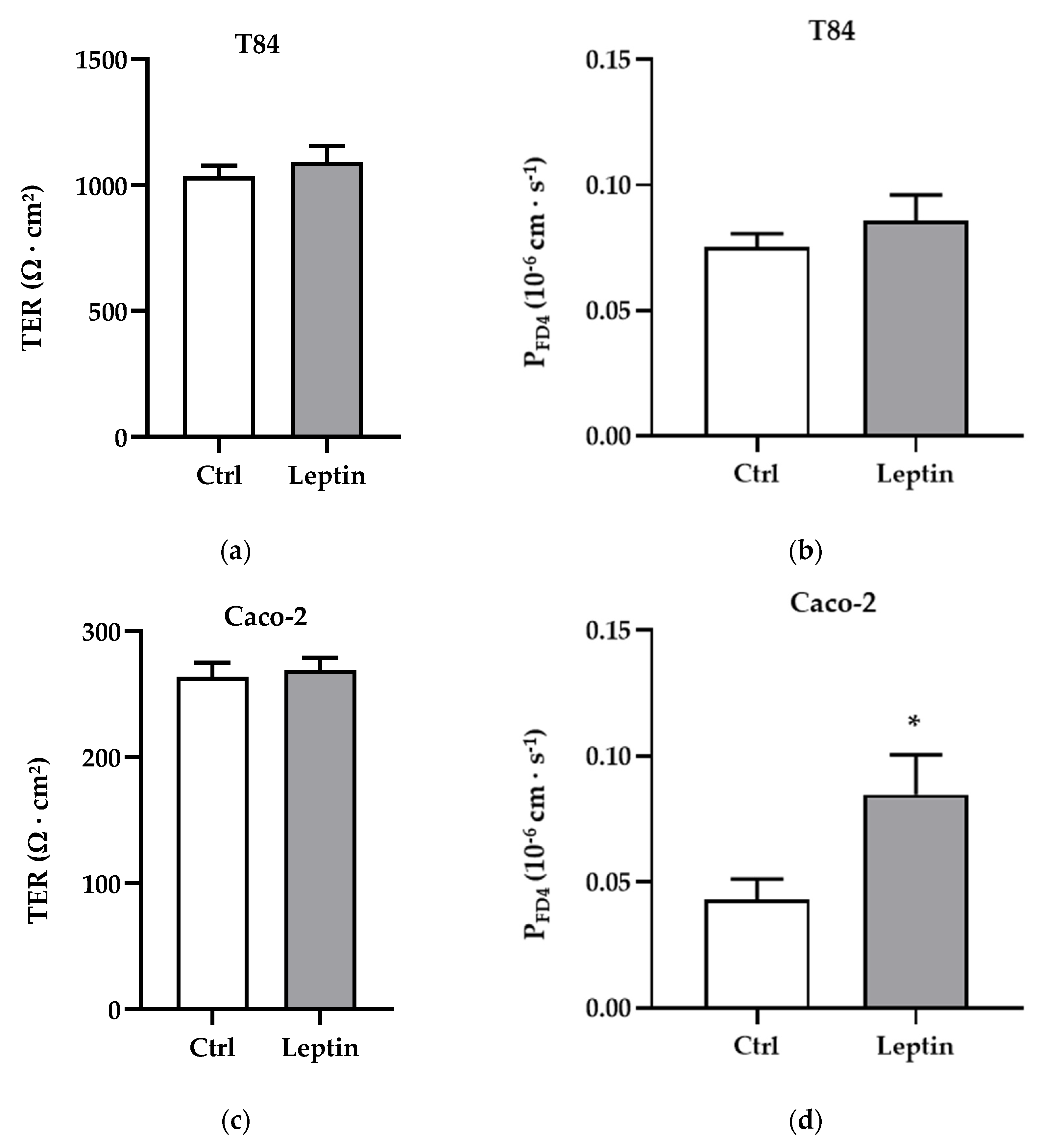
2.4. Barrier Function of T84 and Caco-2 Cells Treated with Leptin
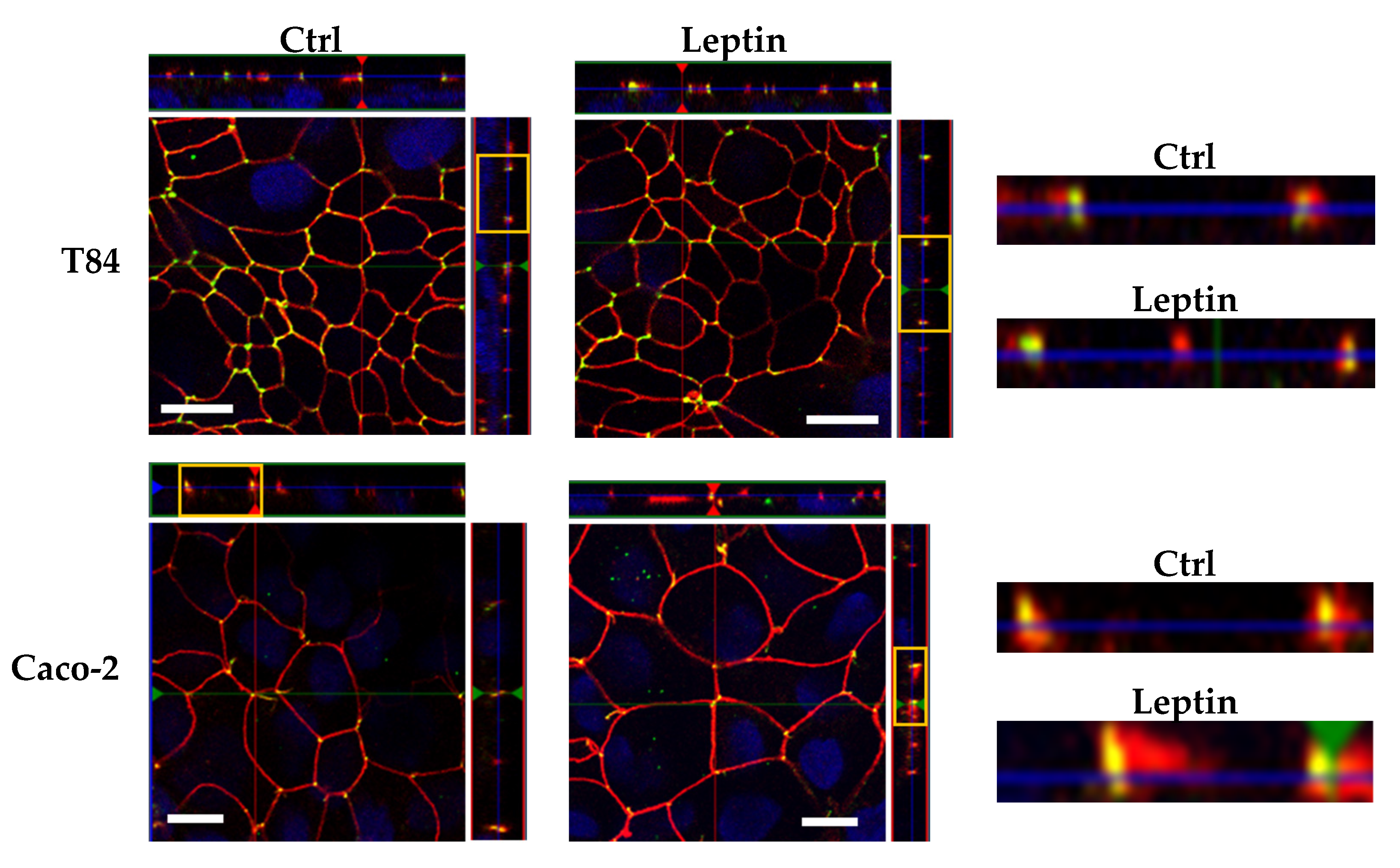
2.5. Tricellulin Localization after Leptin Treatment
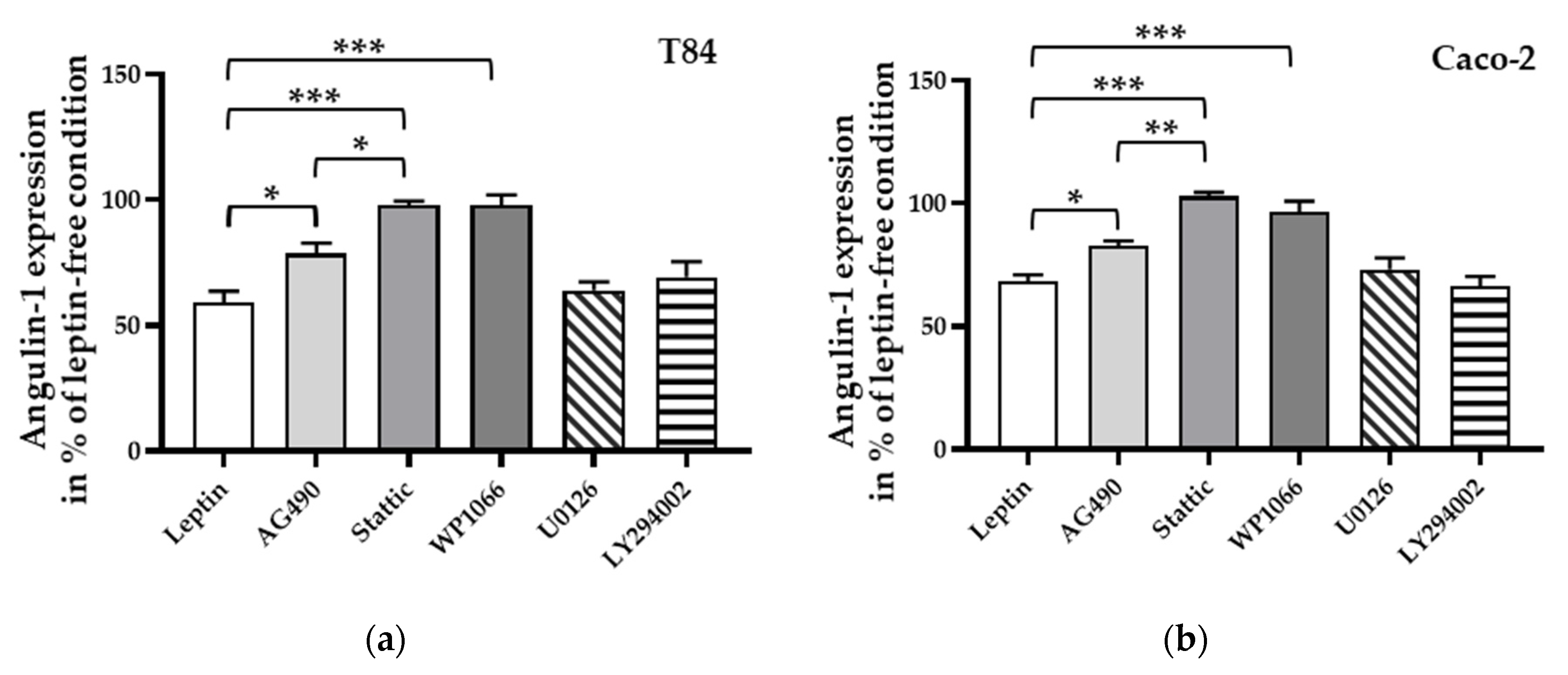
2.6. Signaling Pathway of Leptin
3. Discussion
3.1. The Involvement of Angulin-1 in CD
3.2. Leptin Affecting the Expression of Angulin-1
3.3. Leptin-Regulated Downregulation of Angulin-1 via STAT3 Pathway
3.4. Concluding Remarks
4. Materials and Methods
4.1. Patients and Study Criteria
4.2. Cell Lines
4.3. Cytokines and Inhibitors Experiments
4.4. Western Blotting
4.5. RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR (qRT-PCR)
4.6. Immunofluorescent Staining
4.7. Electrophysiological and Paracellular Flux Measurements
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| bTJ | Bicellular tight junction |
| CD | Crohn’s disease |
| Cldn | Claudin |
| DAPI | 4’,6-diamidino-2-phenylindole |
| ERK | Extracellular signal-regulated kinases |
| FD4 | FITC-dextran 4 kDa |
| FFPE | Formalin-fixed paraffin-embedded |
| FITC | Fluorescein isothiocyanate |
| IBD | Inflammatory bowel disease |
| IFN | Interferon |
| IL | Interleukin |
| JAK | Janus kinase |
| LR | Leptin receptor |
| LSM | Lasor scanning microscope |
| LSR | Lipolysis-stimulated lipoprotein receptor |
| MAPK | Mitogen-activated protein kinase |
| PI3K | Phosphoinositide 3-kinase |
| SES-CD | Simple endoscopic score for CD |
| STAT | Signal transducer and activator of transcription |
| TER | Transepithelial resistance |
| TJ | Tight junction |
| tTJ | Tricellular tight junction |
| TNF | Tumor necrosis factor |
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend Your Fences: The Epithelial Barrier and its Relationship With Mucosal Immunity in Inflammatory Bowel Disease. Cell Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Crohn, B.B.; Ginzburg, L.; Oppenheimer, G.D. Regional ileitis: A pathologic and clinical entity. Am. J. Med. 1952, 13, 583–590. [Google Scholar] [CrossRef]
- Sitaraman, S.; Liu, X.; Charrier, L.; Gu, L.H.; Ziegler, T.R.; Gewirtz, A.; Merlin, D. Colonic leptin: Source of a novel proinflammatory cytokine involved in IBD. FASEB J. 2004, 18, 696–698. [Google Scholar] [CrossRef]
- Barbier, M.; Vidal, H.; Desreumaux, P.; Dubuquoy, L.; Bourreille, A.; Colombel, J.F.; Cherbut, C.; Galmiche, J.P. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol. Clin. Biol. 2003, 27, 987–991. [Google Scholar] [CrossRef]
- Paul, G.; Schäffler, A.; Neumeier, M.; Fürst, A.; Bataillle, F.; Buechler, C.; Müller-Ladner, U.; Schölmerich, J.; Rogler, G.; Herfarth, H. Profiling adipocytokine secretion from creeping fat in Crohn’s disease. Inflamm. Bowel Dis. 2006, 12, 471–477. [Google Scholar] [CrossRef]
- Diamond, J.M. Twenty-first Bowditch lecture. The epithelial junction: Bridge, gate, and fence. Physiologist 1977, 20, 10–18. [Google Scholar]
- Farquhar, M.G.; Palade, G.E. Cell junctions in amphibian skin. J. Cell. Biol. 1965, 26, 263–291. [Google Scholar] [CrossRef] [Green Version]
- Staehelin, L.A.; Mukherjee, T.M.; Williams, A.W. Freeze-etch appearance of the tight junctions in the epithelium of small and large intestine of mice. Protoplasma 1969, 67, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Staehelin, L.A. Further observations on the fine structure of freeze-cleaved tight junctions. J. Cell. Sci. 1973, 13, 763–786. [Google Scholar] [PubMed]
- Ikenouchi, J.; Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S.; Tsukita, S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell. Biol. 2005, 171, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Krug, S.M.; Amasheh, S.; Richter, J.F.; Milatz, S.; Gunzel, D.; Westphal, J.K.; Huber, O.; Schulzke, J.D.; Fromm, M. Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell. 2009, 20, 3713–3724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krug, S.M.; Bojarski, C.; Fromm, A.; Lee, I.M.; Dames, P.; Richter, J.F.; Turner, J.R.; Fromm, M.; Schulzke, J.D. Tricellulin is regulated via interleukin-13-receptor alpha2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal. Immunol. 2018, 11, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Higashi, T.; Tokuda, S.; Kitajiri, S.; Masuda, S.; Nakamura, H.; Oda, Y.; Furuse, M. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2—Tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J. Cell. Sci. 2013, 126 (Pt 4), 966–977. [Google Scholar] [CrossRef] [Green Version]
- Yen, F.T.; Mann, C.J.; Guermani, L.M.; Hannouche, N.F.; Hubert, N.; Hornick, C.A.; Bordeau, V.N.; Agnani, G.; Bihain, B.E. Identification of a lipolysis-stimulated receptor that is distinct from the LDL receptor and the LDL receptor-related protein. Biochemistry 1994, 33, 1172–1180. [Google Scholar] [CrossRef]
- Masuda, S.; Oda, Y.; Sasaki, H.; Ikenouchi, J.; Higashi, T.; Akashi, M.; Nishi, E.; Furuse, M. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J. Cell. Sci. 2011, 124 (Pt 4), 548–555. [Google Scholar] [CrossRef] [Green Version]
- El Hajj, A.; Yen, F.T.; Oster, T.; Malaplate, C.; Pauron, L.; Corbier, C.; Lanhers, M.-C.; Claudepierre, T. Age-related changes in regiospecific expression of Lipolysis Stimulated Receptor (LSR) in mice brain. PLoS ONE 2019, 14, e0218812. [Google Scholar] [CrossRef]
- Xie, T.; Stathopoulou, M.G.; Akbar, S.; Oster, T.; Siest, G.; Yen, F.T.; Visvikis-Siest, S. Effect of LSR polymorphism on blood lipid levels and age-specific epistatic interaction with the APOE common polymorphism. Clin. Genet. 2018, 93, 846–852. [Google Scholar] [CrossRef]
- Akbar, S.; Pincon, A.; Lanhers, M.C.; Claudepierre, T.; Corbier, C.; Gregory-Pauron, L.; Malaplate-Armand, C.; Visvikis, A.; Oster, T.; Yen, F.T. Expression profile of hepatic genes related to lipid homeostasis in LSR heterozygous mice contributes to their increased response to high-fat diet. Physiol. Genom. 2016, 48, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Akbar, S.; Stathopoulou, M.G.; Oster, T.; Masson, C.; Yen, F.T.; Visvikis-Siest, S. Epistatic interaction of apolipoprotein E and lipolysis-stimulated lipoprotein receptor genetic variants is associated with Alzheimer’s disease. Neurobiol. Aging 2018, 69, 292.e1–292.e5. [Google Scholar] [CrossRef]
- Sugase, T.; Takahashi, T.; Serada, S.; Fujimoto, M.; Ohkawara, T.; Hiramatsu, K.; Koh, M.; Saito, Y.; Tanaka, K.; Miyazaki, Y.; et al. Lipolysis-stimulated lipoprotein receptor overexpression is a novel predictor of poor clinical prognosis and a potential therapeutic target in gastric cancer. Oncotarget 2018, 9, 32917–32928. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, K.; Serada, S.; Enomoto, T.; Takahashi, Y.; Nakagawa, S.; Nojima, S.; Morimoto, A.; Matsuzaki, S.; Yokoyama, T.; Takahashi, T.; et al. LSR Antibody Therapy Inhibits Ovarian Epithelial Tumor Growth by Inhibiting Lipid Uptake. Cancer Res. 2018, 78, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Shimada, H.; Abe, S.; Kohno, T.; Satohisa, S.; Konno, T.; Takahashi, S.; Hatakeyama, T.; Arimoto, C.; Kakuki, T.; Kaneko, Y.; et al. Loss of tricellular tight junction protein LSR promotes cell invasion and migration via upregulation of TEAD1/AREG in human endometrial cancer. Sci. Rep. 2017, 7, 37049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, H.; Satohisa, S.; Kohno, T.; Konno, T.; Takano, K.-I.; Takahashi, S.; Hatakeyama, T.; Arimoto, C.; Saito, T.; Kojima, T. Downregulation of lipolysis-stimulated lipoprotein receptor promotes cell invasion via claudin-1-mediated matrix metalloproteinases in human endometrial cancer. Oncol. Lett. 2017, 14, 6776–6782. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Satohisa, S.; Kohno, T.; Takahashi, S.; Hatakeyama, T.; Konno, T.; Tsujiwaki, M.; Saito, T.; Kojima, T. The roles of tricellular tight junction protein lipolysis-stimulated lipoprotein receptor in malignancy of human endometrial cancer cells. Oncotarget 2016, 7, 27735–27752. [Google Scholar] [CrossRef] [Green Version]
- Reaves, D.K.; Fagan-Solis, K.D.; Dunphy, K.; Oliver, S.D.; Scott, D.W.; Fleming, J.M. The role of lipolysis stimulated lipoprotein receptor in breast cancer and directing breast cancer cell behavior. PLoS ONE 2014, 9, e91747. [Google Scholar] [CrossRef]
- Reaves, D.K.; Hoadley, K.A.; Fagan-Solis, K.D.; Jima, D.D.; Bereman, M.; Thorpe, L.; Hicks, J.; McDonald, D.; Troester, M.A.; Perou, C.M.; et al. Nuclear Localized LSR: A Novel Regulator of Breast Cancer Behavior and Tumorigenesis. Mol. Cancer Res. 2017, 15, 165–178. [Google Scholar] [CrossRef] [Green Version]
- García, J.M.; Peña, C.; García, V.; Domínguez, G.; Muñoz, C.; Silva, J.; Millán, I.; Diaz, R.; Lorenzo, Y.; Rodriguez, R.; et al. Prognostic value of LISCH7 mRNA in plasma and tumor of colon cancer patients. Clin. Cancer Res. 2007, 13, 6351–6358. [Google Scholar] [CrossRef] [Green Version]
- Nagahama, M.; Takehara, M.; Kobayashi, K. Interaction of Clostridium perfringens Iota Toxin and Lipolysis-Stimulated Lipoprotein Receptor (LSR). Toxins (Basel) 2018, 10, 405. [Google Scholar] [CrossRef] [Green Version]
- Hemmasi, S.; Czulkies, B.A.; Schorch, B.; Veit, A.; Aktories, K.; Papatheodorou, P. Interaction of the Clostridium difficile Binary Toxin CDT and Its Host Cell Receptor, Lipolysis-stimulated Lipoprotein Receptor (LSR). J. Biol. Chem. 2015, 290, 14031–14044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenger, C.; Hanse, M.; Pratte, D.; Mbala, M.L.; Akbar, S.; Koziel, V.; Escanye, M.C.; Kriem, B.; Malaplate-Armand, C.; Olivier, J.L.; et al. Up-regulation of hepatic lipolysis stimulated lipoprotein receptor by leptin: A potential lever for controlling lipid clearance during the postprandial phase. Faseb J. 2010, 24, 4218–4228. [Google Scholar] [CrossRef]
- Krug, S.M.; Schulzke, J.D.; Fromm, M. Tight junction, selective permeability, and related diseases. Semin. Cell. Dev. Biol. 2014, 36, 166–176. [Google Scholar] [CrossRef]
- Zeissig, S.; Burgel, N.; Gunzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Myers, M.G.; Cowley, M.A.; Munzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef] [Green Version]
- Banks, A.S.; Davis, S.M.; Bates, S.H.; Myers, M.G., Jr. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 2000, 275, 14563–14572. [Google Scholar] [CrossRef] [Green Version]
- Plum, L.; Rother, E.; Munzberg, H.; Wunderlich, F.T.; Morgan, D.A.; Hampel, B.; Shanabrough, M.; Janoschek, R.; Konner, A.C.; Alber, J.; et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell. Metab. 2007, 6, 431–445. [Google Scholar] [CrossRef] [Green Version]
- Krug, S.M. Contribution of the tricellular tight junction to paracellular permeability in leaky and tight epithelia. Ann. N. Y. Acad. Sci. 2017, 1397, 219–230. [Google Scholar] [CrossRef]
- Weidinger, C.; Ziegler, J.F.; Letizia, M.; Schmidt, F.; Siegmund, B. Adipokines and Their Role in Intestinal Inflammation. Front. Immunol. 2018, 9, 1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tartaglia, L.A. The leptin receptor. J. Biol. Chem. 1997, 272, 6093–6096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaab, M.; Kratzsch, J. The soluble leptin receptor. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 661–670. [Google Scholar] [CrossRef]
- Hekerman, P.; Zeidler, J.; Bamberg-Lemper, S.; Knobelspies, H.; Lavens, D.; Tavernier, J.; Joost, H.G.; Becker, W. Pleiotropy of leptin receptor signalling is defined by distinct roles of the intracellular tyrosines. Febs J. 2005, 272, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Bjorbak, C.; Lavery, H.J.; Bates, S.H.; Olson, R.K.; Davis, S.M.; Flier, J.S.; Myers, M.G., Jr. SOCS3 mediates feedback inhibition of the leptin receptor via Tyr985. J. Biol. Chem. 2000, 275, 40649–40657. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, A.; Yasukawa, H.; Shouda, T.; Kitamura, T.; Dikic, I.; Yoshimura, A. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J. Biol. Chem. 2000, 275, 29338–29347. [Google Scholar] [CrossRef] [Green Version]
- Bruno, A.; Conus, S.; Schmid, I.; Simon, H.U. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J. Immunol. 2005, 174, 8090–8096. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Riejos, P.; Goberna, R.; Sánchez-Margalet, V. Leptin promotes cell survival and activates Jurkat T lymphocytes by stimulation of mitogen-activated protein kinase. Clin. Exp. Immunol. 2008, 151, 505–518. [Google Scholar] [CrossRef]
- Lord, G.M.; Matarese, G.; Howard, J.K.; Baker, R.J.; Bloom, S.R.; Lechler, R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998, 394, 897–901. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Shi, F.D.; Zou, H.; Matarese, G.; La Cava, A. Cutting edge: Leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J. Immunol. 2013, 190, 3054–3058. [Google Scholar] [CrossRef] [Green Version]
- Genser, L.; Aguanno, D.; Soula, H.A.; Dong, L.; Trystram, L.; Assmann, K.; Salem, J.E.; Vaillant, J.C.; Oppert, J.M.; Laugerette, F.; et al. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J. Pathol. 2018, 246, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Gomollon, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreusel, K.M.; Fromm, M.; Schulzke, J.D.; Hegel, U. Cl- secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am. J. Physiol. 1991, 261, C574–C582. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | Controls (n = 24) | CD (n = 19) |
|---|---|---|
| Age (median, range) | 54 (24–66) | 35 (25–64) |
| Gender (male/female) | 8/16 | 5/14 |
| SES-CD, n | ||
| Remission (0–2) | - | 5 |
| Active (> 2) | - | 14 |
| Cell Line | Basic Medium | Source | Supplements |
|---|---|---|---|
| T84 | DMEM: F-12 medium | Sigma-Aldrich, Steinheim, Germany | 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin |
| Caco-2 | MEM with glutamax | Sigma-Aldrich, Steinheim, Germany | 15% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin |
| Concentration | Source | |
|---|---|---|
| Cytokines | ||
| TNFα | 500 U/mL | PeproTech, Hamburg, Germany |
| IFNγ | 1000 U/mL | PeproTech, Hamburg, Germany |
| Leptin | 200 ng/mL | PeproTech, Hamburg, Germany |
| IL-1β | 100 ng/mL | PeproTech, Hamburg, Germany |
| IL-6 | 10 ng/mL | Miltenyi Biotec, Bergisch Gladbach, Germany |
| IL-12 | 100 ng/mL | PeproTech, Hamburg, Germany |
| IL-17A | 100 ng/mL | PeproTech, Hamburg, Germany |
| IL-17F | 100 ng/mL | Miltenyi Biotec, Bergisch Gladbach, Germany |
| IL-21 | 100 ng/mL | Miltenyi Biotec, Bergisch Gladbach, Germany |
| IL-22 | 50 ng/mL | PeproTech, Hamburg, Germany |
| IL-23 | 100 ng/mL | PeproTech, Hamburg, Germany |
| IL-33 | 100 ng/mL | Miltenyi Biotec, Bergisch Gladbach, Germany |
| Inhibitors | ||
| AG490 | 100 μM | Calbiochem, Darmstadt, Germany |
| LY294002 | 10 μM | Calbiochem, Darmstadt, Germany |
| Stattic | 20 μM | Calbiochem, Darmstadt, Germany |
| U0126 | 10 μM | Cell signaling Technology, Frankfurt am Main, Germany |
| WP1066 | 5 μM | Calbiochem, Darmstadt, Germany |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.-C.E.; Bojarski, C.; Branchi, F.; Fromm, M.; Krug, S.M. Leptin Downregulates Angulin-1 in Active Crohn’s Disease via STAT3. Int. J. Mol. Sci. 2020, 21, 7824. https://doi.org/10.3390/ijms21217824
Hu J-CE, Bojarski C, Branchi F, Fromm M, Krug SM. Leptin Downregulates Angulin-1 in Active Crohn’s Disease via STAT3. International Journal of Molecular Sciences. 2020; 21(21):7824. https://doi.org/10.3390/ijms21217824
Chicago/Turabian StyleHu, Jia-Chen E., Christian Bojarski, Federica Branchi, Michael Fromm, and Susanne M. Krug. 2020. "Leptin Downregulates Angulin-1 in Active Crohn’s Disease via STAT3" International Journal of Molecular Sciences 21, no. 21: 7824. https://doi.org/10.3390/ijms21217824







